Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries Using an Electronic Nose and GC-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
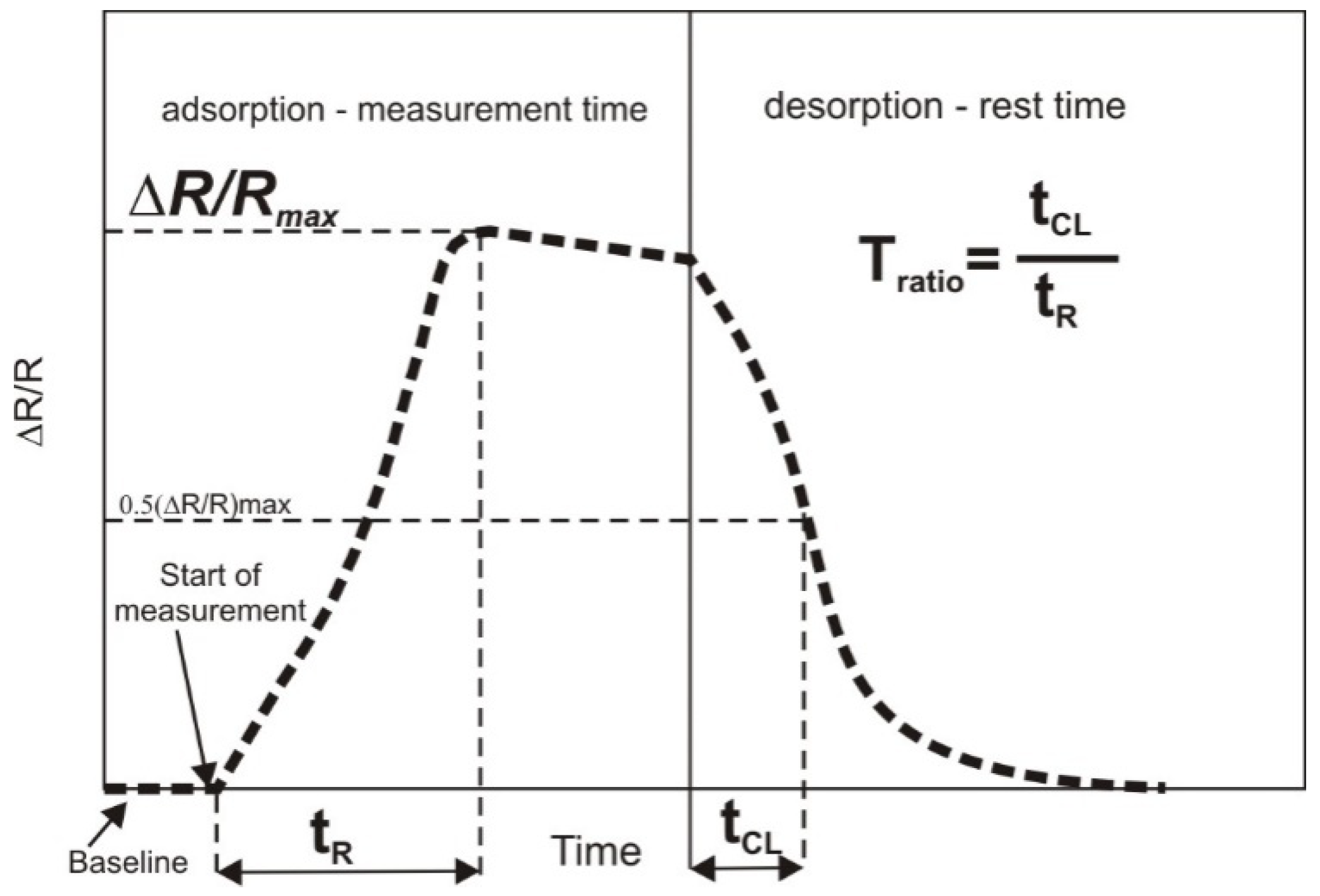
2.2. Electronic Nose
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4. Chemometrics and Statistical Analysis
3. Results and Discussion
3.1. GC-MS Analysis
3.2. Electronic Nose and Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coffee Market Report. April 2018. Available online: http://www.ico.org/documents/cy2017-18/cmr-0418-e.pdf (accessed on 13 May 2018).
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.J.; Tan, L.H.; Zhao, J.P.; Hu, R.S.; Lu, M.Q. Characterization of fatty acid, amino acid and volatile compound compositions and bioactive components of seven coffee (Coffea robusta) cultivars grown in Hainan province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef]
- Dobrzański, B., Jr. Coffee Quality and Properties; Scientific Publisher FRNA, Polish Academy of Sciences: Lublin, Poland, 2009; p. 181. ISBN 978-83-60489-16-1. [Google Scholar]
- Yang, N.; Liu, C.J.; Liu, X.K.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defect. Food Chem. 2016, 211, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems (Review). Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Toledo, P.R.A.B.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship between the Different Aspects Related to Coffee Quality and Their Volatile Compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef] [Green Version]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D’Ambrosio, L.; Chatelain, K.; Bongartz, A. Comparison of nine common coffee extraction methods: Instrumental and sensory analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef] [Green Version]
- Amanpour, A.; Selli, S. Differentiation of volatile profiles and odor activity values of Turkish coffee and French press coffee. J. Food Process. Preserv. 2015, 40, 1116–1124. [Google Scholar] [CrossRef]
- Michishita, T.; Akiyama, M.; Hirano, Y.; Ikeda, M.; Sagara, Y.; Araki, T. Gas chromatography/olfactometry and electronic nose analyses of retronasal aroma of espresso and correlation with sensory evaluation by an artificial neural network. J. Food Sci. 2010, 75, S477–S489. [Google Scholar] [CrossRef]
- Knysak, D. Volatile compounds profiles in unroasted Coffea arabica and Coffea canephora beans from different countries. Food Sci. Technol. 2017, 37, 444–448. [Google Scholar] [CrossRef] [Green Version]
- Peris, M.; Escuder-Gilabert, L. Electronic noses and tongues to access food authentication and adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Brassanello, D.; Liberto, E.; Cordero, C.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C. Coffee aroma: Chemometric comparison of the chemical information provided by three different samplings combined with GC-MS to describe the sensory properties in cup. Food Chem. 2017, 214, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Rusinek, R.; Gancarz, M.; Krekora, M.; Nawrocka, A. A novel method for generation of a fingerprint using electronic nose on the example of rapeseed spoilage. J. Food Sci. 2019, 84, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusinek, R.; Siger, A.; Gawrysiak-Witulska, M.; Rokosik, E.; Malaga-Toboła, U.; Gancarz, M. Application of an electronic nose for determination of pre-pressing treatment of rapeseed based on the analysis of volatile compounds contained in pressed oil. Int. J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Rusinek, R.; Gancarz, M.; Nawrocka, A. Application of an electronic nose with novel method for generation of smellprints for testing the suitability for consumption of wheat bread during 4-day storage. LWT Food Sci. Technol. 2020, 117, 108665. [Google Scholar] [CrossRef]
- Gancarz, M.; Nawrocka, A.; Rusinek, R. Identification of volatile organic compounds and their concentrations using a novel method analysis of MOS sensors signal. J. Food Sci. 2019, 84, 2077–2085. [Google Scholar] [CrossRef]
- Lippolis, V.; Pascale, M.; Cervellieri, S.; Damascelli, A.; Visconti, A. Screening of deoxynivalenol contamination in durum wheat by MOS-based electronic nose and identification of the relevant pattern of volatile compounds. Food Cont. 2014, 37, 263–271. [Google Scholar] [CrossRef]
- Jeleń, H.H. Use of solid phase microextraction (SPME) for profiling fungal volatile metabolites. Lett. Appl. Microbiol. 2003, 36, 263–267. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Dong, W.J.; Zhao, J.P.; Hu, R.S.; Dong, Y.P.; Tan, L.H. Differentiation of Chinese robusta coffees according to species, using a combined electronic nose and tongue, with the aid of chemometrics. Food Chem. 2017, 229, 743–751. [Google Scholar] [CrossRef]
- Bröhan, M.; Huybrighs, T.; Wouters, C.; Van der Bruggen, B. Influence of storage conditions on aroma compounds in coffee pads using static headspace GC-MS. Food Chem. 2009, 116, 480–483. [Google Scholar] [CrossRef]
- Costa Freitas, A.M.; Mosca, A.I. Coffee geographic origin—An aid to coffee differentiation. Food Res. Int. 1999, 32, 565–573. [Google Scholar] [CrossRef]
- Korhońová, M.; Hron, K.; Klimcíková, D.; Müller, L.; Bednář, P.; Barták, P. Coffee aroma—Statistical analysis of compositional data. Talanta 2009, 80, 710–715. [Google Scholar] [CrossRef] [PubMed]




| No. | Name of Compounds | Rtime * | Country of Origin | ||||
|---|---|---|---|---|---|---|---|
| Brazil, % | Ethiopia, % | Guatemala, % | Costa Rica, % | Peru, % | |||
| 1 | 2-Buten-1-ol | 1.10 | 1.7 | 2.1 | 2.5 | 2.3 | 2.2 |
| 2 | 2-oxopropanal | 1.17 | 14.1 | 3.0 | 8.6 | 10.0 | 16.4 |
| 3 | Methyl-D3 1-diderterio-2-propenyl ether | 1.35 | 6.3 | 11.4 | 8.3 | 7.7 | 6.1 |
| 4 | Acetalaldechyde | 1.45 | 4.9 | 8.5 | 3.4 | 4.9 | 5.9 |
| 5 | Pirydine | 1.76 | 28.7 | 15.8 | 15.4 | 13.5 | 21.9 |
| 6 | Butan-2-one | 2.31 | 6.6 | 10.3 | 8.3 | 9.9 | 7.0 |
| 7 | 2-methylpirimidine | 2.54 | 2.9 | 9.3 | 10.8 | 10.5 | 7.7 |
| 8 | 2-furanccarboxaldehyde | 2.64 | 10.9 | 10.6 | 7.1 | 7.8 | 5.7 |
| 9 | 2-furanmethanol | 2.92 | 5.5 | 8.1 | 7.9 | 8.4 | 7.7 |
| 10 | Acetic acid ethenyl ester | 3.16 | 3.2 | 3.5 | 3.9 | 3.8 | 4.2 |
| 11 | 4.6-dimethylpyrimidine | 4.25 | 5.5 | 6.2 | 7.6 | 6.8 | 4.4 |
| 12 | 2-pethylpyrazine | 4.35 | 2.5 | 2.4 | 3.3 | 3.1 | 1.8 |
| 13 | Cis-ocimene | 4.87 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 |
| 14 | 5-methylfuran-2-carbaldehyde | 5.78 | 1.7 | 3.2 | 3.7 | 3.8 | 2.6 |
| 15 | 2-furylmethyl acetale | 6.91 | 2.3 | 2.2 | 3.4 | 3.5 | 3.5 |
| 16 | N.N-dimethylpyridyn-4-amine | 7.06 | 1.4 | 1.6 | 2.8 | 2.0 | 1.5 |
| 17 | 1-methyl-2-cyano-2-piperidine | 7.23 | 0.9 | 1.4 | 2.5 | 1.8 | 1.3 |
| 18 | 2.5-dimethyl-3-ethylpyrazine | 9.73 | 0.6 | 0.3 | 0.3 | 0.1 | 0.0 |
| 19 | Heptasiloxan | 34.36 | 0.3 | 0.2 | 0.3 | 0.1 | 0.2 |
| Sum, Σ (%) | 100 | 100 | 100 | 100 | 100 | ||
| Country of Origin | |||||
|---|---|---|---|---|---|
| Parameter | Brazil | Ethiopia | Guatemala | Costa Rica | Peru |
| 2602 ΔR/Rmax | 3.17 | 2.22 | 1.17 | 1.88 | 1.98 |
| SD | 0.06 | 0.04 | 0.02 | 0.04 | 0.04 |
| AS-MLV-P2 ΔR/Rmax | 2.13 | 1.33 * | 0.57 | 0.86 | 1.35 * |
| SD | 0.04 | 0.03 | 0.01 | 0.02 | 0.03 |
| 2603 ΔR/Rmax | 1.77 | 1.36 | 0.65 | 1.05 | 1.11 |
| SD | 0.04 | 0.03 | 0.01 | 0.02 | 0.02 |
| 2612 ΔR/Rmax | 0.30 | 0.05 * | 0.02 | 0.07 | 0.06 * |
| SD | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 2610 ΔR/Rmax | 0.58 | 0.28 | 0.14 | 0.22 | 0.22 |
| SD | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 2611 ΔR/Rmax | 0.49 | 0.26 | 0.12 | 0.20 | 0.17 |
| SD | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 2620 ΔR/Rmax | 1.12 | 0.54 | 0.25 | 0.42 | 0.40 |
| SD | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| 2600 ΔR/Rmax | 1.08 | 0.50 | 0.27 | 0.47 | 0.44 |
| SD | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| 2602 Tratio | 2.19 | 1.46 * | 1.80 | 1.38 | 1.48 * |
| SD | 0.04 | 0.03 | 0.04 | 0.03 | 0.03 |
| AS-MLV-P2 Tratio | 2.29 | 6.15 | 2.57 | 1.27 | 3.11 |
| SD | 0.05 | 0.12 | 0.05 | 0.03 | 0.06 |
| 2603 Tratio | 2.33 | 1.50 * | 1.80 | 1.34 | 1.50 * |
| SD | 0.05 | 0.04 | 0.04 | 0.03 | 0.03 |
| 2612 Tratio | 2.35 | 2.58 | 3.00 | 1.40 | 2.82 |
| SD | 0.05 | 0.05 | 0.06 | 0.03 | 0.06 |
| 2610 Tratio | 8.80 | 3.20 | 3.00 | 1.32 | 3.39 |
| SD | 0.18 | 0.06 | 0.06 | 0.03 | 0.07 |
| 2611 Tratio | 8.00 | 3.00 * | 3.00 * | 1.44 | 3.21 |
| SD | 0.16 | 0.06 | 0.07 | 0.03 | 0.06 |
| 2620 Tratio | 3.14 | 1.73 | 3.97 | 1.22 | 2.50 |
| SD | 0.06 | 0.03 | 0.08 | 0.02 | 0.05 |
| 2600 Tratio | 2.79 | 1.78 | 2.30 | 1.27 | 1.62 |
| SD | 0.06 | 0.04 | 0.05 | 0.03 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marek, G.; Dobrzański, B., Jr.; Oniszczuk, T.; Combrzyński, M.; Ćwikła, D.; Rusinek, R. Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries Using an Electronic Nose and GC-MS. Sensors 2020, 20, 2124. https://doi.org/10.3390/s20072124
Marek G, Dobrzański B Jr., Oniszczuk T, Combrzyński M, Ćwikła D, Rusinek R. Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries Using an Electronic Nose and GC-MS. Sensors. 2020; 20(7):2124. https://doi.org/10.3390/s20072124
Chicago/Turabian StyleMarek, Gancarz, Bohdan Dobrzański, Jr., Tomasz Oniszczuk, Maciej Combrzyński, Daniel Ćwikła, and Robert Rusinek. 2020. "Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries Using an Electronic Nose and GC-MS" Sensors 20, no. 7: 2124. https://doi.org/10.3390/s20072124







