Intra- and Inter-Rater Reliability of Manual Feature Extraction Methods in Movement Related Cortical Potential Analysis
Abstract
:1. Introduction
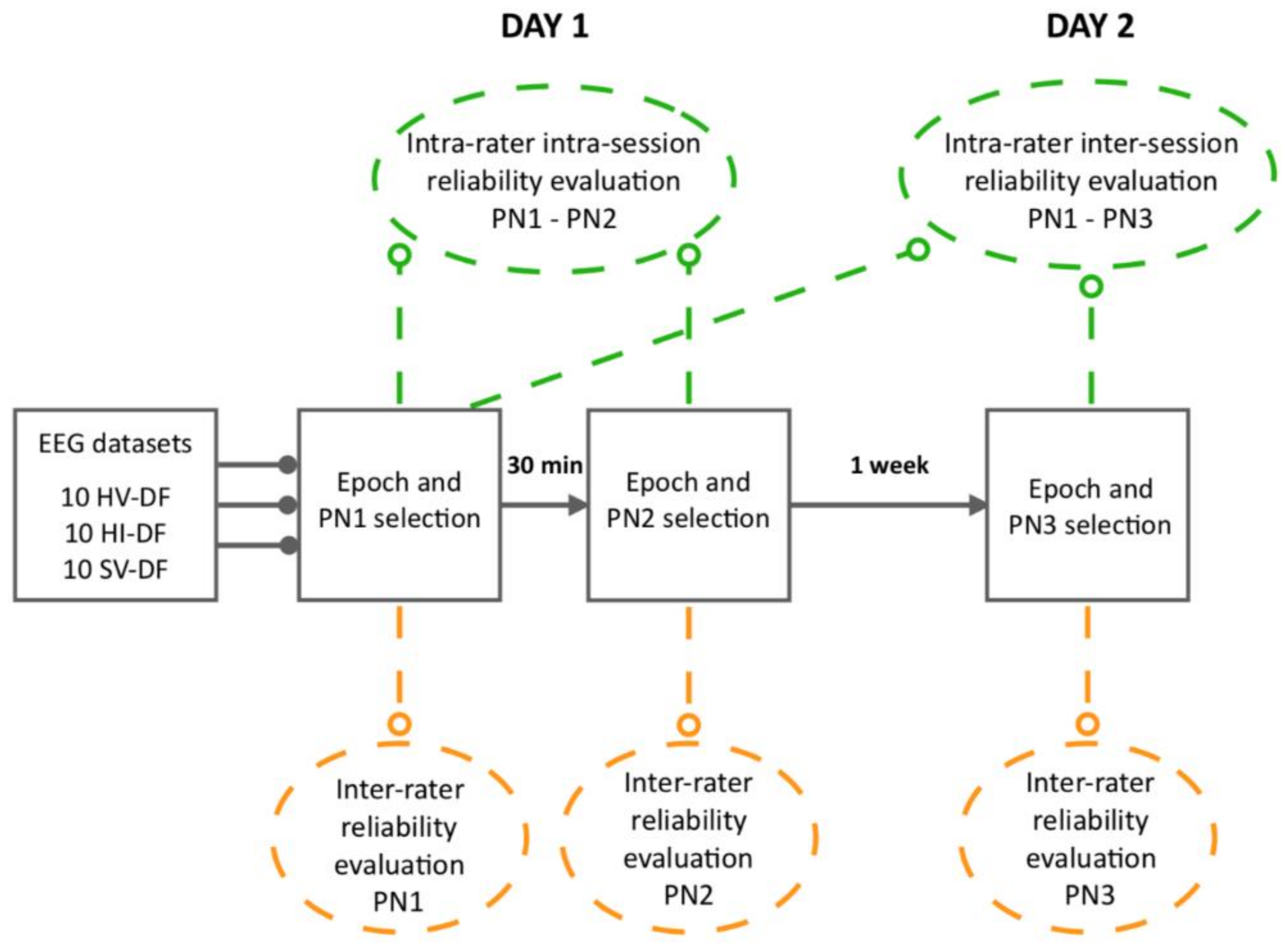
2. Method
2.1. Study Design
2.2. Participants
2.3. Ethical Procedures
2.4. Experimental Procedures
2.4.1. Externally-Cued Movement Paradigm
2.4.2. EEG Data Acquisition
2.4.3. EEG Data Processing
2.4.4. MRCP evaluation by EEG Experts
2.5. Statistical Analysis
2.5.1. Primary Analysis: Average MRCP PN Labelling
2.5.2. Secondary Analysis: Epoch Selection
3. Results
3.1. Primary Findings: Average MRCP PN Labelling
3.1.1. Intra-rater Reliability (Intra- and Inter-session)
Relative Reliability
Absolute Reliability
3.1.2. Inter-rater Reliability
Relative Reliability
Absolute Reliability
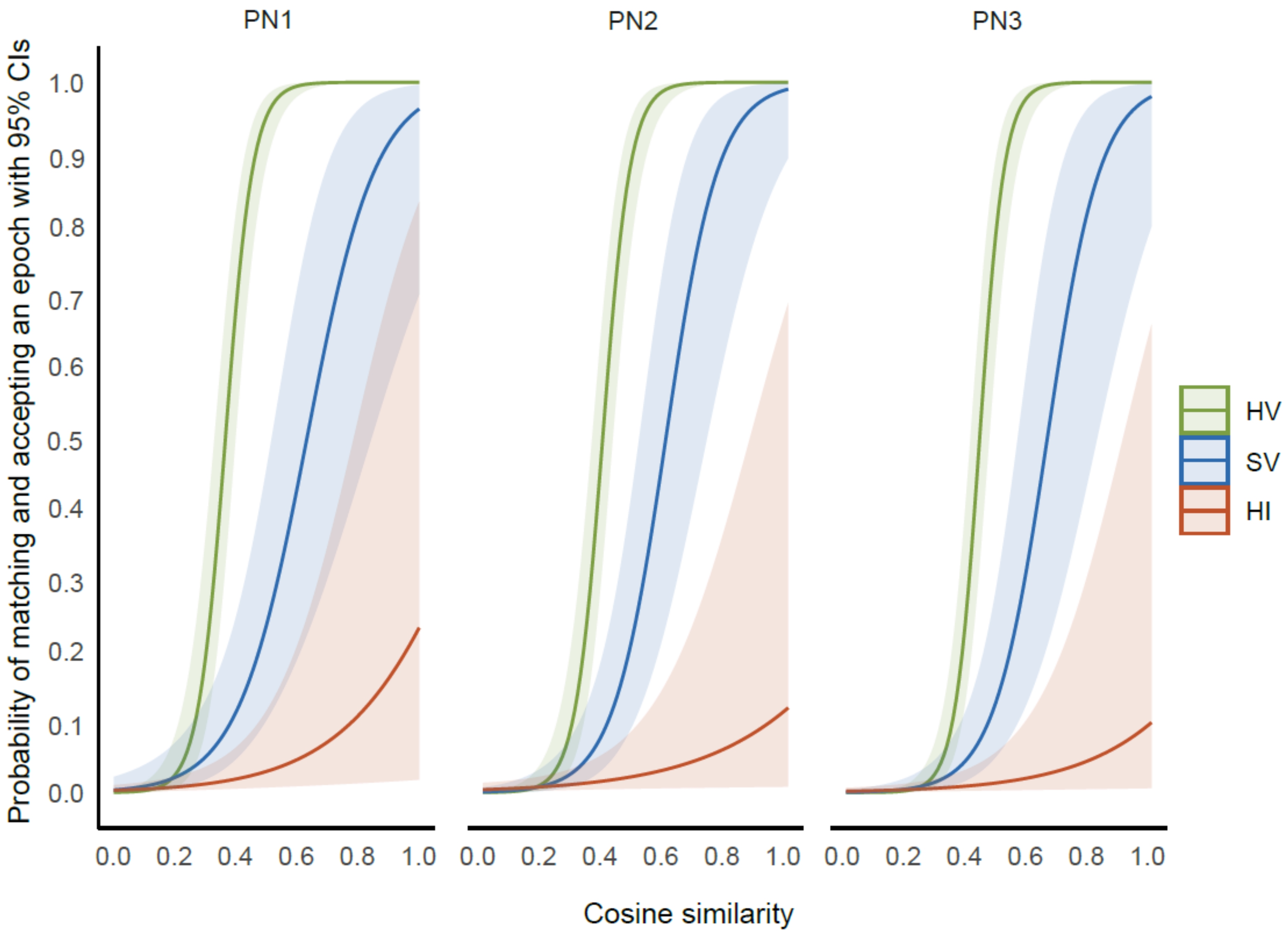
3.2. Secondary Findings: Epoch Selection
3.2.1. Cosine Similarity across Conditions
3.2.2. Intra-rater Reliability (Intra- and Inter-session)
3.2.3. Inter-Rater Reliability
4. Discussion
4.1. Methodological Limitations
4.2. Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olejniczak, P. Neurophysiologic basis of EEG. J. Clin. Neurophysiol. 2006, 23, 186–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sur, S.; Sinha, V.K. Event-related potential: An overview. Ind. Psychiatry J. 2009, 18, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Boudewyn, M.A.; Luck, S.J.; Farrens, J.L.; Kappenman, E.S. How many trials does it take to get a significant ERP effect? It depends. Psychophysiology 2018, 55, e13049. [Google Scholar] [CrossRef]
- Wright, D.J.; Holmes, P.S.; Smith, D. Using the movement-related cortical potential to study motor skill learning. J. Motor Behav. 2011, 43, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.C.; Barry, R.J.; Connolly, J.F.; Fischer, C.; Michie, P.T.; Näätänen, R.; Polich, J.; Reinvang, I.; Petten, C.V. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 2009, 120, 1883–1908. [Google Scholar] [CrossRef]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; The MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Mrachacz-Kersting, N.; Kristensen, S.R.; Niazi, I.K.; Farina, D. Precise temporal association between cortical potentials evoked by motor imagination and afference induces cortical plasticity. J. Physiol. 2012, 590, 1669–1682. [Google Scholar] [CrossRef] [Green Version]
- Carlowitz-Ghori, K.V.; Bayraktaroglu, Z.; Hohlefeld, F.U.; Losch, F.; Curio, G.; Nikulin, V.V. Corticomuscular coherence in acute and chronic stroke. Clin. Neurophysiol. 2014, 125, 1182–1191. [Google Scholar] [CrossRef]
- Fang, Y.; Daly, J.J.; Sun, J.; Hvorat, K.; Fredrickson, E.; Pundik, S.; Sahgal, V.; Yue, G.H. Functional corticomuscular connection during reaching is weakened following stroke. Clin. Neurophysiol. 2009, 120, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Azuma, H.; Hori, S.; Nakanishi, M.; Fujimoto, S.; Ichikawa, N.; Furukawa, T.A. An intervention to improve the interrater reliability of clinical EEG interpretations. Psychiatry Clin. Neurosci. 2003, 57, 485–489. [Google Scholar] [CrossRef]
- Volavka, J. The reliability of visual EEG assessment. Electroencephalogr. Clin. Neurophysiol. 1970, 31, 294. [Google Scholar]
- van Donselaar, C.A.; Schimsheimer, R.J.; Geerts, A.T.; Declerck, A.C. Value of the electroencephalogram in adult patients with untreated idiopathic first seizures. Arch. Neurol. 1992, 49, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Biurrun Manresa, J.A.; Arguissain, F.G.; Redondo, D.E.M.; Mørch, C.D.; Andersen, O.K. On the Agreement between Manual and Automated Methods for Single-Trial Detection and Estimation of Features from Event-Related Potentials. PLoS ONE 2015, 10, e0134127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatem, S.M.; Ragé, M.; Gierasimowicz, A.; Plaghki, L.; Bouhassira, D.; Attal, N.; Iannetti, G.D.; Mouraux, A. Automated single-trial assessment of laser-evoked potentials as an objective functional diagnostic tool for the nociceptive system. Clin. Neurophysiol. 2012, 123, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Rovsing, C.; Rovsing, H.; Cremoux, S.; Signal, N.; Allen, K.; Tayor, D.; Niazi, I.K. Quantification of Movement-Related EEG Correlates Associated with Motor Training: A Study on Movement-Related Cortical Potentials and Sensorimotor Rhythms. Front. Hum. Neurosci. 2017, 11, 604. [Google Scholar] [CrossRef] [Green Version]
- Woodman, G.F. A brief introduction to the use of event-related potentials in studies of perception and atten. Atten. Percept. Psychophys. 2010, 72, 2031–2046. [Google Scholar] [CrossRef]
- Olsen, S.; Signal, N.; Niazi, I.K.; Christensen, T.; Jochumsen, M.; Taylor, D. Paired Associative Stimulation Delivered by Pairing Movement-Related Cortical Potentials With Peripheral Electrical Stimulation: An Investigation of the Duration of Neuromodulatory Effects. Neuromodulation 2018, 21, 362–367. [Google Scholar] [CrossRef]
- Mrachacz-Kersting, N.; Voigt, M.; Stevenson, A.J.T.; Aliakbaryhosseinabadi, S.; Jiang, N.; Dremstrup, K.; Farina, D. The effect of type of afferent feedback timed with motor imagery on the induction of cortical plasticity. Brain Res. 2017, 1674, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Alder, G.; Signal, N.; Vandal, A.C.; Niazi, I.K. Fine-tuning the delivery of a novel neuromodulatory intervention. Presented at Stroke Rehab: From No-Tech to Go-Tech, Christchurch, New Zealand, 29–31 January 2018. [Google Scholar]
- Mrachacz-Kersting, N.; Stevenson, A.J.S.; Jørgensen, H.R.M.; Severinsen, K.E.; Aliakbaryhosseinabadi, S.; Jiang, N.; Farina, D. Brain state-dependent stimulation boosts functional recovery following stroke. Ann. Neurol. 2019, 85, 84–95. [Google Scholar] [CrossRef]
- Mrachacz-Kersting, N.; Jiang, N.; Stevenson, A.J.T.; Niazi, I.K.; Kostic, V.; Pavlovic, A.; Radovanovic, S.; Djuric-Jovicic, M.; Agosta, F.; Dremstrup, K.; et al. Efficient neuroplasticity induction in chronic stroke patients by an associative brain-computer interface. J. Neurophysiol. 2016, 115, 1410–1421. [Google Scholar] [CrossRef]
- Carson, R.G.; Kennedy, N.C. Modulation of human corticospinal excitability by paired associative stimulation. Front. Hum. Neurosci. 2013, 7, 823. [Google Scholar] [CrossRef] [Green Version]
- Suppa, A.; Quartarone, A.; Siebner, H.; Chen, R.; Lazzaro, L.D.; Giudice, P.D.; Paulus, W.; Rothwell, J.C.; Ziemann, U.; Classen, J. The associative brain at work: Evidence from paired associative stimulation studies in humans. Clin. Neurophysiol. 2017, 128, 2140–2164. [Google Scholar] [CrossRef]
- Alder, G.; Signal, N.; Olsen, S.; Taylor, D. A Systematic Review of Paired Associative Stimulation (PAS) to Modulate Lower Limb Corticomotor Excitability: Implications for Stimulation Parameter Selection and Experimental Design. Front. Neurosci. 2019, 13, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibasaki, H.; Hallett, M. What is the Bereitschaftspotential? Clin. Neurophysiol. 2006, 117, 2341–2356. [Google Scholar] [CrossRef]
- Jankelowitz, S.K.; Colebatch, J.G. Movement-related potentials associated with self-paced, cued and imagined arm movements. Exp. Brain Res. 2002, 147, 98–107. [Google Scholar] [CrossRef]
- Walter, W.; Cooper, R.; Aldridge, V.J.; Mccallum, W.C.; Winter, A.L. Contingent negative variation: An electric sign of sensori-motor association and expectancy in the human brain. Nature 1964, 203, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Ridding, M.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Lahr, J.; Paßmann, S.; List, J.; Vach, W.; Flöel, A.; Klöppel, S. Effects of different analysis strategies on paired associative stimulation. A pooled data analysis from three research labs. PLoS ONE 2016, 11, e0154880. [Google Scholar] [CrossRef] [PubMed]
- Müller-Dahlhaus, J.F.M.; Orekhov, Y.; Liu, Y.; Ziemann, U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp. Brain Res. 2008, 187, 467–475. [Google Scholar] [CrossRef]
- Hamada, M.; Murase, N.; Hasan, A.; Balaratnam, M.; Rothwell, J.C. The role of interneuron networks in driving human motor cortical plasticity. Cereb. Cortex 2012, 23, 1593–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sale, M.V.; Ridding, M.C.; Nordstrom, M.A. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp. Brain Res. 2007, 181, 615–626. [Google Scholar] [CrossRef]
- Voti, P.L.; Conte, A.; Suppa, A.; Iezzi, E.; Bologna, M.; Aniello, M.S.; Defazio, D.; Rothwell, J.C.; Berardelli, A. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp. Brain Res. 2011, 212, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Signal, N.; Nedergaard, R.W.; Taylor, D.; Haavik, H.; Niazi, I.K. Induction of Long-term Depression-like Plasticity by Pairings of Motor Imagination and Peripheral Electrical Stimulation. Front. Hum. Neurosci. 2015, 9, 644. [Google Scholar] [CrossRef] [Green Version]
- Jochumsen, M.; Niazi, I.K.; Signal, N.; Nedergaard, R.W.; Holt, K.; Haavik, H.; Taylor, D. Pairing voluntary movement and muscle-located electrical stimulation increases cortical excitability. Front. Hum. Neurosci. 2016, 10, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [CrossRef]
- McGraw, K.O.; Wong, S.P. Forming Inferences about Some Intraclass Correlation Coefficients. Psychol. Methods 1996, 1, 30. [Google Scholar] [CrossRef]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 3rd ed.; F.A Davis Company: Philadelphia, PA, USA, 2015. [Google Scholar]
- Atkinson, G.; Nevill, A.M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef]
- Rashid, U.; Niazi, I.K.; Jochumsen, M.; Krol, L.R.; Signal, N.; Taylor, D. Automated Labeling of Movement-Related Cortical Potentials Using Segmented Regression. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1282–1291. [Google Scholar] [CrossRef]
- Douglas, B.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Yilmaz, O.; Cho, W.; Braun, C.; Birbaumer, N.; Ramos-Murguialday, A. Movement related cortical potentials in severe chronic stroke. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013. [Google Scholar]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Byblow, W.D.; Steyvers, M.; Levin, O.; Swinnen, S.P. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp. Brain Res. 2006, 168, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Shi, F.Y.; Guan, Y.; Wu, Y.; Zhang, L.L.; Shen, C.; Zeng, Y.W.; Wang, D.H.; Zhang, J. The effect of motor imagery with specific implement in expert badminton player. Neuroscience 2014, 275, 102–112. [Google Scholar] [CrossRef]
- Bisio, A.; Avanzino, L.; Biggio, M.; Ruggeri, P.; Bove, M. Motor training and the combination of action observation and peripheral nerve stimulation reciprocally interfere with the plastic changes induced in primary motor cortex excitability. Neuroscience 2017, 348, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Avanzino, L.; Gueugneau, N.; Bisio, A.; Ruggeri, P.; Papaxanthis, C.; Bove, M. Motor cortical plasticity induced by motor learning through mental practice. Front. Behav. Neurosci. 2015, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonassi, G.; Biggio, M.; Bisio, A.; Ruggeri, P.; Bove, M.; Avanzino, L. Provision of somatosensory inputs during motor imagery enhances learning-induced plasticity in human motor cortex. Sci. Rep. 2017, 7, 9300. [Google Scholar] [CrossRef]
- Do Nascimento, O.F.; Nielsen, K.D.; Voigt, M. Movement-related parameters modulate cortical activity during imaginary isometric plantar-flexions. Exp. Brain Res. 2006, 171, 78–90. [Google Scholar] [CrossRef]
- Taylor, M.J. Bereitschaftspotential during the acquisition of a skilled motor task. Electroencephalogr. Clin. Neurophysiol. 1978, 45, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Chiang, H.; Slobounov, S.M.; Ray, W. Practice-related modulations of force enslaving and cortical activity as revealed by EEG. Clin. Neurophysiol. 2004, 115, 1033–1043. [Google Scholar] [CrossRef]
- Smith, A.L.; Staines, W.R. Cortical and behavioral adaptations in response to short-term inphase versus antiphase bimanual movement training. Exp. Brain Res. 2010, 205, 465–477. [Google Scholar] [CrossRef]
- Smith, A.L.; Staines, W.R. Externally cued inphase bimanual training enhances preparatory premotor activity. Clin. Neurophysiol. 2012, 123, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.J.; Holmes, P.; Smith, D. Reduced motor cortex activity during movement preparation following a period of motor skill practice. PLoS ONE 2012, 7, e51886. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.J.; Holmes, P.S.; Russo, F.D.; Loporto, M.; Smith, D. Differences in cortical activity related to motor planning between experienced guitarists and non-musicians during guitar playing. Hum. Mov. Sci. 2012, 31, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Niemann, J.; Winker, T.; Gerling, L.; Landwehrmeyer, B.; Jung, R. Changes of slow cortical negative DC-potentials during the acquisition of a complex finger motor task. Exp. Brain Res. 1991, 85, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Francesco, D.R.; Sabrina, P.; Teresa, A.; Donatella, S. Effect of practice on brain activity: An investigation in top-level rifle shooters. Med. Sci. Sports Exerc. 2005, 37, 1586–1593. [Google Scholar] [CrossRef] [Green Version]
- Fattapposta, F.; Amabile, G.; Cordischi, M.V.; Venanzio, D.D.; Foti, A.; Pierelli, F.; D’Alessio, C.; Pigozzi, F.; Parisi, A.; Morrocutti, C. Long-term practice effects on a new skilled motor learning: An electrophysiological study. Electroencephalogr. Clin. Neurophysiol. 1996, 99, 495–507. [Google Scholar] [CrossRef]
- Hatta, A.; Nishihira, Y.; Higashiura, T.; Kim, S.R.; Kaneda, T. Long-term motor practice induces practice-dependent modulation of movement-related cortical potentials (MRCP) preceding a self-paced non-dominant handgrip movement in kendo players. Neurosci. Lett. 2009, 459, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Mori, A.; Nara, M. Two types of movement-related cortical potentials preceding wrist extension in humans. Neuroreport 2001, 12, 2221–2225. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Los Angeles, CA, USA, 2018. [Google Scholar]
- Lenth, R. emmeans: Estimated Marginal Means, Aka Least-Squares Means R Package Version 1.3.0. 2018. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 February 2020).



| Healthy Participants | Participants with Stroke | |
|---|---|---|
| Mean age (years) | 28.6 (21–52) | 67 (57–78) |
| Gender (males:females) | 8:12 | 2:3 |
| Lesion | ||
| -Hemisphere (right:left) | 3:2 | |
| -Type (ischemic:haemorrhagic) | 4:1 | |
| Mean time since stroke (years) | 7 (1–17) | |
| Mean gait speed (m/s) | 0.4 (0.2–0.75) | |
| m/s = metres per second. Bracketed age and m/s data represent ranges. | ||
| Intra-session Reliability: PN1 and PN2 | ||||||
|---|---|---|---|---|---|---|
| Healthy Voluntary DF | Healthy Imagined DF | Stroke Voluntary DF | ||||
| ICC | SEM | ICC | SEM | ICC | SEM | |
| E1 | 1.00 (0.99–1.00) | 7.7 | 0.61 (0.34–0.89) | 155.54 | 0.84 (0.46–0.96) | 78.14 |
| E2 | 0.99 (0.97–0.99) | 18.9 | 0.90 (0.67–0.97) | 63.55 | 0.91 (0.70–0.98) | 46.98 |
| E3 | 0.90 (0.67–0.97) | 72.6 | 0.75 (0.30–0.93) | 75.42 | 0.93 (0.75–0.98) | 27.43 |
| E4 | 1.00 (0.99–1.00) | 6.99 | 0.63 (0.09–0.89) | 120.38 | 1.00 (0.99–1.00) | 3.84 |
| E5 | 1.00 (0.99–1.00) | 5.06 | 0.63 (0.06–0.89) | 180.21 | 0.95 (0.80–0.99) | 37.9 |
| Inter-session Reliability: PN1 and PN3 | ||||||
| E1 | 0.99 (0.95–1.00) | 19.61 | 0.55 (−0.03–0.86) | 184.61 | 0.90 (0.65–0.98) | 57.47 |
| E2 | 0.98 (0.93–0.97) | 30.56 | 0.76 (0.28–0.93) | 115.10 | 0.94 (0.77–0.98) | 41.22 |
| E3 | 0.98 (0.93–1.00) | 32.61 | 0.48 (−0.09–0.83) | 116.01 | 0.85 (0.50–0.96) | 55.81 |
| E4 | 0.95 (0.83–0.99) | 52.76 | 0.05 (−0.60–0.64) | 279.2 | 0.80 (0.42–0.95) | 72.81 |
| E5 | 1.00 (0.98–1.00) | 15.32 | 0.68 (0.10–0.91) | 193.39 | 0.83 (0.46–0.96) | 65.91 |
| Inter-rater reliability: PN1, PN2, PN3 | ||||||
|---|---|---|---|---|---|---|
| Healthy Voluntary DF | Healthy Imagined DF | Stroke Voluntary DF | ||||
| ICC | SEM | ICC | SEM | ICC | SEM | |
| PN1 | 0.99 (0.97–1.00) | 26.13 | 0.40 (0.14–0.74) | 187.89 | 0.84 (0.67–0.95) | 58.71 |
| PN2 | 0.95 (0.88–0.98) | 51.15 | 0.76 (0.53–0.92) | 104.81 | 0.88 (0.74–0.96) | 62.12 |
| PN3 | 0.97 (0.92–0.99) | 39.75 | 0.2 (0.00–0.60) | 246.51 | 0.78 (0.67–0.87) | 79.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alder, G.; Signal, N.; Rashid, U.; Olsen, S.; Niazi, I.K.; Taylor, D. Intra- and Inter-Rater Reliability of Manual Feature Extraction Methods in Movement Related Cortical Potential Analysis. Sensors 2020, 20, 2427. https://doi.org/10.3390/s20082427
Alder G, Signal N, Rashid U, Olsen S, Niazi IK, Taylor D. Intra- and Inter-Rater Reliability of Manual Feature Extraction Methods in Movement Related Cortical Potential Analysis. Sensors. 2020; 20(8):2427. https://doi.org/10.3390/s20082427
Chicago/Turabian StyleAlder, Gemma, Nada Signal, Usman Rashid, Sharon Olsen, Imran Khan Niazi, and Denise Taylor. 2020. "Intra- and Inter-Rater Reliability of Manual Feature Extraction Methods in Movement Related Cortical Potential Analysis" Sensors 20, no. 8: 2427. https://doi.org/10.3390/s20082427
APA StyleAlder, G., Signal, N., Rashid, U., Olsen, S., Niazi, I. K., & Taylor, D. (2020). Intra- and Inter-Rater Reliability of Manual Feature Extraction Methods in Movement Related Cortical Potential Analysis. Sensors, 20(8), 2427. https://doi.org/10.3390/s20082427








