Gait Characteristics under Imposed Challenge Speed Conditions in Patients with Parkinson’s Disease During Overground Walking
Abstract
:1. Introduction
2. Materials and Methods
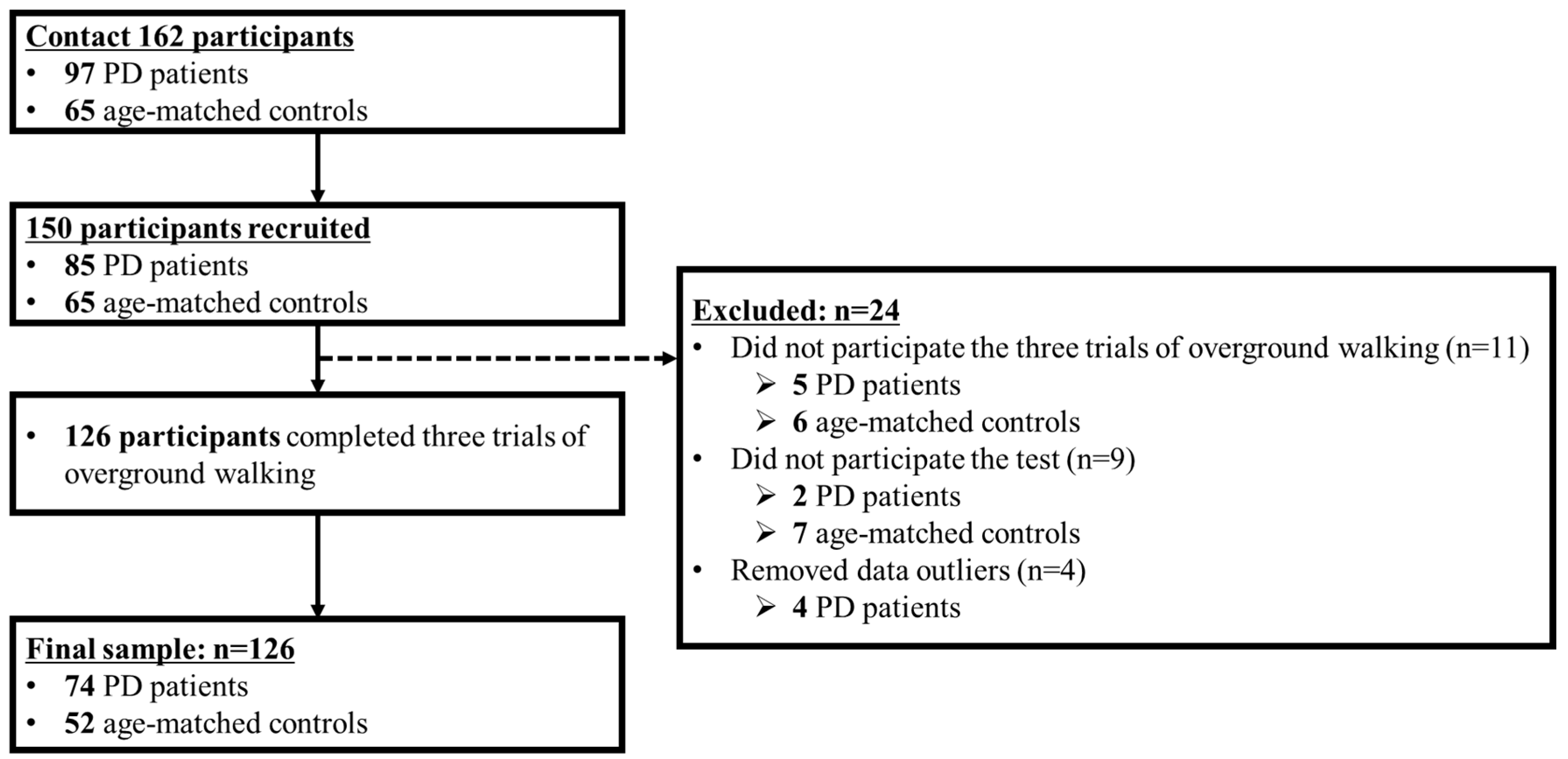
2.1. Participants
2.2. Instrumentation
2.3. Test Procedures
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Reliability of Estimation and Measurement Speeds
3.2. Group Differences: PD Patients vs. Controls
3.3. Speed Differences: Slower, Preferred, and Faster Speed Conditions
3.4. Classifier Variables for PD Patients and Controls
3.5. Relationship between Clinical Data and Gait Variables of PD Patients
4. Discussion
5. Conclusions
Ethical Statement
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henderson, E.J.; Lord, S.R.; Brodie, M.A.; Gaunt, D.M.; Lawrence, A.D.; Close, J.C.; Whone, A.I.; Ben-Shlomo, Y. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. Neurol. 2016, 15, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Cole, M.H.; Sweeney, M.; Conway, Z.J.; Blackmore, T.; Silburn, P.A. Imposed faster and slower walking speeds influence gait stability differently in Parkinson fallers. Arch. Phys. Med. Rehabil. 2017, 98, 639–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creaby, M.W.; Cole, M.H. Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Raffegeau, T.E.; Krehbiel, L.M.; Kang, N.; Thijs, F.J.; Altmann, L.J.; Cauraugh, J.H.; Hass, C.J. A meta-analysis: Parkinson’s disease and dual-task walking. Parkinsonism Relat. Disord. 2019, 62, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Quach, L.; Galica, A.M.; Jones, R.N.; Procter-Gray, E.; Manor, B.; Hannan, M.T.; Lipsitz, L.A. The nonlinear relationship between gait speed and falls: The maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J. Am. Geriatr. Soc. 2011, 59, 1069–1073. [Google Scholar] [CrossRef] [Green Version]
- Rennie, L.; Löfgren, N.; Moe-Nilssen, R.; Opheim, A.; Dietrichs, E.; Franzén, E. The reliability of gait variability measures for individuals with Parkinson’s disease and healthy older adults–the effect of gait speed. Gait Posture 2018, 62, 505–509. [Google Scholar] [CrossRef]
- Plotnik, M.; Bartsch, R.P.; Zeev, A.; Giladi, N.; Hausdorff, J.M. Effects of walking speed on asymmetry and bilateral coordination of gait. Gait Posture 2013, 38, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.J.; Peterson, D.S.; Earhart, G.M. Gait coordination in Parkinson disease: Effects of step length and cadence manipulations. Gait Posture 2013, 38, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.U.; Hausdorff, J.M.; Ferrucci, L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: Results from the Baltimore longitudinal study of ageing. Age Ageing 2010, 39, 688–694. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.J.; Wang, M.J. The change of gait parameters during walking at different percentage of preferred walking speed for healthy adults aged 20–60 years. Gait Posture 2010, 31, 131–135. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Giladi, N.; Peretz, C.; Herman, T.; Gruendlinger, L.; Hausdorff, J.M. Effect of gait speed on gait rhythmicity in Parkinson’s disease: Variability of stride time and swing time respond differently. J. Neuroeng. Rehabil. 2005, 2, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almarwani, M.; VanSwearingen, J.M.; Perera, S.; Sparto, P.J.; Brach, J.S. Challenging the motor control of walking: Gait variability during slower and faster pace walking conditions in younger and older adults. Arch. Gerontol. Geriatr. 2016, 66, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Luzi, S.; Murer, K.; de Bie, R.A.; de Bruin, E.D. Concurrent validity of a trunk tri-axial accelerometer system for gait analysis in older adults. Gait Posture 2009, 29, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Kim, Y.K.; Joo, J.Y.; Jeong, S.H.; Jeon, J.H.; Jung, D.Y. Effects of walking speed and age on the directional stride regularity and gait variability in treadmill walking. J. Mech. Sci. Technol. 2016, 30, 2899–2906. [Google Scholar] [CrossRef]
- Lee, M.; Youm, C.; Jeon, J.; Cheon, S.M.; Park, H. Validity of shoe-type inertial measurement units for Parkinson’s disease patients during treadmill walking. J. Neuroeng. Rehabil. 2018, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Plotnik, M.; Giladi, N.; Dagan, Y.; Hausdorff, J.M. Postural instability and fall risk in Parkinson’s disease: Impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp. Brain Res. 2011, 210, 529–538. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, C.O.; Kim, K.J.; Jeon, J.; Chang, H.; Kim, E.S.; Park, H. Quantitative analysis of the bilateral coordination and gait asymmetry using inertial measurement unit-based gait analysis. PLoS ONE 2019, 14, e0222913. [Google Scholar] [CrossRef] [Green Version]
- Noh, B.; Youm, C.; Lee, M.; Park, H. Age-specific differences in gait domains and global cognitive function in older women: Gait characteristics based on gait speed modification. PeerJ 2020, 8, e8820. [Google Scholar] [CrossRef] [Green Version]
- Nonnekes, J.; Růžička, E.; Nieuwboer, A.; Hallett, M.; Fasano, A.; Bloem, B.R. Compensation strategies for gait impairments in Parkinson disease: A review. JAMA Neurol. 2019, 76, 718–725. [Google Scholar] [CrossRef]
- Plotnik, M.; Giladi, N.; Hausdorff, J.M. A new measure for quantifying the bilateral coordination of human gait: Effects of aging and Parkinson’s disease. Exp. Brain Res. 2007, 181, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Neptune, R.R.; Sasaki, K.; Kautz, S.A. The effect of walking speed on muscle function and mechanical energetics. Gait Posture 2008, 28, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasirzade, A.; Sadeghi, H.; Mokhtarinia, H.R.; Rahimi, A. A review of selected factors affecting gait symmetry. Phys. Treat. -Specif. Phys. Ther. J. 2017, 7, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Moon, Y.; Sung, J.; An, R.; Hernandez, M.E.; Sosnoff, J.J. Gait variability in people with neurological disorders: A systematic review and meta-analysis. Hum. Mov. Sci. 2016, 47, 197–208. [Google Scholar] [CrossRef]
- Young, P.A.; Young, P.H.; Tolbert, D.L. Basic Clinical Neuroscience, 3rd ed.; Lippincott Williams & Wilkins: St. Louis, MO, USA, 2015; pp. 88–103. [Google Scholar]
- Hausdorff, J.M.; Cudkowicz, M.E.; Firtion, R.; Wei, J.Y.; Goldberger, A.L. Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov. Disord. 1998, 13, 428–437. [Google Scholar] [CrossRef]
- Wuehr, M.; Schniepp, R.; Pradhan, C.; Ilmberger, J.; Strupp, M.; Brandt, T.; Jahn, K. Differential effects of absent visual feedback control on gait variability during different locomotion speeds. Exp. Brain Res. 2013, 224, 287–294. [Google Scholar] [CrossRef]
- Nascimbeni, A.; Minchillo, M.; Salatino, A.; Morabito, U.; Ricci, R. Gait attentional load at different walking speeds. Gait Posture 2015, 41, 304–306. [Google Scholar] [CrossRef]
- Den Otter, A.R.; Geurts, A.C.H.; Mulder, T.; Duysens, J. Speed related changes in muscle activity from normal to very slow walking speeds. Gait Posture 2004, 19, 270–278. [Google Scholar] [CrossRef] [Green Version]


| Variables | PD (n = 74) | Controls (n = 52) |
|---|---|---|
| Sex (male/female) | 45/29 | 27/25 |
| Age (years) | 69.6 ± 5.7 | 71.4 ± 4.9 |
| Height (cm) | 159.8 ± 8.8 | 160.2 ± 9.4 |
| Body weight (kg) | 61.5 ± 8.7 | 64.6 ± 9.7 |
| MMSE score | 26.9 ± 2.4 | 27.0 ± 2.2 |
| UPDRS total score | 61.6 ± 18.4 | - |
| UPDRS part 3 total score | 40.9 ± 13.3 | - |
| PIGD (score) | 4.2 ± 3.2 | - |
| Hoehn & Yahr scale | 2.3 ± 0.3 | - |
| L-dopa dose (mg/day) | 788.7 ± 503.8 | - |
| Non-Freezer (male/female) | 15/5 | - |
| NFOGQ (score) | 14.8 ± 7.0 |
| Variables | PD (n = 76) | Controls (n = 52) |
|---|---|---|
| Slower speed | ||
| Estimated/measured (m/s) | 0.86/0.89 | 1.03/1.05 |
| ICC (2,1) | 0.828 | 0.895 |
| p-value | < 0.001 | < 0.001 |
| Faster speed | ||
| Estimated/measured (m/s) | 1.29/1.27 | 1.55/1.60 |
| ICC (2,1) | 0.905 | 0.945 |
| p-value | < 0.001 | < 0.001 |
| Variables | Slower Speed | Preferred Speed | Faster Speed | Significance for Groups | Significance for Speeds | ||||
|---|---|---|---|---|---|---|---|---|---|
| PD Patients | Controls | PD Patients | Controls | PD Patients | Controls | PD Patients | Controls | ||
| Walking speed (m/s) | 0.89 ± 0.21 | 1.05 ± 0.20 | 1.08 ± 0.21 | 1.29 ± 0.23 | 1.27 ± 0.28 | 1.60 ± 0.27 | A, B, C | d, e, f | d, e, f |
| Stride length (m) | 1.02 ± 0.22 | 1.20 ± 0.19 | 1.11 ± 0.22 | 1.33 ± 0.17 | 1.21 ± 0.24 | 1.46 ± 0.19 | A, B, C | d, e, f | d, e, f |
| Single support phase (%) | 38.72 ± 1.90 | 39.16 ± 1.66 | 39.87 ± 1.66 | 40.26 ± 1.63 | 41.06 ± 1.84 | 41.73 ± 1.80 | C | d, e, f | d, e, f |
| Double support phase (%) | 22.44 ± 3.84 | 21.56 ± 3.06 | 20.10 ± 3.26 | 19.41 ± 2.99 | 17.64 ± 3.46 | 16.37 ± 2.96 | C | d, e, f | d, e, f |
| Stance phase (%) | 61.16 ± 2.39 | 60.72 ± 1.70 | 59.97 ± 1.90 | 59.67 ± 1.58 | 58.70 ± 2.00 | 58.10 ± 1.45 | N/S | d, e, f | d, e, f |
| GA (%) | 3.79 ± 3.57 | 2.49 ± 2.58 | 2.88 ± 2.24 | 2.08 ± 2.12 | 2.78 ± 3.10 | 2.44 ± 2.35 | A, B | N/S | N/S |
| CV of stride length (%) | 3.05 ± 1.48 | 2.47 ± 1.00 | 2.31 ± 1.40 | 1.86 ± 0.81 | 2.51 ± 3.78 | 1.85 ± 0.76 | A, B | d | d, e |
| CV of single support phase (%) | 4.69 ± 3.14 | 4.34 ± 2.26 | 3.10 ± 1.70 | 2.65 ± 0.90 | 2.68 ± 1.50 | 2.19 ± 0.71 | C | d, e | d, e, f |
| CV of double support phase (%) | 11.38 ± 6.00 | 9.48 ± 4.25 | 9.10 ± 5.21 | 7.98 ± 3.68 | 9.40 ± 4.58 | 7.48 ± 3.75 | C | d, e | e |
| CV of stance phase (%) | 4.51 ± 2.40 | 3.62 ± 1.69 | 2.91 ± 1.84 | 2.48 ± 1.28 | 2.67 ± 1.74 | 1.99 ± 0.85 | A, C | d, e | d, e, f |
| Variables | UPDRS Total | UPDRS Part 3 | L-Dopa Dose |
|---|---|---|---|
| Slower Speed | |||
| Stance phase | 0.251 * | 0.185 | 0.010 |
| GA | 0.246 * | 0.333 ** | −0.174 |
| CV of stride length | 0.263 * | 0.219 | 0.016 |
| CV of single support phase | 0.232 * | 0.237 * | −0.039 |
| CV of stance phase | 0.275 * | 0.299 * | −0.074 |
| Preferred Speed | |||
| Single support phase | −0.229 * | −0.175 | 0.031 |
| Double support phase | 0.262 * | 0.161 | 0.022 |
| Stance phase | 0.250 * | 0.123 | 0.064 |
| Faster Speed | |||
| CV of double support phase | 0.202 | 0.299 ** | −0.179 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Youm, C.; Noh, B.; Park, H.; Cheon, S.-M. Gait Characteristics under Imposed Challenge Speed Conditions in Patients with Parkinson’s Disease During Overground Walking. Sensors 2020, 20, 2132. https://doi.org/10.3390/s20072132
Lee M, Youm C, Noh B, Park H, Cheon S-M. Gait Characteristics under Imposed Challenge Speed Conditions in Patients with Parkinson’s Disease During Overground Walking. Sensors. 2020; 20(7):2132. https://doi.org/10.3390/s20072132
Chicago/Turabian StyleLee, Myeounggon, Changhong Youm, Byungjoo Noh, Hwayoung Park, and Sang-Myung Cheon. 2020. "Gait Characteristics under Imposed Challenge Speed Conditions in Patients with Parkinson’s Disease During Overground Walking" Sensors 20, no. 7: 2132. https://doi.org/10.3390/s20072132
APA StyleLee, M., Youm, C., Noh, B., Park, H., & Cheon, S.-M. (2020). Gait Characteristics under Imposed Challenge Speed Conditions in Patients with Parkinson’s Disease During Overground Walking. Sensors, 20(7), 2132. https://doi.org/10.3390/s20072132








