Assessment of an Assistive Control Approach Applied in an Active Knee Orthosis Plus Walker for Post-Stroke Gait Rehabilitation
Abstract
:1. Introduction
1.1. Lower-Limb Powered Devices
1.2. Admittance Controller
2. Materials
2.1. Advanced Lower-Limb Orthosis for Rehabilitation (ALLOR)
2.2. Controller
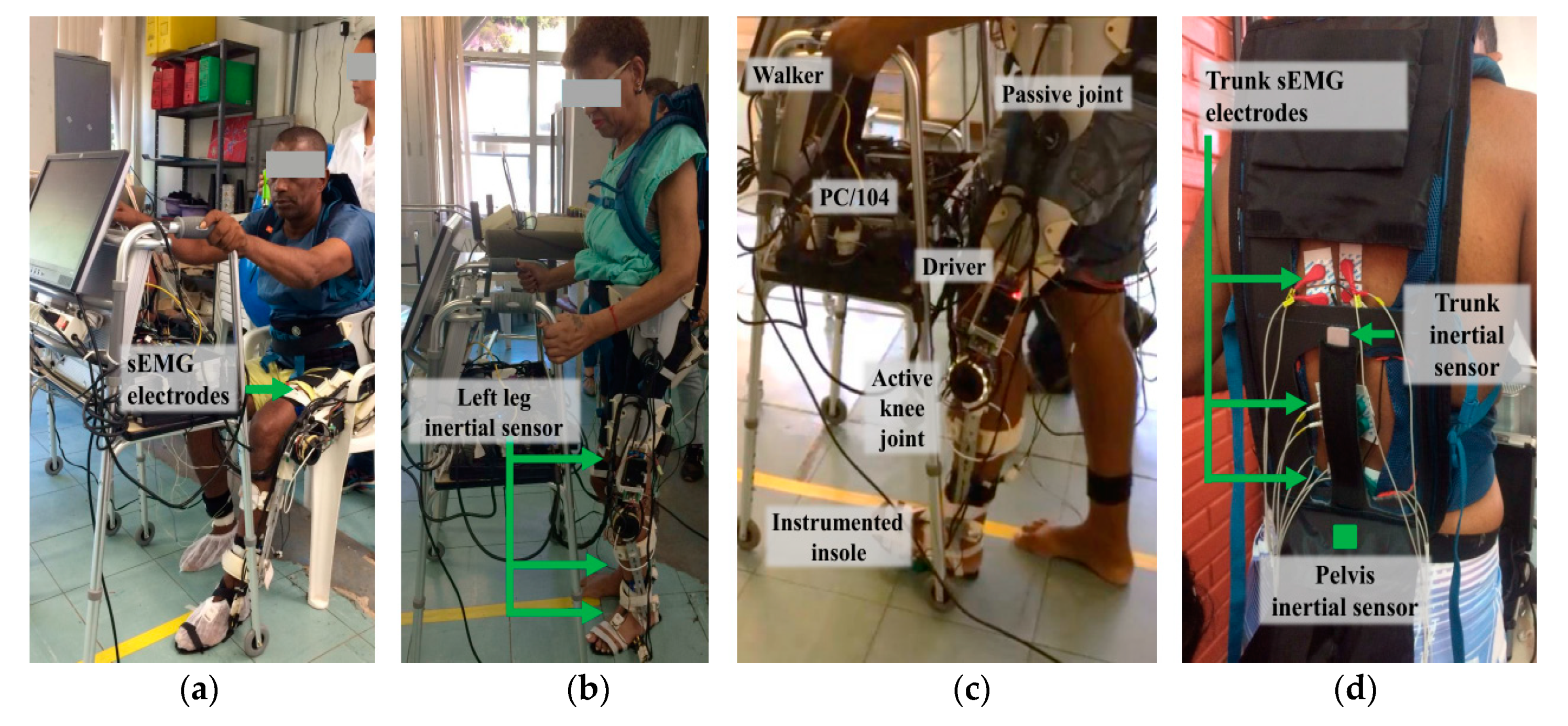
2.3. sEMG and Inertial Sensors
3. Methodology
3.1. Subjects
3.2. Experimental Protocol
3.3. Data Processing
3.3.1. Bilateral Muscular Analysis and Fatigue
3.3.2. Bilateral Muscular Analysis and Fatigue
3.4. Statistical Analysis
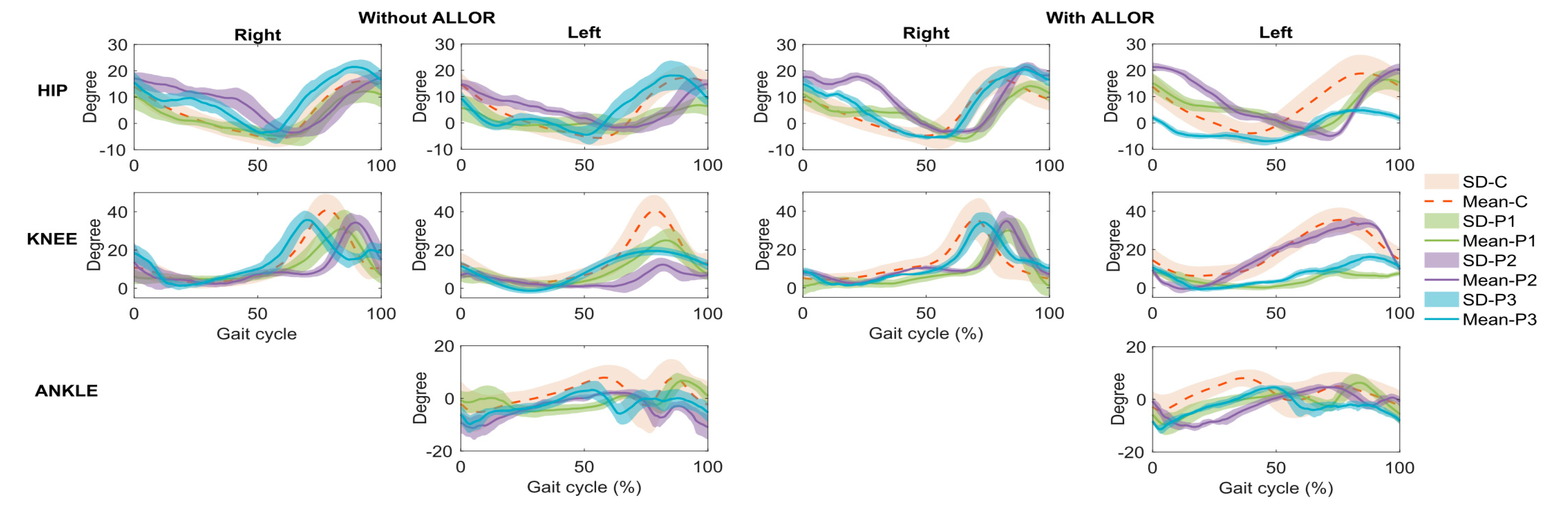
4. Results
5. Discussion
6. Conclusions
7. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statements
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Balaban, B.; Tok, F. Gait disturbances in patients with stroke. PM&R 2014, 6, 635–642. [Google Scholar]
- Lewek, M.D.; Bradley, C.E.; Wutzke, C.J.; Zinder, S.M. The relationship between spatiotemporal gait asymmetry and balance in individuals with chronic stroke. J. Appl. Biomech. 2014, 30, 3136. [Google Scholar] [CrossRef] [Green Version]
- Weerdesteyn, V.; Niet, M.; van Duijnhoven, H.J.R.; Geurts, A.C.H. Falls in individuals with stroke. J. Rehabil. Res. Dev. 2008, 45, 1195–1213. [Google Scholar] [CrossRef]
- Peñas, C.F.; Cleland, J.A.; Huijbregts, P.A. Neck and Arm Pain Syndromes; E-Book: Evidence-informed Screening, Diagnosis and Management; Elsevier: London, UK, 2011. [Google Scholar]
- Peñas, C.F.; Cleland, J.A. Manual Therapy for Musculoskeletal Pain Syndromes E-Book: An Evidence- and Clinical-Informed Approach; Elsevier: London, UK, 2015. [Google Scholar]
- Gillen, G. Stroke Rehabilitation, 4th ed.; Elsevier: Maryland Heights, MS, USA, 2016. [Google Scholar]
- Chaitow, L.; DeLany, J. Clinical Application of Neuromuscular Techniques, 2nd ed.; The Lower Body; Elsevier: London, UK, 2011; Volume 2. [Google Scholar]
- Rafiaei, M.; Bahramizadeh, M.; Arazpour, M.; Samadian, M.; Hutchins, S.W.; Farahmand, F.; Mardani, M.A. The gait and energy efficiency of stance control knee–ankle–foot orthoses: A literature review. Prosthet. Orthot. Int. 2016, 40, 202–214. [Google Scholar] [CrossRef]
- Zacharias, B.; Kannenberg, A. Clinical benefits of stance control orthosis systems: An analysis of the scientific literature. J. Prosthet. Orthot. 2012, 24, 2–7. [Google Scholar] [CrossRef]
- Ir, M.; Ao, N.A. Stance-control-orthoses with electromechanical actuation mechanism: Usefulness, design analysis and directions to overcome challenges. J. Neurol. Neurosci. 2015, 6, 49. [Google Scholar] [CrossRef]
- Standards Roadmap: Neurotechnologies for Brain-Machine Interfacing. IEEE SA Industry Connections Activity No. IC17-007 2020. Available online: https://standards.ieee.org/content/dam/ieee-standards/standards/web/documents/presentations/ieee-neurotech-for-bmi-standards-roadmap.pdf (accessed on 25 March 2020).
- Grasmücke, D.; Zieriacks, A.; Jansen, O.; Fisahn, C.; Sczesny-Kaiser, M.; Wessling, M.; Meindl, R.C.; Schildhauer, T.A.; Aach, M. Against the odds: What to expect in rehabilitation of chronic spinal cord injury with a neurologically controlled Hybrid Assistive Limb exoskeleton. A subgroup analysis of 55 patients according to age and lesion level. Neurosurg. Focus 2017, 42, E15. [Google Scholar] [CrossRef]
- Pons, J.L. Wearable Robots: Biomechatronic Exoskeletons; John Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- García-Cossio, E.; Severens, M.; Nienhuis, B.; Duysens, J.; Desain, P.; Keijsers, N.; Farquhar, J. Decoding sensorimotor rhythms during robotic-assisted treadmill walking for brain computer interface (BCI) applications. PLoS ONE 2015, 10, e0137910. [Google Scholar]
- Arazpour, M.; Hutchins, S.W.; Ahmadi, B.M. The efficacy of powered orthoses on walking in persons with paraplegia. Prosthet. Orthot. Int. 2015, 39, 90–99. [Google Scholar] [CrossRef]
- Rupal, B.S.; Rafique, S.; Singla, A.; Singla, E.; Isaksson, M.; Virk, G.S. Lower-limb exoskeletons: Research trends and regulatory guidelines in medical and non-medical applications. Int. J. Adv. Robot. Syst. 2017, 14. [Google Scholar] [CrossRef]
- Contreras-Vidal, J.L.; Bhagat, N.; Brantley, J.; Cruz-Garza, J.G.; He, Y.; Manley, Q.; Nakagome, S.; Nathan, K.; Tan, S.H.; Zhu, F.; et al. Powered exoskeletons for bipedal locomotion after spinal cord injury. J. Neural Eng. 2016, 13, 031001. [Google Scholar] [CrossRef]
- Miller, L.E.; Zimmermann, A.K.; Herbert, W.G. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: Systematic review with meta-analysis. Med. Devices 2016, 9, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Lajeunesse, V.; Vincent, C.; Routhier, F.; Careau, E.; Michaud, F. Exoskeletons’ design and usefulness evidence according to a systematic review of lower limb exoskeletons used for functional mobility by people with spinal cord injury. Disabil. Rehabil. Assist. Technol. 2016, 11, 535–547. [Google Scholar] [CrossRef]
- Louie, D.R.; Eng, J.J. Powered robotic exoskeletons in post-stroke rehabilitation of gait: A scoping review. J. Neuroeng. Rehabil. 2016, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Eguren, D.; Luu, T.P.; Contreras-Vidal, J.L. Risk management and regulations for lower limb medical exoskeletons: A review. Med. Devices 2017, 10, 89–107. [Google Scholar] [CrossRef] [Green Version]
- 1A1-1 Empower, Ottobock. Available online: https://professionals.ottobock.com.au/ Products/Prosthetics/Prosthetics-Lower-Limb/Feet/1A1-1-Empower/p/1A1-1 (accessed on 25 March 2020).
- Power Knee, Ossur. Available online: https://www.ossur.com/en-us/prosthetics/knees (accessed on 25 March 2020).
- Young, A.J.; Ferris, D.P. State of the art and future directions for lower limb robotic exoskeletons. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 171–182. [Google Scholar] [CrossRef]
- Chen, B.; Ma, H.; Qin, L.Y.; Gao, F.; Chan, K.M.; Law, S.W.; Qin, L.; Liao, W.H. Recent developments and challenges of lower extremity exoskeletons. J. Orthop. Transl. 2016, 5, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Farris, R.J.; Quintero, H.A.; Murray, S.A.; Ha, K.H.; Hartigan, C.; Goldfarb, M. A preliminary assessment of legged mobility provided by a lower limb exoskeleton for persons with paraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Tucker, M.R.; Olivier, J.; Pagel, A.; Bleuler, H.; Bouri, M.; Lambercy, O.; del R Millán, J.; Riener, R.; Vallery, H.; Gassert, R. Control strategies for active lower extremity prosthetics and orthotics: A review. J. Neuroeng. Rehabil. 2015, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Buerger, S.P.; Hogan, N. Impedance and interaction control. In Robotics and Automation Handbook; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Newman, W.S. Stability and performance limits of interaction controllers. J. Dyn. Syst. Meas. Control 1992, 114, 563–570. [Google Scholar] [CrossRef]
- Hussain, S.; Xie, S.Q.; Jamwal, P.K. Adaptive impedance control of a robotic orthosis for gait rehabilitation. IEEE Trans. Cybern. 2013, 43, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J. Mechanical impedance and its relations to motor control, limb dynamics, and motion biomechanics. J. Med. Biol. Eng. 2015, 35, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Xie, S.Q.; Das, R.; Zhu, G.L. Control strategies for effective robot assisted gait rehabilitation: The state of art and future prospects. Med. Eng. Phys. 2014, 36, 1555–1566. [Google Scholar] [CrossRef]
- Tucker, M.; Moser, A.; Lambercy, O.; Sulzer, J.; Gassert, R. Design of a wearable perturbator for human knee impedance estimation during gait. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Seattle, WA, USA, 24–26 June 2013; pp. 1–6. [Google Scholar]
- Villa-Parra, A.C.; Delisle-Rodriguez, D.; Botelho, T.; Villarejo-Mayor, J.J.; López-Delis, A.; Carelli, R.; Frizera-Neto, A.; Bastos-Filho, T. Control of a robotic knee exoskeleton for assistance and rehabilitation based on motion intention from sEMG. Res. Biomed. Eng. 2018, 34, 198–210. [Google Scholar] [CrossRef]
- Villa-Parra, A.C.; Delisle-Rodriguez, D.; Lima, J.S.; Frizera-Neto, A.; Bastos, T. Knee impedance modulation to control an active orthosis using insole sensors. Sensors 2017, 17, 2751. [Google Scholar] [CrossRef] [Green Version]
- Knaepen, K.; Beyl, P.; Duerinck, S.; Hagman, F.; Lefeber, D.; Meeusen, R. Human-robot interaction: Kinematics and muscle activity inside a powered compliant knee exoskeleton. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 1128–1137. [Google Scholar] [CrossRef]
- Kammen, K.; Boonstra, A.M.; Woude, L.H.V.; Reinders-Messelink, H.A.; Otter, R. Differences in muscle activity and temporal step parameters between Lokomat guided walking and treadmill walking in post-stroke hemiparetic patients and healthy walkers. J. Neuroeng. Rehabil. 2017, 14, 32. [Google Scholar] [CrossRef]
- Coenen, P.; Werven, G.; Nunen, M.P.; Dieën, J.H.; Gerrits, K.H.; Janssen, T.W. Robot-assisted walking vs overground walking in stroke patients: An evaluation of muscle activity. J. Rehabil. Med. 2012, 44, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Kammen, K.; Boonstra, A.; Reinders-Messelink, H.; Otter, R. The combined effects of body weight support and gait speed on gait related muscle activity: A comparison between walking in the Lokomat exoskeleton and regular treadmill walking. PLoS ONE 2014, 9, e107323. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European recommendations for surface electromyography. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Vargas-Valencia, L.S.; Elias, A.; Rocon, E.; Bastos-Filho, T.; Frizera, A. An IMU-to-body alignment method applied to human gait analysis. Sensors 2016, 16, 2090. [Google Scholar] [CrossRef] [Green Version]
- Palermo, E.; Rossi, S.; Marini, F.; Patanè, F.; Cappa, P. Experimental evaluation of accuracy and repeatability of a novel body-to-sensor calibration procedure for inertial sensor-based gait analysis. Measurement 2014, 52, 145–155. [Google Scholar] [CrossRef]
- Cutti, A.G.; Ferrari, A.; Garofalo, P.; Raggi, M.; Cappello, A.; Ferrari, A. “Outwalk”: A protocol for clinical gait analysis based on inertial and magnetic sensors. Med. Biol. Eng. Comput. 2010, 48, 17–25. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Shahar, N.; Schwartz, I.; Portnoy, S. Differences in muscle activity and fatigue of the upper limb between task-specific training and robot assisted training among individuals post stroke. J. Biomech. 2019, 89, 28–33. [Google Scholar] [CrossRef]


| Weight | 3.4 kg |
| User’s Heights | 1.5–1.85 m |
| User’s Weights | 50–95 kg |
| Control Levels | High: Human Movement Intention Recognition (HMIR) through acquisition of sEMG from trunk muscles |
| Middle: Finite State Machine (FSM) to switch the following classes of movement: Stand-Up (SU), Sit-Down (SD), Knee Flexion-Extension (F/E), Walking (W), Rest in Stand-Up Position (RSU) and Rest in Sit-Down Position (RSD) | |
| Low: Admittance Controller, Speed Controller, Proportional Integral (PI) Controller |
| Joint | P1 | P2 | P3 | |||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |
| Without ALLOR | ||||||
| Hip | 0.91 | 0.49 | 0.51 | 0.52 | 0.78 | 0.83 |
| Knee | 0.82 | 0.77 | 0.43 | 0.24 | 0.77 | 0.72 |
| Ankle | 0.09 | 0.41 | 0.46 | |||
| With ALLOR | ||||||
| Hip | 0.46 | 0.44 | 0.31 | 0.22 | 0.87 | 0.42 |
| Knee | 0.33 | 0.10 | 0.26 | 0.92 | 0.86 | 0.30 |
| Ankle | 0.33 | 0.09 | 0.34 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Parra, A.C.; Lima, J.; Delisle-Rodriguez, D.; Vargas-Valencia, L.; Frizera-Neto, A.; Bastos, T. Assessment of an Assistive Control Approach Applied in an Active Knee Orthosis Plus Walker for Post-Stroke Gait Rehabilitation. Sensors 2020, 20, 2452. https://doi.org/10.3390/s20092452
Villa-Parra AC, Lima J, Delisle-Rodriguez D, Vargas-Valencia L, Frizera-Neto A, Bastos T. Assessment of an Assistive Control Approach Applied in an Active Knee Orthosis Plus Walker for Post-Stroke Gait Rehabilitation. Sensors. 2020; 20(9):2452. https://doi.org/10.3390/s20092452
Chicago/Turabian StyleVilla-Parra, Ana Cecilia, Jessica Lima, Denis Delisle-Rodriguez, Laura Vargas-Valencia, Anselmo Frizera-Neto, and Teodiano Bastos. 2020. "Assessment of an Assistive Control Approach Applied in an Active Knee Orthosis Plus Walker for Post-Stroke Gait Rehabilitation" Sensors 20, no. 9: 2452. https://doi.org/10.3390/s20092452





