Tunable Chemical Grafting of Three-Dimensional Poly (3, 4-ethylenedioxythiophene)/Poly (4-styrenesulfonate)-Multiwalled Carbon Nanotubes Composite with Faster Charge-Carrier Transport for Enhanced Gas Sensing Performance
Abstract
:1. Introduction
2. Materials and Methods
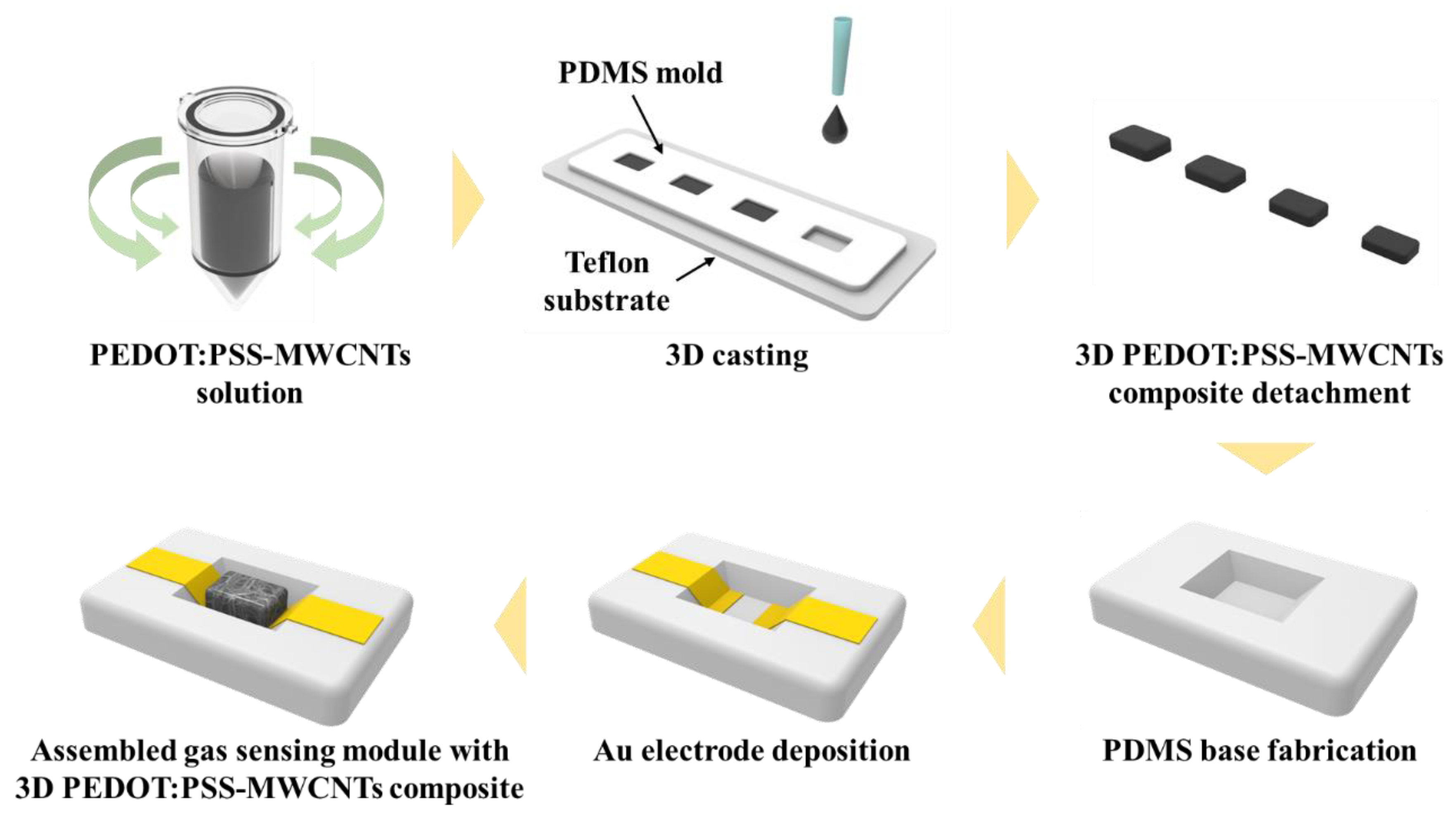
2.1. Fabrication of 3D PEDOT:PSS-MWCNT Composite
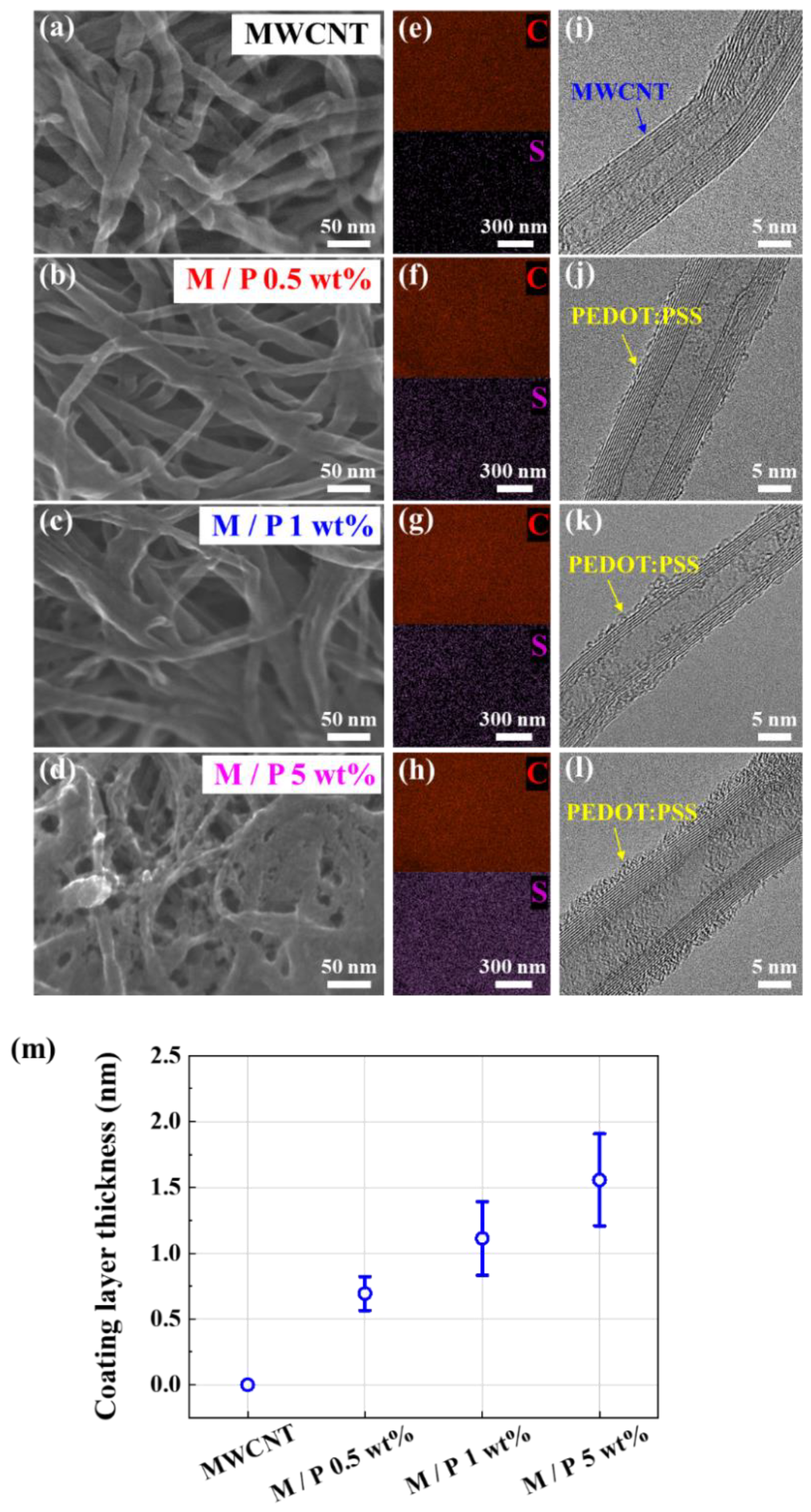
2.2. Characterization of 3D PEDOT:PSS-MWCNT Composites
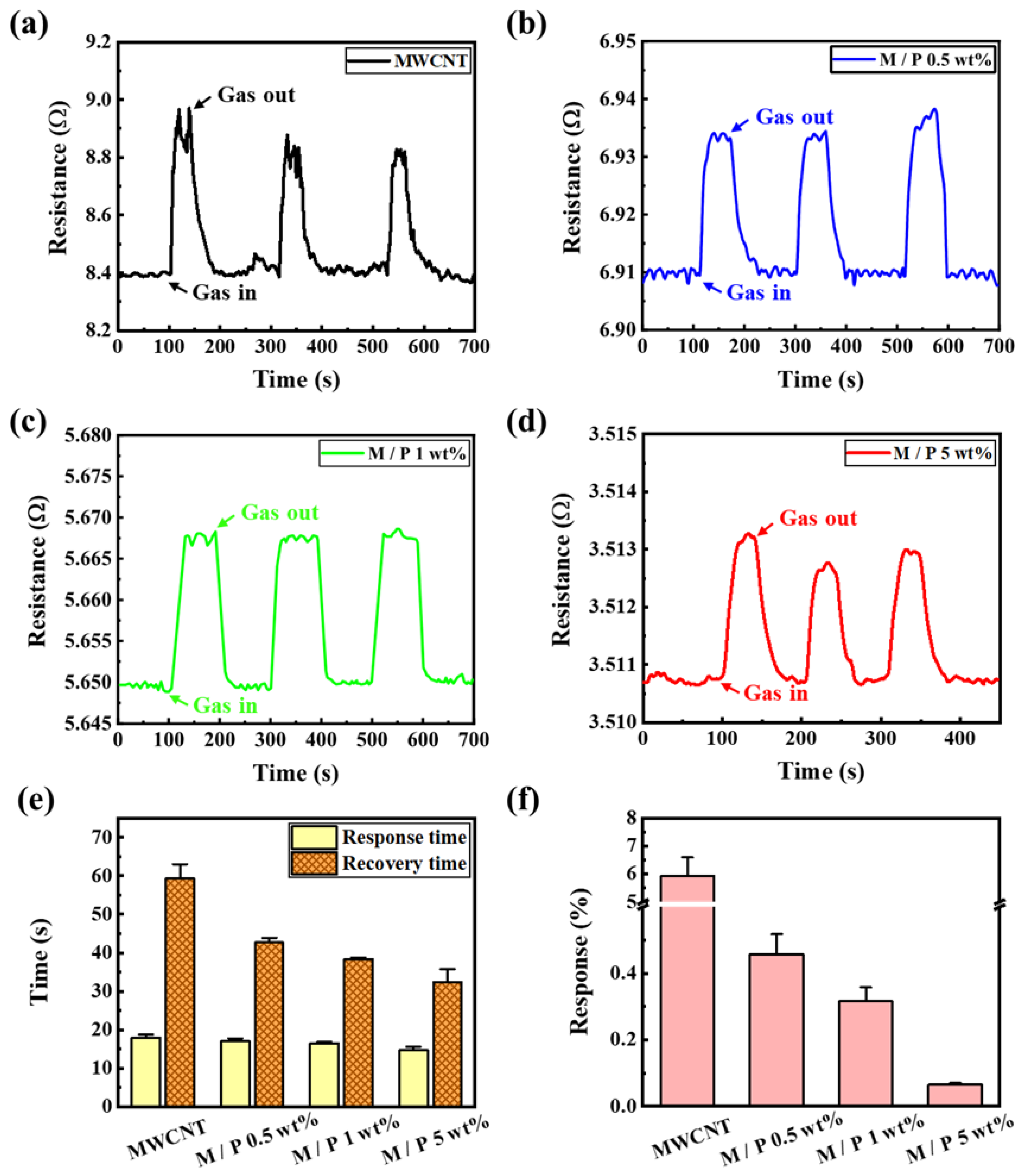
2.3. Testing of Gas-Sensing Performance
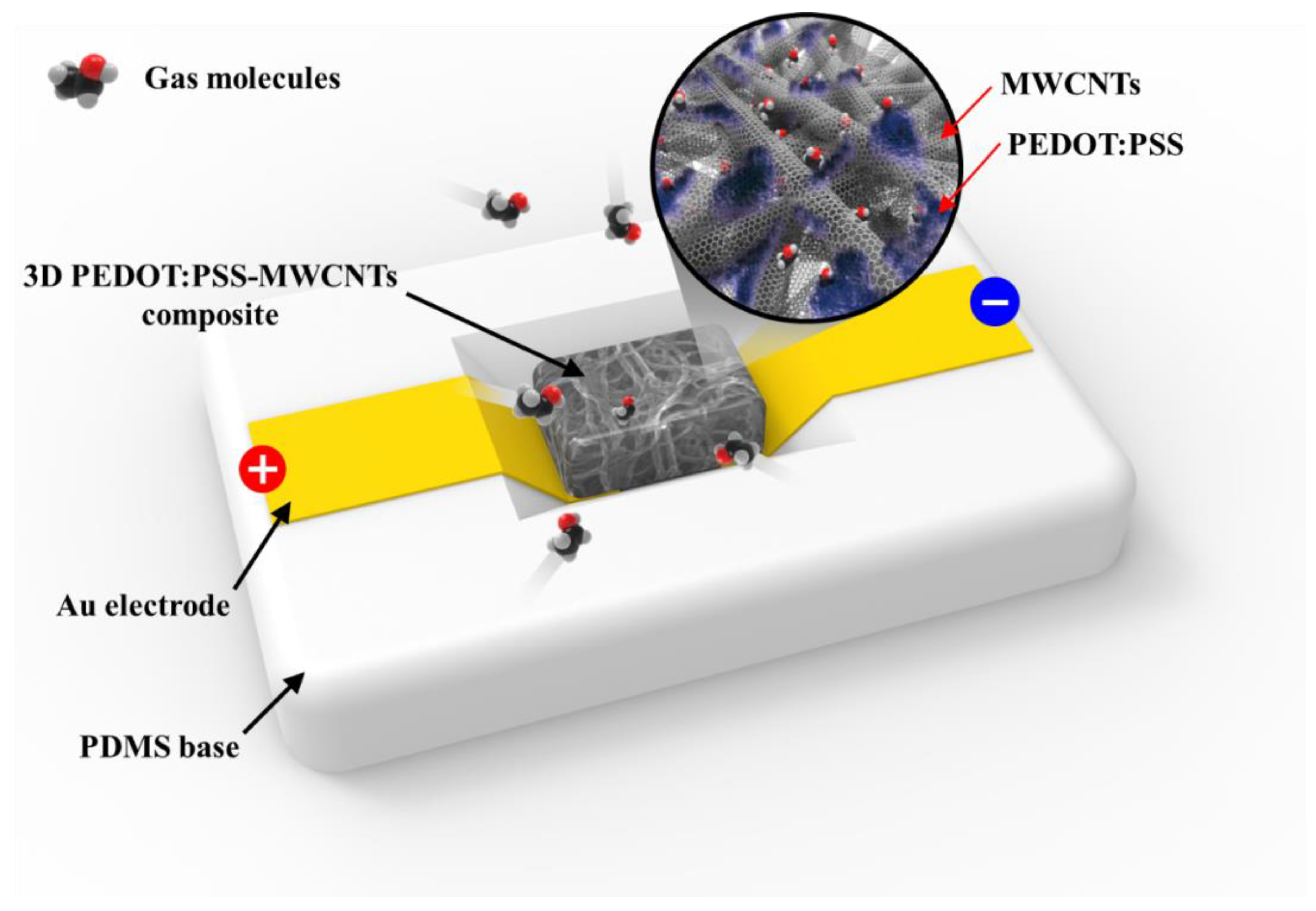
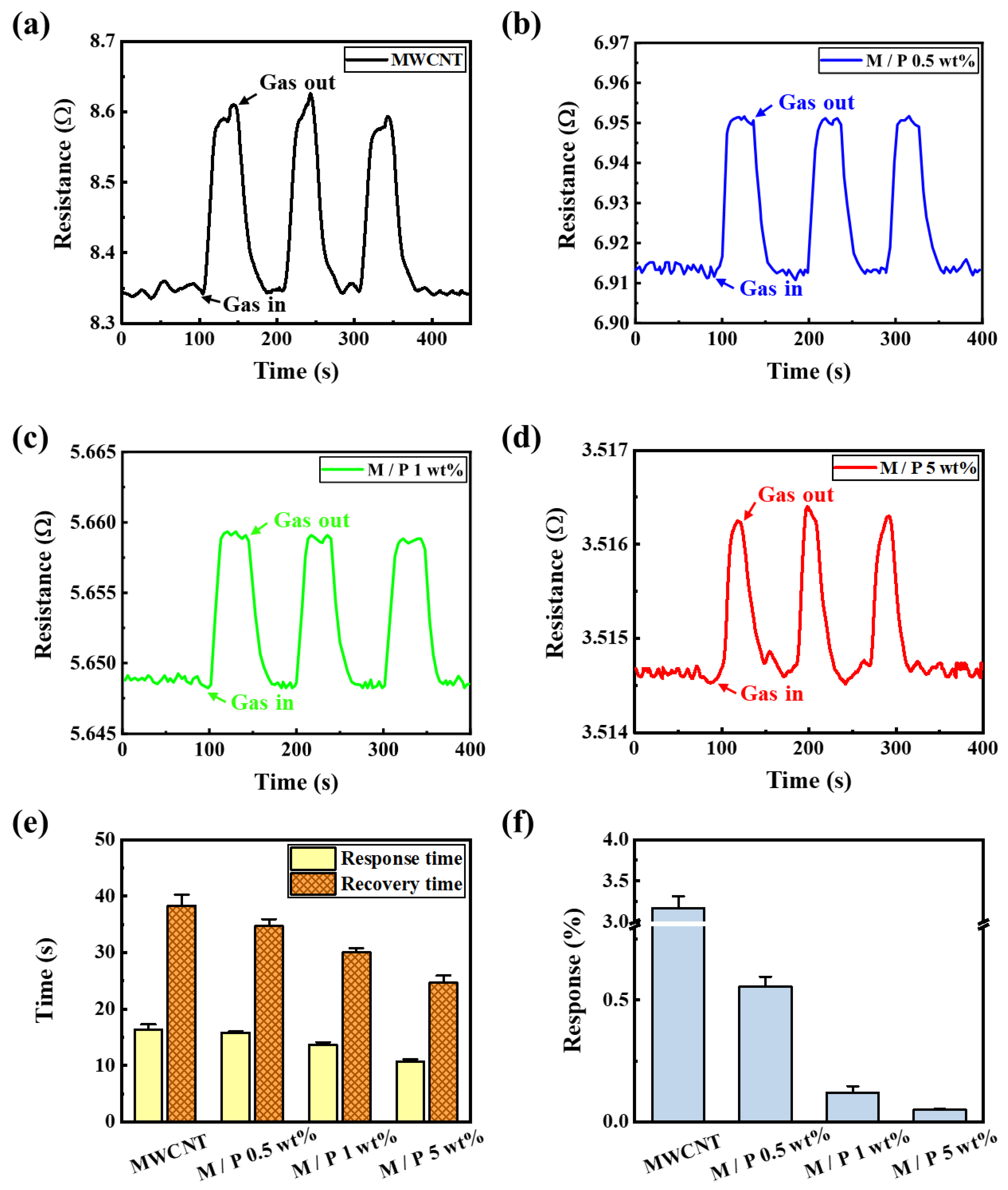
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, G.; Ocola, L.E.; Chen, J. Room-temperature gas sensing based on electron transfer between discrete tin oxide nanocrystals and multiwalled carbon nanotubes. Adv. Mater. 2009, 21, 2487–2491. [Google Scholar] [CrossRef]
- Modi, A.; Koratkar, N.; Lass, E.; Wei, B.; Ajayan, P.M. Miniaturized gas ionization sensors using carbon nanotubes. Nature 2003, 424, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Ceseracciu, L.; Biso, M.; Ansaldo, A.; Futaba, D.N.; Hata, K.; Barone, A.C.; Ricci, D. Mechanics and actuation properties of bucky gel-based electroactive polymers. Sens. Actuators B Chem. 2011, 156, 949–953. [Google Scholar] [CrossRef]
- Cha, S.I.; Kim, K.T.; Lee, K.H.; Mo, C.B.; Jeong, Y.J.; Hong, S.H. Mechanical and electrical properties of cross-linked carbon nanotubes. Carbon 2008, 46, 482–488. [Google Scholar] [CrossRef]
- Mathur, R.; Pande, S.; Singh, B.; Dhami, T. Electrical and mechanical properties of multi-walled carbon nanotubes reinforced PMMA and PS composites. Polym. Compos. 2008, 29, 717–727. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Sharma, S.; Hussain, S.; Singh, S.; Islam, S. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sens. Actuators B Chem. 2014, 194, 213–219. [Google Scholar] [CrossRef]
- Wu, H.X.; Tong, R.; Qiu, X.Q.; Yang, H.F.; Lin, Y.H.; Cai, R.F.; Qian, S.X. Functionalization of multiwalled carbon nanotubes with polystyrene under atom transfer radical polymerization conditions. Carbon 2007, 45, 152–159. [Google Scholar] [CrossRef]
- Leghrib, R.; Pavelko, R.; Felten, A.; Vasiliev, A.; Cané, C.; Gràcia, I.; Pireaux, J.-J.; Llobet, E. Gas sensors based on multiwall carbon nanotubes decorated with tin oxide nanoclusters. Sens. Actuators B Chem. 2010, 145, 411–416. [Google Scholar] [CrossRef]
- Sharma, S.; Hussain, S.; Sengupta, K.; Islam, S.S. Development of MWCNTs/alumina composite-based sensor for trace level ammonia gas sensing. Appl. Phys. A 2012, 111, 965–974. [Google Scholar] [CrossRef]
- Dhall, S.; Jaggi, N.; Nathawat, R. Functionalized multiwalled carbon nanotubes based hydrogen gas sensor. Sens. Actuators A Phys. 2013, 201, 321–327. [Google Scholar] [CrossRef]
- Adjizian, J.J.; Leghrib, R.; Koos, A.A.; Suarez-Martinez, I.; Crossley, A.; Wagner, P.; Grobert, N.; Llobet, E.; Ewels, C.P. Boron- and nitrogen-doped multi-wall carbon nanotubes for gas detection. Carbon 2014, 66, 662–673. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Cosio, M.S.; Pellicanò, A.; Brunetti, B.; Fuenmayor, C.A. A simple hydroxylated multi-walled carbon nanotubes modified glassy carbon electrode for rapid amperometric detection of bisphenol A. Sens. Actuators B Chem. 2017, 246, 673–679. [Google Scholar] [CrossRef]
- Zhang, Y.; Bunes, B.R.; Wu, N.; Ansari, A.; Rajabali, S.; Zang, L. Sensing methamphetamine with chemiresistive sensors based on polythiophene-blended single-walled carbon nanotubes. Sens. Actuators B Chem. 2018, 255, 1814–1818. [Google Scholar] [CrossRef]
- Majumdar, S.; Nag, P.; Devi, P.S. Enhanced performance of CNT/SnO2 thick film gas sensors towards hydrogen. Mater. Chem. Phys. 2014, 147, 79–85. [Google Scholar] [CrossRef]
- Ko, H.; Park, S.; Park, S.; Lee, C. Enhanced NO2 Gas Sensing Properties of WO3-Coated Multiwall Carbon Nanotube Sensors. J. Nanosci. Nanotechnol. 2015, 15, 5295–5300. [Google Scholar] [CrossRef]
- Yaqoob, U.; Phan, D.T.; Uddin, A.S.M.I.; Chung, G.S. Highly flexible room temperature NO2 sensor based on MWCNTs-WO3 nanoparticles hybrid on a PET substrate. Sens. Actuators B Chem. 2015, 221, 760–768. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Na, H.G.; Kang, S.Y.; Choi, S.-W.; Kim, S.S.; Kim, H.W. Selective detection of low concentration toluene gas using Pt-decorated carbon nanotubes sensors. Sens. Actuators B Chem. 2016, 227, 157–168. [Google Scholar] [CrossRef]
- Eising, M.; Cava, C.E.; Salvatierra, R.V.; Zarbin, A.J.G.; Roman, L.S. Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor. Sens. Actuators B Chem. 2017, 245, 25–33. [Google Scholar] [CrossRef]
- Şenocak, A.; Göl, C.; Basova, T.V.; Demirbaş, E.; Durmuş, M.; Al-Sagur, H.; Kadem, B.; Hassan, A. Preparation of single walled carbon nanotube-pyrene 3D hybrid nanomaterial and its sensor response to ammonia. Sens. Actuators B Chem. 2018, 256, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.; Yang, Z.; Qiu, L.; Sun, X.; Zhang, Z.; Ren, J.; Peng, H. Oriented PEDOT: PSS on aligned carbon nanotubes for efficient dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 13268–13273. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Gao, C.; Zhang, J.; Sun, H. Influence of MWCNTs doping on the structure and properties of PEDOT: PSS films. Int. J. Photoenergy 2009, 2009, 1–5. [Google Scholar]
- Li, J.; Liu, J.C.; Gao, C.J. On the mechanism of conductivity enhancement in PEDOT/PSS film doped with multi-walled carbon nanotubes. J. Polym. Res. 2010, 17, 713–718. [Google Scholar] [CrossRef]
- Pathak, C.; Singh, J.; Singh, R. Preparation of novel graphene-PEDOT: PSS nanocomposite films and fabrication of heterojunction diodes with n-Si. Chem. Phys. Lett. 2018, 694, 75–81. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, Y.; Zhang, L.; Zhu, J.; Zhao, X.; Zhang, W. Flexible and Highly Sensitive Hydrogen Sensor Based on Organic Nanofibers Decorated by Pd Nanoparticles. Sensors 2019, 19, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova Cháfer, J.; García Aboal, R.; Atienzar, P.; Llobet, E. Gas Sensing Properties of Perovskite Decorated Graphene at Room Temperature. Sensors 2019, 19, 4563. [Google Scholar] [CrossRef] [Green Version]
- Shim, M.; Javey, A.; Shi Kam, N.W.; Dai, H. Polymer functionalization for air-stable n-type carbon nanotube field-effect transistors. J. Am. Chem. Soc. 2001, 123, 11512–11513. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Sun, M.; Gong, Z.; Guo, Y.; Wu, F.; Li, W.; Ding, W. Influence of Charge Carriers Concentration and Mobility on the Gas Sensing Behavior of Tin Dioxide Thin Films. Coatings 2019, 9, 591. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Jung, K.; Kim, J.; Kwon, K.; Lee, C.J.; Yun, K.; Min, N. Percolated pore networks of oxygen plasma-activated multi-walled carbon nanotubes for fast response, high sensitivity capacitive humidity sensors. Nanotechnology 2013, 24, 085501. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.; Bhatia, R.; Prasad, V.; Rajanna, K. Ethanol sensing using CuO/MWNT thin film. Sens. Actuators B Chem. 2011, 158, 229–234. [Google Scholar] [CrossRef]

| Materials | Fabrication Method | Targeted Analyte | Working Temperature (°C) | Recovery Time (s) | Ref. |
|---|---|---|---|---|---|
| CNT/SnO2 | Oxidative functionalization | CO | RT | - | [10] |
| MWCNT/Al2O3 | Acid treatment | NH3 | RT | - | [11] |
| F-MWCNTs | Acid treatment | H2 | RT | 100 | [12] |
| N-MWCNTs | Film deposition | NO2 | 150 | 2400 | [13] |
| PANI/MWCNTs | Oxidative polymerization | NH3 | RT | 35–62 | [14] |
| MWCNTs-COOH | Chemical modification | Bisphenol A | RT | 10 | [15] |
| P3CT/CNTs | Drop-casting | NMPEA | RT | 40 | [16] |
| CNT/SnO2 | Wet chemical method | H2 | 200 | >120 | [17] |
| CNT/WO3 | Sputter deposition | NO2 | RT | >300 | [18] |
| MWCNTs-WO3NPs | Hydrothermal synthetization | NO2 | RT | 1620 | [19] |
| Pt-MWCNTs | Sputter deposition | C7H8 | 150 | >70 | [20] |
| PANI:CNTs | Interfacial polymerization | NH3 | RT | 46 | [21] |
| SWCNTs-Pyrene 3D Hybrid | Drop-casting | NH3 | 22 | 20 | [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jang, Y.; Lee, G.W.; Yang, S.Y.; Jung, J.; Oh, J. Tunable Chemical Grafting of Three-Dimensional Poly (3, 4-ethylenedioxythiophene)/Poly (4-styrenesulfonate)-Multiwalled Carbon Nanotubes Composite with Faster Charge-Carrier Transport for Enhanced Gas Sensing Performance. Sensors 2020, 20, 2470. https://doi.org/10.3390/s20092470
Kim H, Jang Y, Lee GW, Yang SY, Jung J, Oh J. Tunable Chemical Grafting of Three-Dimensional Poly (3, 4-ethylenedioxythiophene)/Poly (4-styrenesulfonate)-Multiwalled Carbon Nanotubes Composite with Faster Charge-Carrier Transport for Enhanced Gas Sensing Performance. Sensors. 2020; 20(9):2470. https://doi.org/10.3390/s20092470
Chicago/Turabian StyleKim, Hyojae, Yeongseok Jang, Gyeong Won Lee, Seung Yun Yang, Jinmu Jung, and Jonghyun Oh. 2020. "Tunable Chemical Grafting of Three-Dimensional Poly (3, 4-ethylenedioxythiophene)/Poly (4-styrenesulfonate)-Multiwalled Carbon Nanotubes Composite with Faster Charge-Carrier Transport for Enhanced Gas Sensing Performance" Sensors 20, no. 9: 2470. https://doi.org/10.3390/s20092470




