Is Continuous Heart Rate Monitoring of Livestock a Dream or Is It Realistic? A Review
Abstract
1. Introduction
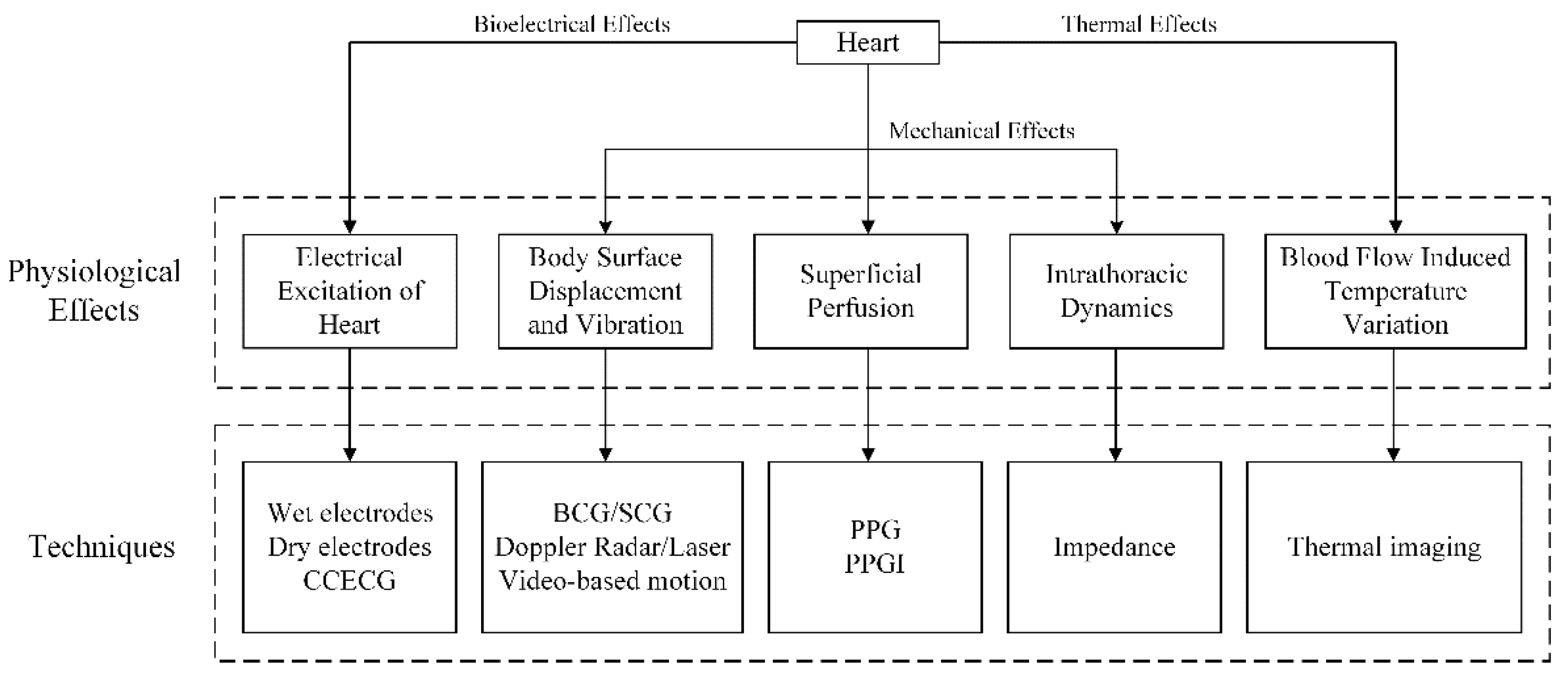
2. Physiological Effects
3. Available Techniques to Monitor HR
3.1. Electrocardiography (ECG)
3.2. Photoplethysmography (PPG)
3.3. Photoplethysmographic Imaging (PPGI)
3.4. Video-Based Motion
3.5. Thermal Imaging
3.6. Ballistocardiography (BCG) and Seismocardiography (SCG)
3.7. Doppler Radar and Laser
3.8. Impedance
3.9. Animal HR Monitoring Techniques
4. Transferable HR Monitoring Techniques in Livestock
4.1. Evaluation Criteria
4.2. Transferable Feasibility from Human to Livestock of Various Techniques
5. Challenge and Future Work
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCG | Ballistocardiography |
| CCECG | Capacitively Coupled ECG |
| ECG | Electrocardiography |
| FAO | Food and Agriculture Organization of the United Nations |
| HR | Heart Rate |
| PLF | Precision Livestock Farming |
| PPG | Photoplethysmography |
| PPGI | Photoplethysmographic Imaging |
| SCG | Seismocardiography |
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT. Livestock Primary. 2018. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 2 February 2020).
- McLeod, A. World Livestock 2011-Livestock in Food Security; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011. [Google Scholar]
- Norton, T.; Berckmans, D. Engineering advances in Precision Livestock Farming. Biosyst. Eng. 2018, 173, 1–3. [Google Scholar] [CrossRef]
- European Commission. Horizon 2020, Work Programme 2018–2020. Food Security, Sustainable Agriculture and Forestry, Marine, Maritime and Inland Water Research and the Bioeconomy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- National Academics of Sciences, Engineering, and Medicine. Science Breakthroughs to Advance Food and Agricultural Research by 2030; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Brüser, C.; Antink, C.H.; Wartzek, T.; Walter, M.; Leonhardt, S. Ambient and Unobtrusive Cardiorespiratory Monitoring Techniques. IEEE Rev. Biomed. Eng. 2015, 8, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Seidman, D.S.; Jonathan, M.; Zivanit, E.; Arie, L.; Vreman, H.J.; Stevenson, D.K.; Rena, G. A prospective randomized controlled study of phototherapy using blue and blue-green light-emitting devices, and conventional halogen-quartz phototherapy. J. Perinatol. 2003, 23, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, D.; Foussier, J.; Jia, J.; Leonhardt, S.; Walter, M. Noncontact Monitoring of Cardiorespiratory Activity by Electromagnetic Coupling. IEEE Trans. Biomed. Eng. 2013, 60, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Kranjec, J.; Begus, S.; Gersak, G.; Drnovsek, J. Non-contact heart rate and heart rate variability measurements: A review. Biomed. Signal Process. 2014, 13, 102–112. [Google Scholar] [CrossRef]
- Nemati, E.; Deen, M.J.; Mondal, T. A Wireless Wearable ECG Sensor for Long-Term Applications. IEEE Commun. Mag. 2012, 50, 36–43. [Google Scholar] [CrossRef]
- Gruetzmann, A.; Hansen, S.; Mueller, J. Novel dry electrodes for ECG monitoring. Physiol. Meas. 2007, 28, 1375–1390. [Google Scholar] [CrossRef]
- Yokus, M.A.; Jur, J.S. Fabric-Based Wearable Dry Electrodes for Body Surface Biopotential Recording. IEEE Trans. Biomed. Eng. 2016, 63, 423–430. [Google Scholar] [CrossRef]
- Fuhrhop, S.; Lamparth, S.; Heuer, S. A Textile Integrated Long-Term ECG Monitor with Capacitively Coupled Electrodes. In Proceedings of the 2009 IEEE Biomedical Circuits and Systems Conference, Beijing, China, 26–28 November 2009; pp. 21–24. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, S.M.; Lee, C.K.; Jeong, K.S.; Cho, G.; Yoo, S.K. Wearable ECG Monitoring System Using Conductive Fabrics and Active Electrodes. In Proceedings of the 13th International Conference on Human-Computer Interaction, San Diego, CA, USA, 19–24 July 2009; pp. 778–783. [Google Scholar] [CrossRef]
- Aleksandrowicz, A.; Leonhardt, S. Wireless and Non-contact ECG Measurement System—The “Aachen SmartChair”. Acta Polytech. 2007, 47, 32. [Google Scholar] [CrossRef]
- Yong, G.L.; Kim, K.K.; Park, S. ECG measurement on a chair without conductive contact. IEEE Trans. Biomed. Eng. 2006, 53, 956–959. [Google Scholar] [CrossRef]
- Lim, Y.G.; Kim, K.K.; Park, K.S. ECG Recording on a Bed During Sleep Without Direct Skin-Contact. IEEE Trans. Biomed. Eng. 2007, 54, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ha, C.; Zhang, Z. Design and evaluation of a ubiquitous chest-worn cardiopulmonary monitoring system for healthcare application: A pilot study. Med. Biol. Eng. Comput. 2017, 55, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.F.; Zhang, Y.T. Contactless and continuous monitoring of heart electric activities through clothes on a sleeping bed. In Proceedings of the 2008 International Conference on Information Technology and Applications in Biomedicine, Shenzhen, China, 30–31 May 2008; pp. 282–285. [Google Scholar] [CrossRef]
- Gargiulo, G.; Bifulco, P.; Cesarelli, M.; Ruffo, M.; Romano, M.; Calvo, R.A.; Jin, C.; Schaik, A.V. An ultra-high input impedance ECG amplifier for long-term monitoring of athletes. Med. Devices 2010, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chang, C.-L.; Chang, C.-W.; Lai, S.-C.; Chien, T.-F.; Huang, H.-Y.; Chiou, J.-C.; Luo, C.-H. A low-power bio-potential acquisition system with flexible PDMS dry electrodes for portable ubiquitous healthcare applications. Sensors 2013, 13, 3077–3091. [Google Scholar] [CrossRef] [PubMed]
- Rawstorn, J.C.; Gant, N.; Warren, I.; Doughty, R.N.; Lever, N.; Poppe, K.K.; Maddison, R. Measurement and Data Transmission Validity of a Multi-Biosensor System for Real-Time Remote Exercise Monitoring Among Cardiac Patients. Jmir Rehabil. Assist. Technol. 2015, 2, e2. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Xiao, X.; Chen, X.; Lin, H.; Wu, W.; Chen, S. A low-power and miniaturized electrocardiograph data collection system with smart textile electrodes for monitoring of cardiac function. Australas Phys. Eng. Sci. Med. 2016, 39, 1029–1040. [Google Scholar] [CrossRef]
- Dionisi, A.; Marioli, D.; Sardini, E.; Serpelloni, M. Autonomous Wearable System for Vital Signs Measurement with Energy-Harvesting Module. IEEE Trans. Instrum. Meas. 2016, 65, 1423–1434. [Google Scholar] [CrossRef]
- Li, M.; Kim, Y.T. Design of a Wireless Sensor System with the Algorithms of Heart Rate and Agility Index for Athlete Evaluation. Sensors 2017, 17, 373. [Google Scholar] [CrossRef]
- Hertzman, A.B. The blood supply of various skin areas as estimated by the photoelectric plethysmograph. Am. J. Physiol. Leg. Content 1938, 124, 328–340. [Google Scholar] [CrossRef]
- Kamal, A.A.; Harness, J.B.; Irving, G.; Mearns, A.J. Skin photoplethysmography—A review. Comput. Methods Programs Biomed. 1989, 28, 257–269. [Google Scholar] [CrossRef]
- Challoner, A.V.J.; Ramsay, C.A. A photoelectric plethysmograph for the measurement of cutaneous blood flow. Phys. Med. Biol. 1974, 19, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.B.; Asada, H. Low variance adaptive filter for cancelling motion artifact in wearable photoplethysmogram sensor signals. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 652–655. [Google Scholar]
- Park, S.M.; Kim, J.Y.; Ko, K.E.; Jang, I.H.; Sim, K.B. Real-Time Heart Rate Monitoring System based on Ring-Type Pulse Oximeter Sensor. J. Electr. Eng. Technol. 2013, 8, 376–384. [Google Scholar] [CrossRef]
- Poh, M.-Z.; Swenson, N.C.; Picard, R.W. Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 786–794. [Google Scholar] [CrossRef]
- Celka, P.; Verjus, C.; Vetter, R.; Renevey, P.; Neuman, V. Motion resistant earphone located infrared based heart rate measurement device. In Proceedings of the 2nd IASTED International Conference on Biomedical Engineering BioMED 2004, Innsbruck, Austria, 16–18 February 2004. [Google Scholar]
- Wang, L.; Lo, B.P.L.; Yang, G.-Z. Multichannel Reflective PPG Earpiece Sensor with Passive Motion Cancellation. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 235–241. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zheng, Y.P. Home-Telecare of the elderly living alone using a new designed ear-wearable sensor. In Proceedings of the 2008 5th International Summer School and Symposium on Medical Devices and Biosensors, Hong Kong, China, 1–3 June 2008; pp. 71–74. [Google Scholar] [CrossRef]
- Mendelson, Y.; Duckworth, R.J.; Comtois, G. A wearable reflectance pulse oximeter for remote physiological monitoring. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 912–915. [Google Scholar] [CrossRef]
- Maeda, Y.; Sekine, M.; Tamura, T. Relationship Between Measurement Site and Motion Artifacts in Wearable Reflected Photoplethysmography. J. Med. Syst. 2011, 35, 969–976. [Google Scholar] [CrossRef]
- Vogel, S.; Hulsbusch, M.; Hennig, T.; Blazek, V.; Leonhardt, S. In-ear vital signs monitoring using a novel microoptic reflective sensor. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 882–889. [Google Scholar] [CrossRef]
- Yousefi, R.; Nourani, M.; Ostadabbas, S.; Panahi, I. A motion-tolerant adaptive algorithm for wearable photoplethysmographic biosensors. IEEE J. Biomed. Health 2014, 18, 670–681. [Google Scholar] [CrossRef]
- Alzahrani, A.; Hu, S.; Azorin-Peris, V.; Barrett, L.; Esliger, D.; Hayes, M.; Akbare, S.; Achart, J.; Kuoch, S. A multi-channel opto-electronic sensor to accurately monitor heart rate against motion artefact during exercise. Sensors 2015, 15, 25681–25702. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J.; Zhang, J.; Zhou, G.; Luo, N.; Zhao, N. Flexible Organic/Inorganic Hybrid Near-Infrared Photoplethysmogram Sensor for Cardiovascular Monitoring. Adv. Mater. 2017, 29, 975. [Google Scholar] [CrossRef]
- Lee, E.M.; Shin, J.Y.; Hong, J.H.; Cha, E.J.; Lee, T.S. Glass-type wireless PPG measuring system. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1433–1436. [Google Scholar] [CrossRef]
- Rhee, S.; Yang, B.-H.; Asada, H.H. Artifact-resistant power-efficient design of finger-ring plethysmographic sensors. IEEE Trans. Biomed. Eng. 2001, 48, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Maria Lopez-Silva, S.; Giannetti, R.; Luisa Dotor, M.; Pedro Silveira, J.; Golmayo, D.; Miguel-Tobal, F.; Bilbao, A.; Galindo, M.; Martin-Escudero, P. Heuristic Algorithm for Photoplethysmographic Heart Rate Tracking During Maximal Exercise Test. J. Med. Biol. Eng. 2012, 32, 181–188. [Google Scholar] [CrossRef]
- Shin, K.; Kim, Y.; Bae, S.; Park, K.; Kim, S. A Novel Headset with a Transmissive PPG Sensor for Heart Rate Measurement. In Proceedings of the 13th International Conference on Biomedical Engineering, Singapore, 3–6 December 2008; pp. 519–522. [Google Scholar]
- Poh, M.-Z.; Kim, K.; Goessling, A.; Swenson, N.; Picard, R. Cardiovascular Monitoring Using Earphones and a Mobile Device. IEEE Pervasive Comput. 2012, 11, 18–26. [Google Scholar] [CrossRef]
- Leboeuf, S.F.; Aumer, M.E.; Kraus, W.E.; Johnson, J.L.; Duscha, B. Earbud-based sensor for the assessment of energy expenditure, HR, and VO2max. Med. Sci. Sports Exerc. 2014, 46, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Fatema, E.-A.; Ismail, N.M. Are Currently Available Wearable Devices for Activity Tracking and Heart Rate Monitoring Accurate, Precise, and Medically Beneficial? Healthc. Inform. Res. 2015, 21, 315–320. [Google Scholar] [CrossRef]
- Kamisalic, A.; Fister, I., Jr.; Turkanovic, M.; Karakatic, S. Sensors and Functionalities of Non-Invasive Wrist-Wearable Devices: A Review. Sensors 2018, 18, 1714. [Google Scholar] [CrossRef]
- Jeon, E.; Park, H.-A. Development of a Smartphone Application for Clinical-Guideline-Based Obesity Management. Healthc. Inform. Res. 2015, 21, 10–20. [Google Scholar] [CrossRef]
- Zhang, Z. Undergraduate Students Compete in the IEEE Signal Processing Cup: Part 3 [SP Education]. IEEE Signal Process. Mag. 2015, 32, 113–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Pi, Z.; Liu, B. TROIKA: A general framework for heart rate monitoring using wrist-type photoplethysmographic signals during intensive physical exercise. IEEE Trans. Biomed. Eng. 2015, 62, 522–531. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Z. Photoplethysmography-Based Heart Rate Monitoring Using Asymmetric Least Squares Spectrum Subtraction and Bayesian Decision Theory. IEEE Sens. J. 2015, 15, 7161–7168. [Google Scholar] [CrossRef]
- Zhang, Z. Photoplethysmography-Based Heart Rate Monitoring in Physical Activities via Joint Sparse Spectrum Reconstruction. IEEE Trans. Biomed. Eng. 2015, 62, 1902–1910. [Google Scholar] [CrossRef]
- Zhu, S.; Tan, K.; Zhang, X.; Liu, Z.; Liu, B. MICROST: A mixed approach for heart rate monitoring during intensive physical exercise using wrist-type PPG signals. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 2347–2350. [Google Scholar] [CrossRef]
- Salehizadeh, S.M.A.; Dao, D.; Bolkhovsky, J.; Cho, C.; Mendelson, Y.; Chon, K.H. A Novel Time-Varying Spectral Filtering Algorithm for Reconstruction of Motion Artifact Corrupted Heart Rate Signals During Intense Physical Activities Using a Wearable Photoplethysmogram Sensor. Sensors 2015, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, M.B.; Asadi, E.; Eskandari, M.; Kiani, S.; Marvasti, F. Heart Rate Tracking using Wrist-Type Photoplethysmographic (PPG) Signals during Physical Exercise with Simultaneous Accelerometry. IEEE Signal Process. Lett. 2016, 23, 227–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Zhang, Z. Combining ensemble empirical mode decomposition with spectrum subtraction technique for heart rate monitoring using wrist-type photoplethysmography. Biomed. Signal Process. 2015, 21, 119–125. [Google Scholar] [CrossRef]
- Xiong, J.; Cai, L.; Jiang, D.; Song, H.; He, X. Spectral Matrix Decomposition-Based Motion Artifacts Removal in Multi-Channel PPG Sensor Signals. IEEE Access 2016, 4, 3076–3086. [Google Scholar] [CrossRef]
- Khan, E.; Al Hossain, F.; Uddin, S.Z.; Alam, S.K.; Hasan, M.K. A Robust Heart Rate Monitoring Scheme Using Photoplethysmographic Signals Corrupted by Intense Motion Artifacts. IEEE Trans. Biomed. Eng. 2016, 63, 550–562. [Google Scholar] [CrossRef]
- Xiong, J.; Cai, L.; Wang, F.; He, X. SVM-Based Spectral Analysis for Heart Rate from Multi-Channel WPPG Sensor Signals. Sensors 2017, 17, 506. [Google Scholar] [CrossRef]
- Temko, A. Accurate Heart Rate Monitoring During Physical Exercises Using PPG. IEEE Trans. Biomed. Eng. 2017, 64, 2016–2024. [Google Scholar] [CrossRef]
- Chowdhury, S.S.; Hyder, R.; Bin Hafiz, M.S.; Haque, M.A. Real Time Robust Heart Rate Estimation from Wrist-type PPG Signals Using Multiple Reference Adaptive Noise Cancellation. IEEE J. Biomed. Health 2018, 22, 450–459. [Google Scholar] [CrossRef]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Blazek, V.; Schmitt, H.J. Photoplethysmography imaging: A new noninvasive and noncontact method for mapping of the dermal perfusion changes. In Proceedings of the Optical Techniques and Instrumentation for the Measurement of Blood Composition, Structure, and Dynamics, Amsterdam, The Netherlands, 22 November 2000; pp. 62–70. [Google Scholar] [CrossRef]
- Villarroel, M.; Guazzi, A.; Jorge, J.; Davis, S.; Watkinson, P.; Green, G.; Shenvi, A.; McCormick, K.; Tarassenko, L. Continuous non-contact vital sign monitoring in neonatal intensive care unit. Healthcare Technol. Lett. 2014, 1, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Poh, M.-Z.; Mcduff, D.J.; Picard, R.W. Advancements in noncontact, multiparameter physiological measurements using a webcam. IEEE Trans. Biomed. Eng. 2011, 58, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, H.; Park, K.S. Validation of heart rate extraction using video imaging on a built-in camera system of a smartphone. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2174–2177. [Google Scholar] [CrossRef]
- Sun, Y.; Papin, C.; Azorin-Peris, V.; Kalawsky, R.; Greenwald, S.; Hu, S. Use of ambient light in remote photoplethysmographic systems: Comparison between a high-performance camera and a low-cost webcam. J. Biomed. Opt. 2012, 17, 037005. [Google Scholar] [CrossRef] [PubMed]
- McDuff, D.J.; Estepp, J.R.; Piasecki, A.M.; Blackford, E.B. A survey of remote optical photoplethysmographic imaging methods. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 6398–6404. [Google Scholar] [CrossRef]
- Poh, M.Z.; Mcduff, D.J.; Picard, R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express 2010, 18, 10762–10774. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, S.; Azorin-Peris, V.; Greenwald, S.; Chambers, J.; Zhu, Y. Motion-compensated noncontact imaging photoplethysmography to monitor cardiorespiratory status during exercise. J. Biomed. Opt. 2011, 16, 852. [Google Scholar] [CrossRef]
- de Haan, G.; Jeanne, V. Robust pulse rate from chrominance-based rPPG. IEEE Trans. Biomed. Eng. 2013, 60, 2878–2886. [Google Scholar] [CrossRef]
- Holton, B.D.; Mannapperuma, K.; Lesniewski, P.J.; Thomas, J.C. Signal recovery in imaging photoplethysmography. Physiol. Meas. 2013, 34, 1499–1511. [Google Scholar] [CrossRef]
- Bousefsaf, F.; Maaoui, C.; Pruski, A. Continuous wavelet filtering on webcam photoplethysmographic signals to remotely assess the instantaneous heart rate. Biomed. Signal Process. 2013, 8, 568–574. [Google Scholar] [CrossRef]
- Monkaresi, H.; Calvo, R.A.; Yan, H. A machine learning approach to improve contactless heart rate monitoring using a webcam. IEEE J. Biomed. Health 2014, 18, 1153–1160. [Google Scholar] [CrossRef]
- Veeraraghavan, A.; Sabharwal, A.; Kumar, M. DistancePPG: Robust non-contact vital signs monitoring using a camera. Biomed. Opt. Express 2015, 6, 1565–1588. [Google Scholar] [CrossRef]
- Yu, Y.-P.; Raveendran, P.; Lim, C.-L. Dynamic heart rate measurements from video sequences. Biomed. Opt. Express 2015, 6, 2466–2480. [Google Scholar] [CrossRef] [PubMed]
- Amelard, R.; Clausi, D.A.; Wong, A. Spectral-spatial fusion model for robust blood pulse waveform extraction in photoplethysmographic imaging. Biomed. Opt. Express 2016, 7, 4874–4885. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, X.; Xu, L.; Wang, Z.J. Illumination Variation-Resistant Video-Based Heart Rate Measurement Using Joint Blind Source Separation and Ensemble Empirical Mode Decomposition. IEEE J. Biomed. Health 2017, 21, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Guo, Z.; Chen, X.; Shen, Z.; Wang, Z.J. Video-based human heart rate measurement using joint blind source separation. Biomed. Signal Process. 2017, 31, 309–320. [Google Scholar] [CrossRef]
- Bousefsaf, F.; Maaoui, C.; Pruski, A. Automatic Selection of Webcam Photoplethysmographic Pixels Based on Lightness Criteria. J. Med. Biol. Eng. 2017, 37, 374–385. [Google Scholar] [CrossRef]
- Tayibnapis, I.R.; Yang, Y.-M.; Lim, K.M. Blood Volume Pulse Extraction for Non-Contact Heart Rate Measurement by Digital Camera Using Singular Value Decomposition and Burg Algorithm. Energies 2018, 11, 76. [Google Scholar] [CrossRef]
- Ling, S.-S.; Paramesran, R.; Yu, Y.-P. Dynamic Heart Rate Measurements from Video Sequences using Canonical Component Analysis. Adv. Electr. Comput. Eng. 2019, 19, 41–48. [Google Scholar] [CrossRef]
- Wu, H. Eulerian Video Processing and Medical Applications; Massachusetts Institute of Technology: Cambridge, MA, USA, 2012. [Google Scholar]
- Balakrishnan, G.; Durand, F.; Guttag, J. Detecting Pulse from Head Motions in Video. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Portland, OR, USA, 23–28 June 2013; pp. 3430–3437. [Google Scholar] [CrossRef]
- Hassan, M.A.; Malik, A.S.; Fofi, D.; Saad, N.; Karasfi, B.; Ali, Y.S.; Meriaudeau, F. Heart rate estimation using facial video: A review. Biomed. Signal Process. 2017, 38, 346–360. [Google Scholar] [CrossRef]
- Marc, G.; Nanfei, S.; Arcangelo, M.; Ioannis, P. Contact-free measurement of cardiac pulse based on the analysis of thermal imagery. IEEE Trans. Biomed. Eng. 2007, 54, 1418–1426. [Google Scholar] [CrossRef]
- Inan, O.T.; Migeotte, P.-F.; Park, K.-S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and seismocardiography: A review of recent advances. IEEE J. Biomed. Health 2015, 19, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, K.; Ngai, B.; Akhbardeh, A.; Kaminska, B.; Blaber, A. Comparative analysis of infrasonic cardiac signals. In Proceedings of the 2009 36th Annual Computers in Cardiology Conference (CinC), Park City, UT, USA, 13–16 September 2009; pp. 757–760. [Google Scholar]
- Brüser, C.; Kortelainen, J.M.; Winter, S.; Tenhunen, M.; Parkka, J.; Leonhardt, S. Improvement of force-sensor-based heart rate estimation using multichannel data fusion. IEEE J. Biomed. Health 2015, 19, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Landaeta, R.; Casas, O.; Pallas-Areny, R. Heart rate detection from an electronic weighing scale. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 6282–6285. [Google Scholar] [CrossRef]
- Wang, C.W.; Wang, X.; Long, Z.; Zhang, K.X. Heart Rate Extraction Algorithm Based on Ballistocardiogram. J. Northeast. Univ. 2012, 33, 1103–1106. [Google Scholar]
- Chen, D.W.; Zhu, X.; Nemoto, T.; Kanemitsu, Y.; Kitamura, K.; Yamakoshi, K. Unconstrained detection of respiration rhythm and pulse rate with one under-pillow sensor during sleep. Med. Biol. Eng. Comput. 2005, 43, 306–312. [Google Scholar] [CrossRef]
- Brüser, C.; Kerekes, A.; Winter, S.; Leonhardt, S. Multi-channel optical sensor-array for measuring ballistocardiograms and respiratory activity in bed. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 5042–5045. [Google Scholar] [CrossRef]
- Brüser, C.; Kurt, S.; Stijn, D.W.; Steffen, L. Adaptive beat-to-beat heart rate estimation in ballistocardiograms. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Brüser, C.; Stadlthanner, K.; Brauers, A.; Leonhardt, S. Applying machine learning to detect individual heart beats in ballistocardiograms. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1926–1929. [Google Scholar] [CrossRef]
- Sprager, S.; Zazula, D. Optimization of Heartbeat Detection in Fiber-Optic Unobtrusive Measurements by Using Maximum A Posteriori Probability Estimation. IEEE J. Biomed. Health 2013, 18, 1161–1168. [Google Scholar] [CrossRef]
- Sprager, S.; Zazula, D. Heartbeat and respiration detection from optical interferometric signals by using a multimethod approach. IEEE Trans. Biomed. Eng. 2012, 59, 2922–2929. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Hurnanen, T.; Koskinen, J.; Eriksson, J.; Pankaala, M.; Teras, M.; Koivisto, T. A real-time approach for heart rate monitoring using a Hilbert transform in seismocardiograms. Physiol. Meas. 2016, 37, 1885–1909. [Google Scholar] [CrossRef]
- Hernandez, J.; Li, Y.; Rehg, J.M.; Picard, R.W. BioGlass: Physiological parameter estimation using a head-mounted wearable device. In Proceedings of the 2014 4th International Conference on Wireless Mobile Communication and Healthcare-Transforming Healthcare Through Innovations in Mobile and Wireless Technologies (MOBIHEALTH), Athens, Greece, 3–5 November 2014; pp. 55–58. [Google Scholar] [CrossRef]
- Aubert, X.L.; Brauers, A. Estimation of vital signs in bed from a single unobtrusive mechanical sensor: Algorithms and real-life evaluation. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 4744–4747. [Google Scholar] [CrossRef]
- Paalasmaa, J.; Toivonen, H.; Partinen, M. Adaptive Heartbeat Modeling for Beat-to-Beat Heart Rate Measurement in Ballistocardiograms. IEEE J. Biomed. Health 2015, 19, 1945–1952. [Google Scholar] [CrossRef]
- Park, J.-H.; Jang, D.-G.; Park, J.W.; Youm, S.-K. Wearable Sensing of In-Ear Pressure for Heart Rate Monitoring with a Piezoelectric Sensor. Sensors 2015, 15, 23402–23417. [Google Scholar] [CrossRef]
- Obeid, D.; Sadek, S.; Zaharia, G.; Zein, G.E. Multi-tunable microwave system for touch-less heartbeat detection and heart rate variability extraction. Microw. Opt. Technol. Lett. 2010, 52, 192–198. [Google Scholar] [CrossRef]
- Scalise, L.; Morbiducci, U. Non-contact cardiac monitoring from carotid artery using optical vibrocardiography. Med. Eng. Phys. 2008, 30, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lin, J.S.; Boric-Lubecke, O.; Lubecke, M. Frequency tuning technique for remote detection of heartbeat and respiration using low-power double-sideband transmission in Ka-band. IEEE Trans. Microw. Theory Tech. 2006, 54, 2023–2032. [Google Scholar] [CrossRef]
- Xiao, Y.; Lin, J.; Boriclubecke, O.; Lubecke, V.M. A Ka-Band Low Power Doppler Radar System for Remote Detection of Cardiopulmonary Motion. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, Shanghai, China, 17–18 January 2006; pp. 912–915. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, C.; Lin, J. A Portable Noncontact Heartbeat and Respiration Monitoring System Using 5-GHz Radar. IEEE Sens. J. 2007, 7, 1042–1043. [Google Scholar] [CrossRef]
- Li, C.; Lin, J.; Xiao, Y. Robust overnight monitoring of human vital signs by a non-contact respiration and heartbeat detector. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2235–2238. [Google Scholar] [CrossRef]
- Tavakolian, K.; Zadeh, F.M.; Chuo, Y.; Vaseghi, A.; Kaminska, B. Development of a novel contactless mechanocardiograph device. Int. J. Telemed. Appl. 2008, 2008, 436870. [Google Scholar] [CrossRef][Green Version]
- Obeid, D.; Sadek, S.; Zaharia, G.; Zein, G.E. Doppler Radar for Heartbeat Rate and Heart Rate Variability Extraction. In Proceedings of the 2011 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 24–26 November 2011; pp. 1–4. [Google Scholar] [CrossRef]
- Morbiducci, U.; Scalise, L.; Melis, M.D.; Grigioni, M. Optical Vibrocardiography: A Novel Tool for the Optical Monitoring of Cardiac Activity. Ann. Biomed. Eng. 2007, 35, 45–58. [Google Scholar] [CrossRef]
- Joosen, P.; Norton, T.; Marchant-Forde, J.; Berckmans, D. Animal welfare monitoring by real-time physiological signals. In Proceedings of the 9th European Conference on Precision Livestock Farming EC 2019, Cork, Ireland, 26–29 August 2019; pp. 337–344. [Google Scholar]
- Eberhard, V.B.; Jan, L.; Gérard, D.; Sven, H.; Christine, L.; Jeremy, M.F.; Ruth, M.F.; Michela, M.; Elmar, M.; Armelle, P. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef]
- Konold, T.; Arnold, M.E.; Austin, A.R.; Cawthraw, S.; Hawkins, S.A.C.; Stack, M.J.; Simmons, M.M.; Sayers, A.R.; Dawson, M.; Wilesmith, J.W.; et al. Time and frequency domain analysis of heart rate variability in cattle affected by bovine spongiform encephalopathy. BMC Res. Notes 2012, 4, 259. [Google Scholar] [CrossRef]
- Chen, G.P.; Qin, W.J.; Ding, J.; Wan, M.C.; Guo, L.F.; Wang, W.S. Designing and Validation of the Remote Monitoring System for Pigs’ Survival Based on IoT Technology. Sci. Agric. Sin. 2017, 50, 942–950. [Google Scholar] [CrossRef]
- Jukan, A.; Masip-Bruin, X.; Amla, N. Smart Computing and Sensing Technologies for Animal Welfare: A Systematic Review. ACM Comput. Surv. 2017, 50, 960. [Google Scholar] [CrossRef]
- Jorquera-Chavez, M.; Fuentes, S.; Dunshea, F.R.; Warner, R.D.; Poblete, T.; Jongman, E.C. Modelling and Validation of Computer Vision Techniques to Assess Heart Rate, Eye Temperature, Ear-Base Temperature and Respiration Rate in Cattle. Animals 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Irani, R.; Nasrollahi, K.; Moeslund, T.B. Heartbeat Rate Measurement from Facial Video. IEEE Intell. Syst. 2016, 31, 40–48. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Wang, L. A review of non-contact, low-cost physiological information measurement based on photoplethysmographic imaging. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2088–2091. [Google Scholar] [CrossRef]
- Zanetti, J.M.; Tavakolian, K. Seismocardiography: Past, present and future. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 7004–7007. [Google Scholar] [CrossRef]
- Cho, M.-C.; Kim, J.-Y.; Cho, S. A Bio-Impedance Measurement System for Portable Monitoring of Heart Rate and Pulse Wave Velocity Using Small Body Area. In Proceedings of the 2009 IEEE International Symposium on Circuits and Systems (ISCAS), Taipei, Taiwan, 24–27 May 2009; pp. 3106–3109. [Google Scholar]
- Lee, J.; Cho, S. A Motion-tolerant Heart Rate Detection Method Using Bio-impedance and MUSIC Algorithm. In Proceedings of the 2015 IEEE Sensors, Busan, Korea, 1–4 November 2015; pp. 1901–1904. [Google Scholar]
- Meyer, W.; Schwarz, R.; Neurand, K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr. Probl. Dermatol. 2015, 7, 39–52. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin Res. Technol. 2010, 13, 19–24. [Google Scholar] [CrossRef]
- Lee, Y.; Han, H.; Kim, J. Influence of motion artifacts on photoplethysmographic signals for measuring pulse rates. In Proceedings of the 2008 International Conference on Control, Automation and Systems, Seoul, Korea, 14–17 October 2008; pp. 962–965. [Google Scholar] [CrossRef]
- Thakor, N.V.; Zhu, Y.-S. Applications of adaptive filtering to ECG analysis: Noise cancellation and arrhythmia detection. IEEE Trans. Biomed. Eng. 1991, 38, 785–794. [Google Scholar] [CrossRef]
- Chan, K.W.; Zhang, Y.T. Adaptive reduction of motion artifact from photoplethysmographic recordings using a variable step-size LMS filter. In Proceedings of the Proceedings of IEEE SENSORS 2002, Orlando, FL, USA, 12–14 June 2002; pp. 1343–1346. [Google Scholar] [CrossRef]
- Asada, H.H.; Shaltis, P.; Reisner, A.; Rhee, S.; Hutchinson, R.C. Mobile monitoring with wearable photoplethysmographic biosensors. IEEE Eng. Med. Biol. 2003, 22, 28–40. [Google Scholar] [CrossRef]
- Graybeal, J.M.; Petterson, M.T. Adaptive filtering and alternative calculations revolutionizes pulse oximetry sensitivity and specificity during motion and low perfusion. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 5363–5366. [Google Scholar] [CrossRef]
- Ram, M.R.; Madhav, K.V.; Krishna, E.H.; Komalla, N.R.; Reddy, K.A. A Novel Approach for Motion Artifact Reduction in PPG Signals Based on AS-LMS Adaptive Filter. IEEE Trans. Instrum. Meas. 2012, 61, 1445–1457. [Google Scholar] [CrossRef]
- Kim, B.S.; Yoo, S.K. Motion artifact reduction in photoplethysmography using independent component analysis. IEEE Trans. Biomed. Eng. 2006, 53, 566–568. [Google Scholar] [CrossRef]
- Krishnan, R.; Natarajan, B.B.; Warren, S. Two-stage approach for detection and reduction of motion artifacts in photoplethysmographic data. IEEE Trans. Biomed. Eng. 2010, 57, 1867–1876. [Google Scholar] [CrossRef]
- Yao, J.; Warren, S. A short study to assess the potential of independent component analysis for motion artifact separation in wearable pulse oximeter signals. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 3585–3588. [Google Scholar] [CrossRef]
- Lee, C.M.; Zhang, Y.T. Reduction of motion artifacts from photoplethysmographic recordings using a wavelet denoising approach. In Proceedings of the IEEE EMBS Asian-Pacific Conference on Biomedical Engineering, Kyoto, Japan, 20–22 October 2003; pp. 194–195. [Google Scholar] [CrossRef]
- Foo, J.Y.A. Comparison of wavelet transformation and adaptive filtering in restoring artefact-induced time-related measurement. Biomed. Signal Process. 2006, 1, 93–98. [Google Scholar] [CrossRef]
- Raghuram, M.; Madhav, K.V.; Krishna, E.H.; Reddy, K.A. Evaluation of wavelets for reduction of motion artifacts in photo plethysmographic signals. In Proceedings of the 10th International Conference on Information Science, Signal Processing and their Applications (ISSPA 2010), Kuala Lumpur, Malaysia, 10–13 May 2010; pp. 460–463. [Google Scholar] [CrossRef]
- Hayes, M.J.; Smith, P.R. Artifact Reduction in Photoplethysmography. Appl. Opt. 1998, 37, 7437–7446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, P.; Zhang, Y. Artifact Reduction based on Empirical Mode Decomposition (EMD) in Photoplethysmography for Pulse Rate Detection. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 959–962. [Google Scholar] [CrossRef]
- Sun, X.; Ping, Y.; Li, Y.; Gao, Z.; Zhang, Y.T. Robust Heart Beat Detection from Photoplethysmography Interlaced with Motion Artifacts Based on Empirical Mode Decomposition. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 775–778. [Google Scholar] [CrossRef]
- Garde, A.; Karlen, W.; Dehkordi, P.; Ansermino, J.; Dumont, G. Empirical Mode Decomposition for Respiratory and Heart Rate Estimation from the Photoplethysmogram. In Proceedings of the Computing in Cardiology 2013, Zaragoza, Spain, 22–25 September 2013; pp. 799–802. [Google Scholar]
- Yan, Y.S.; Poon, C.C.; Zhang, Y.T. Reduction of motion artifact in pulse oximetry by smoothed pseudo Wigner-Ville distribution. J. Neuroeng. Rehabil. 2005, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Seyedtabaii, S.; Seyedtabaii, L. Kalman Filter Based Adaptive Reduction of Motion Artifact from Photoplethysmographic Signal. Int. J. Circuit Theory Appl. 2008, 2, 1. [Google Scholar] [CrossRef]
- Hayes, M.J.; Smith, P.R. A new method for pulse oximetry possessing inherent insensitivity to artifact. IEEE Trans. Biomed. Eng. 2001, 48, 452–461. [Google Scholar] [CrossRef]
- Fukushima, H.; Kawanaka, H.; Bhuiyan, M.S.; Oguri, K. Estimating heart rate using wrist-type Photoplethysmography and acceleration sensor while running. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2901–2904. [Google Scholar] [CrossRef]
- Biswas, D.; Simoes-Capela, N.; Van Hoof, C.; Van Helleputte, N. Heart Rate Estimation from Wrist-Worn Photoplethysmography: A Review. IEEE Sens. J. 2019, 19, 6560–6570. [Google Scholar] [CrossRef]
- Presura, C.N.; Roovers, D.A.C.M. Device and Method for Estimating the Heart Rate during Motion. U.S. Patent 09,770,176, 26 September 2017. [Google Scholar]
- Kyriacou, P.A. Direct Pulse Oximetry Within the Esophagus, on the Surface of Abdominal Viscera, and on Free Flaps. Anesth. Analg. 2013, 117, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. The Optics of Human Skin. J. Investig. Dermatol. 1981, 77, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Sekine, M.; Tamura, T. The Advantages of Wearable Green Reflected Photoplethysmography. J. Med. Syst. 2011, 35, 829–834. [Google Scholar] [CrossRef]
- Delgado-Gonzalo, R.; Parak, J.; Tarniceriu, A.; Renevey, P.; Korhonen, I. Evaluation of Accuracy and Reliability of PulseOn Optical Heart Rate Monitoring Device. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 430–433. [Google Scholar] [CrossRef]
- Mohapatra, P.; Preejith, S.; Sivaprakasam, M. A novel sensor for wrist based optical heart rate monitor. In Proceedings of the 2017 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Turin, Italy, 22–25 May 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Spigulis, J. Optical noninvasive monitoring of skin blood pulsations. Appl. Opt. 2005, 44, 1850–1857. [Google Scholar] [CrossRef]
- Daniel, R.K.; Priest, D.L.; Wheatley, D.C. Etiologic factors in pressure sores: An experimental model. Arch. Phys. Med. Rehabil. 1981, 62, 492–498. [Google Scholar] [CrossRef]
- Teng, X.F.; Zhang, Y.T. The effect of contacting force on photoplethysmographic signals. Physiol. Meas. 2004, 25, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Hsiu, H.; Hsu, C.L.; Wu, T.L. Effects of different contacting pressure on the transfer function between finger photoplethysmographic and radial blood pressure waveforms. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.L.; Fuller, M.R.; Boitani, L.; Fuller, T.K. A Critical Review of the Effects of Marking on the Biology of Vertebrates; Columbia University Press: New York, NY, USA, 2000; pp. 15–64. [Google Scholar]
| Citation | Technique | Implementation | Movement | Power Consumption | Quantitative Result |
|---|---|---|---|---|---|
| Wu and Zhang [19] | CCECG | Integrated into a bedsheet | Sleep | NA | Root mean square error (RMSE): 0.66 ± 0.57 bpm |
| Gargiulo et al. [20] | Dry electrodes | Integrated into a chest strap | Exercise | 33 mA (including transmission) | Correlation: larger than 0.96 |
| Nemati et al. [10] | CCECG | Integrated into a stretchable cloth | Motionless | less than 25 mA | NA |
| Chen et al. [21] | Flexible dry electrodes | Integrated into a wrist band | NA | 84.83 mW | NA |
| Rawstorn et al. [22] | CCECG | Integrated into a harness (chest strap) | Exercise | NA | Mean bias: −0.30 ± 4.53 bpm (sinus rhythm) 1.10 ± 9.75 (atrial fibrillation) |
| Dai et al. [23] | Flexible dry electrodes | Integrated into a garment | Sitting | 29.74 mW | Accuracy: 98.55% |
| Dionisi et al. [24] | CCECG | Integrated into a T-shirt | Walking | 17 mW (flexible solar panel) | Mean bias: 0.38 bpm |
| Zheng et al. [18] | CCECG | Integrated into chest strap | Exercise | 2.1 mA | Mean bias: 0.60 ± 1.48 bpm |
| Li and Kim [25] | Dry electrodes | Integrated into a patch | Exercise | NA | Error rate: within 2% Correlation: 0.97 |
| Citation | Mode | Light Wavelength | Movement | Power Consumption | Quantitative Results |
|---|---|---|---|---|---|
| Rhee et al. [43] | Reflection | Red and Infrared | Shaking finger | Total current consumption: 0.491 mA; RF Transmitter: 0.098 mA; CPU-LED circuit: 0.393 mA | RMSE: 1.23 bpm |
| Maria Lopez-Silva et al. [44] | Transmission | Near infrared (850 nm) | Exercise | NA | : −0.7 bpm : 2.92 bpm LOA: [−6.41, 5.01] bpm |
| Park et al. [31] | Reflection | Red and Infrared | Motionless | Transmit mode: 31 mA; Receiving mode: 26 mA | NA |
| Yousefi et al. [39] | Transmission | Red (660 nm) and Infrared (895 nm) | Exercise | NA | : −0.57 bpm : 3.30 bpm LOA: [−7.0, 5.9] bpm |
| Citation | Sensor | Mode | Light Wavelength | Movement | Quantitative Results |
|---|---|---|---|---|---|
| Wang and Zheng [35] | Ear-worn | Reflection | Infrared | Motionless | RMSE: 1.3 bpm : −0.25 bpm : 0.5 bpm LOA: [−1.23, 0.73] bpm |
| Shin et al. [45] | Transmission | Infrared (940 nm) | Exercise | Error rates: 0.6% (rest); 1.7% (walk); 0.7% (jog); 5.7% (run) | |
| Poh et al. [32] | Reflection | Infrared (940 nm) | Exercise | Stand: : 0.62%; : 4.51%; LOA: [−8.23, 9.46]% Walk: : −0.49%; : 8.65%; LOA: [−17.39, 16.42]% Run: : −0.32%; : 10.63%; LOA: [−21.15, 20.52]% | |
| Poh et al. [46] | Reflection | Infrared | Exercise | Stand: : −0.07 bpm; : 2.56 bpm; Cycle: : −0.67 bpm; : 2.34 bpm; Walk: : 0.51 bpm; : 5.31 bpm; | |
| Leboeuf et al. [47] | Reflection | Infrared | Exercise | : −0.2% : 4.4% | |
| Alzahrani et al. [40] | Patch | Reflection | Green (525 nm) Red (650 nm) IR (870 nm) | Exercise | : 0.85 bpm : 9.20 bpm |
| Method and Citation | Signal Processing Techniques | Error 1 (Mean ± SD) (bpm) | Error 2 (Mean ± SD) (%) | Bland-Altman Analysis (bpm) | Pearson Correlation Coefficient |
|---|---|---|---|---|---|
| SPECTRAP Sun and Zhang [54] | Spectrum subtraction based on asymmetric least squares | 1.50 ± 1.95 | 1.12 ± 1.47 | LOA: [−5.59, 6.01] | 0.995 |
| TROIKA Zhang et al. [52] | Sparse signal reconstruction: single measurement vector (SMV) | 2.34 ± 0.82 | 1.80 | : −1.24 3.07 LOA: [−7.26, 4.79] | 0.992 |
| JOSS Zhang [55] | Joint sparse spectrum reconstruction: multiple measurement vector (MMV) | 1.28 ± 2.61 | 1.01 ± 2.29 | LOA: [−5.94, 5.41] | 0.993 |
| MICROST Zhu et al. [56] | Wavelet and time-domain methods | 2.58 ± 2.70 | 1.85 | 3.73 LOA: [−7.31, 7.31] | 0.988 |
| SpaMA Salehizadeh et al. [57] | Time-varying spectral filtering algorithm | 0.89 ± 0.6 | 0.65 ± 0.4 | NA | 0.98 |
| IMAT Mashhadi et al. [58] | Sparse reconstruction: iterative method with adaptive thresholding | 1.25 | NA | NA | NA |
| Method and Citation | Signal Processing Techniques | Error 1 (Mean ± SD) (bpm) | Error 2 (Mean ± SD) (%) | Bland-Altman Analysis (bpm) | Pearson Correlation Coefficient |
|---|---|---|---|---|---|
| FEEMD Zhang et al. [59] | Fast ensemble empirical mode decomposition (FEEMD) and spectrum subtraction | 1.83 ± 1.21 | 1.4 | 3.62 LOA: [−7.56, 6.61] | 0.989 |
| MC-SMD Xiong et al. [60] | Multi-channel spectral matrix decomposition (MC-SMD) model | 1.11 | 0.80 | : 0.2248 1.9940 LOA: [−3.68, 4.13] | 0.9968 |
| EEMD Khan et al. [61] | Ensemble empirical mode decomposition (EEMD) | 1.02 ± 1.79 | 0.79 | LOA: [−4.10, 3.98] | 0.996 |
| Mix-SVM Xiong et al. [62] | Principle component analysis (PCA) and adaptive filter Sparse signal reconstruction Support vector machine (SVM) spectral analysis | 1.01 | 0.72 | LOA: [−3.46, 3.83] | 0.9972 |
| WFPV Temko [63] | Wiener filter and phase vocoder | 1.02 | 0.81 | NA | 0.997 |
| MURAD Chowdhury et al. [64] | Multiple reference RLS adaptive noise cancellation | 0.9726 ± 1.831 | 0.76 ± 1.5 | LOA: [−3.5665, 3.6112] | 0.9972 |
| Citation | Sensor | Illumination | Distance (m) | Movement | Signal Processing Technique | RMSE (bpm) | Bland-Altman Analysis (bpm) | Pearson Correlation Coefficient |
|---|---|---|---|---|---|---|---|---|
| Poh et al. [72] | Webcam | Ambient light | 0.5 | Slight motion (sitting) | Independent component analysis (ICA) | Sitting still: 2.29 Slight motion: 4.63 | Sitting sill: μ: −0.05; σ: 2.29 LOA: [−4.55, 4.44] Slight motion: μ: 0.64; σ: 4.59 LOA: [−8.35, 4.63] | Sitting still: 0.98 Slight motion: 0.95 |
| Poh et al. [68] | Webcam | Ambient light | 0.5 | Motionless | ICA | 1.24 | NA | 1 |
| Sun et al. [73] | Monochrome CMOS camera | IR (870 nm) | 0.4 | Motionless | Planar motion compensation and blind source separation | NA | μ: 0.33 LOA: [−1.29, 1.96] | 0.9 |
| de Haan and Jeanne [74] | CCD camera | Ambient light | NA | Cycling | Chrominance-based methods | 0.4 | NA | 1 |
| Holton et al. [75] | Webcam | Ambient light | 0.6 | Motionless | ICA | 6.92 | Standard error: 6.51 bpm | 0.89 |
| Citation | Sensor | Illumination | Distance (m) | Movement | Signal Processing Technique | RMSE (bpm) | Bland-Altman Analysis (bpm) | Pearson Correlation Coefficient |
|---|---|---|---|---|---|---|---|---|
| Bousefsaf et al. [76] | Webcam | Ambient light | 1 | Head movements | Continuous wavelet filtering | 2.33 ± 0.73 | μ: 0.02 LOA: [−4.96, 4.99] | 0.853 ± 0.056 |
| Monkaresi et al. [77] | Webcam | Ambient light | NA | Cycling | Machine learning approach | 4.33 | μ: −0.28 σ: 4.33 | 0.97 |
| Veeraraghavan et al. [78] | Camera | Ambient light | 0.5 | Facial movements | Combining skin-color change signals from different facial regions using a weighted average | NA | μ: 0.48 LOA: [−5.73, 6.70] | NA |
| Yu et al. [79] | Camera | Ambient light | 0.6 | Cycling | ICA | 1.97 | NA | 0.99 |
| Amelard et al. [80] | Monochrome camera | NIR | 1.5 | Supine position | Spectral-spatial fusion model | NA | µ: −1.0 σ: 0.70 | 0.9952 |
| Citation | Sensor | Illumination | Distance (m) | Movement | Signal Processing Technique | RMSE (bpm) | Bland-Altman Analysis (bpm) | Pearson Correlation Coefficient |
|---|---|---|---|---|---|---|---|---|
| Cheng et al. [81] | Webcam | Ambient light | 0.5 | Motionless | Joint blind source separation and ensemble empirical mode decomposition (JBSS–EEMD) | NA | μ: 1.15 σ: 8.46 LOA: [−15.43, 17.73] | 0.53 |
| Qi et al. [82] | Webcam | Ambient light | NA | Motionless | Joint blind source separation | 5.0017 | NA | 0.7423 |
| Bousefsaf et al. [83] | Webcam | Ambient light | 1 | Motionless | Segmentation based on lightness criteria | 4.81 | μ: 0.16 LOA: [−10.95, 11.26] | 0.78 |
| Tayibnapis et al. [84] | Webcam | Ambient light | 0.3–1.1 | Motionless | singular value Decomposition and Burg algorithm | 3.34 | μ: 2.15 σ: 2.58 | 0.73 |
| Ling et al. [85] | Camera | Ambient light | 0.6 | Cycling | Canonical component analysis | Experiment 1: 3.70 Experiment 2: 2.33 | NA | Experiment 1: 0.97 Experiment 2: 0.99 |
| Citation | Sensor | Movement | Quantitative Result |
|---|---|---|---|
| Wang et al. [94] | Pressure sensor | NA | Accuracy: 98.22% |
| Aubert and Brauers [103] | Electromechanical film sensors | Supine | Error: 1.25 bpm |
| Paalasmaa et al. [104] | Flexible piezoelectric film | Sleep | Mean absolute error: 0.78 bpm |
| Park et al. [105] | Piezoelectric film | Motionless | Standard deviation: 1.82 bpm |
| Bruser et al. [98] | Strain gauge | NA | Mean error: 0.39 bpm |
| Bruser et al. [97] | Strain gauge | Supine | Mean error: 0.46 bpm (10 s); 0.5 bpm (30 s) |
| Hernandez et al. [102] | Accelerometer, gyroscope, camera | Motionless | (gyroscope) Mean absolute error: 0.83 bpm |
| Tadi et al. [101] | Accelerometer | Supine | Average RMSE error: 0.33 bpm (supine); 0.62 bpm (right lateral); 0.45 bpm (left lateral) |
| Method | Device/Sensor | Distance (m) | Movement | Quantitative Result |
|---|---|---|---|---|
| Xiao et al. [108,109] | Ka-band Doppler radar | 2 | Motionless | Accuracy: 0.5 m, 100%; 1 m, 96%; 1.5 m, 89.3%; 2 m, 81.5%; 2.5 m, 64.6% |
| Xiao et al. [110] | 2.8 | Motionless | Accuracy: 0.5, 1, 1.5, 2, 2.8 m: 98.82%, 91.71%, 92.40%, 85.78%, 81.35% | |
| Li et al. [111] | 0.5–2.5 | Motionless | Accuracy: 0.5 m, 1 m, 1.5 m, 2 m, 2.5 m: 99.1%, 89.8%, 98.9%, 85.2%, 83.3% (front); 96.3%, 89.8%, 89%, 80.5%, 85.7% (left); 100%, 93.2%, 93.8%, 97.4%, 85.1% (right); 97.6%, 100%, 94.3%, 93.6%, 85.5% (back) | |
| Tavakolian et al. [112] | Doppler radar | 0.1 | Motionless | Accuracy: 92.9% |
| Obeid et al. [113] | NA | Motionless | Relative error: 0.5–1.5% | |
| Morbiducci et al. [114] | Laser Doppler vibrometer | 1.5 | Motionless | Bias: 0.006 bpm (male);0.015 bpm (female) |
| Scalise and Morbiducci [107] | 1.5 | Motionless | Mean bias: 0.026 bpm |
| Technique | Measuring Sensor | Distance | Movement | Cost |
|---|---|---|---|---|
| PPG | Phototransistor | mm | Exercise | low |
| PPGI | Camera/webcam | m | Motionless | low |
| Thermal imaging | Thermal imaging camera | m | Motionless | highest |
| BCG/SCG | Pressure sensor, strain gauge, optical sensor, etc. | mm | Motionless | low |
| Video-based motion | Camera/webcam | m | Motionless | low |
| Radar | Microwave sensor | m | Motionless | medium |
| Laser | Laser | m | Motionless | high |
| Wet ECG | Wet electrodes | 0 | Subtle Motion | medium |
| Dry ECG | Dry electrodes | 0 | Exercise | medium |
| CCECG | Capacitively coupled electrodes | mm | Exercise | medium |
| Impedance | Coils/electrodes | cm | Motionless | medium |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, L.; Berckmans, D.; Wang, C.; Li, B. Is Continuous Heart Rate Monitoring of Livestock a Dream or Is It Realistic? A Review. Sensors 2020, 20, 2291. https://doi.org/10.3390/s20082291
Nie L, Berckmans D, Wang C, Li B. Is Continuous Heart Rate Monitoring of Livestock a Dream or Is It Realistic? A Review. Sensors. 2020; 20(8):2291. https://doi.org/10.3390/s20082291
Chicago/Turabian StyleNie, Luwei, Daniel Berckmans, Chaoyuan Wang, and Baoming Li. 2020. "Is Continuous Heart Rate Monitoring of Livestock a Dream or Is It Realistic? A Review" Sensors 20, no. 8: 2291. https://doi.org/10.3390/s20082291
APA StyleNie, L., Berckmans, D., Wang, C., & Li, B. (2020). Is Continuous Heart Rate Monitoring of Livestock a Dream or Is It Realistic? A Review. Sensors, 20(8), 2291. https://doi.org/10.3390/s20082291






