Foliar Elemental Analysis of Brazilian Crops via Portable X-ray Fluorescence Spectrometry
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material Collection from Brazilian Crops
2.2. Conventional Analysis of Plant Leaves: Acid Digestion (AD) Method
2.3. pXRF Analysis
2.4. Statistical Analyses
3. Results and Discussion
3.1. Recoveries of Elements
3.2. General Description of AD and pXRF Data
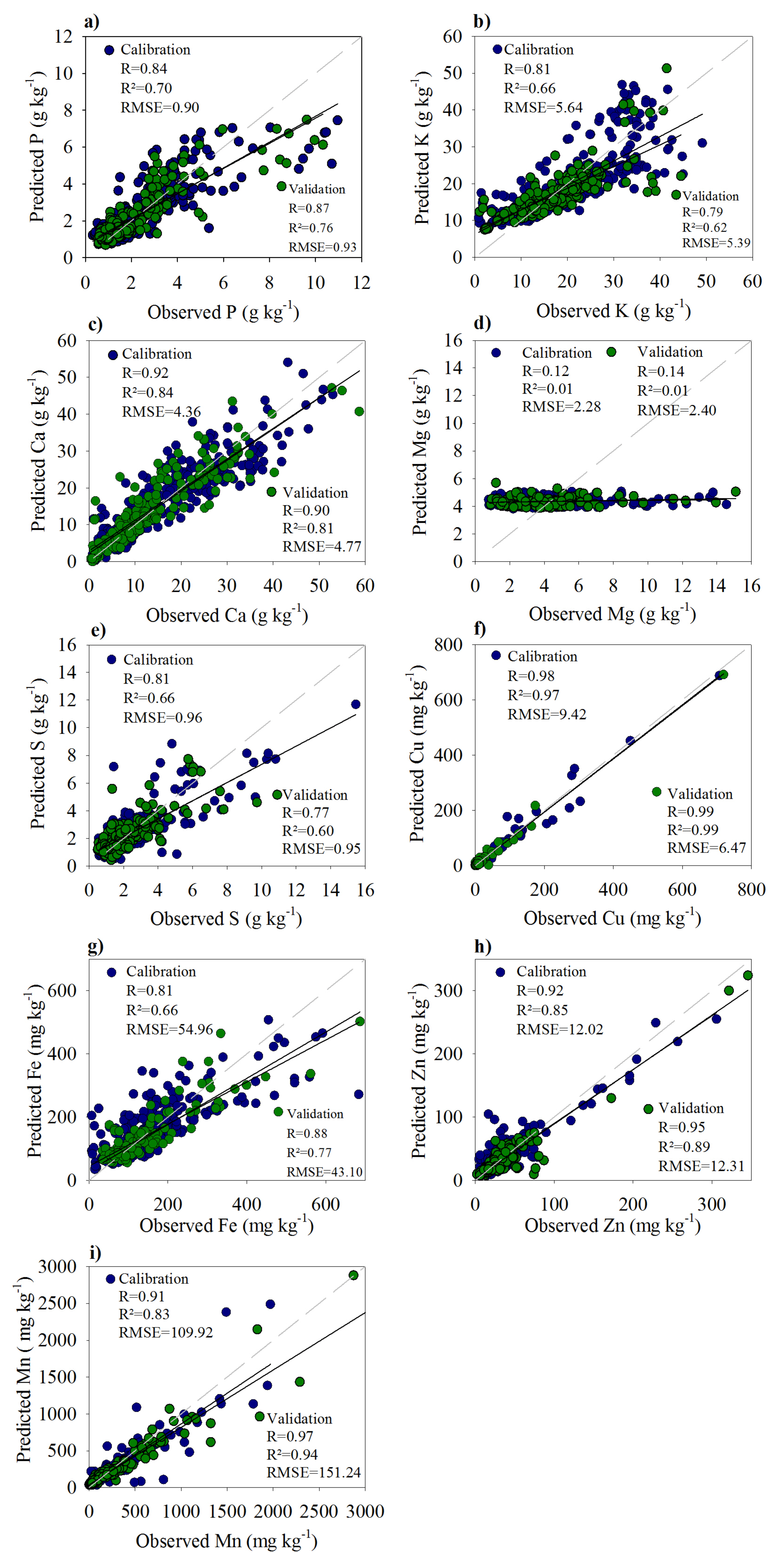
3.3. Correlation between AD and pXRF
3.4. Correlation between pXRF and AD for Each Crop
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Plant Nutrition for Food Security. A Guide for Integrated Nutrient Management; Food and Agriculture Organization of The United Nations (FAO): Rome, Italy, 2006. [Google Scholar]
- White, P.J.; Broadley, M.R.; Gregory, P.J. Managing the nutrition of plants and people. Appl. Environ. Soil Sci. 2012, 2012, 104826. [Google Scholar] [CrossRef] [Green Version]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture: A review. Agron. Sustain. Dev. 2009, 28, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Spann, T.M.; Schumann, A.W. The Role of Plant Nutrients in Disease Development with Emphasis on Citrus and Huanglongbing. Proc. Fla. State Hortic Soc. 2009, 122, 169–171. [Google Scholar]
- Huber, D.; Römheld, V.; Weinmann, M. Relationship between Nutrition, Plant Diseases and Pests. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 283–298. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought Tolerance: Role of Organic Osmolytes, Growth Regulators, and Mineral Nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; Volume 1, pp. 16–43. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Foreing Agricultural Service Home Page. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 20 January 2020).
- United States Department of Agriculture, Foreing Agricultural Service Home Page. Available online: https://apps.fas.usda.gov/psdonline/circulars/coffee.pdf (accessed on 20 January 2020).
- Lopes, A.S.; Guilherme, L.G. A career perspective on soil management in the Cerrado region of Brazil. Adv. Agron. 2016, 137, 1–72. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Guimarães, P.T.G.; Alvarez, V.V.H. Recomendação Para o uso de Corretivos e Fertilizantes em Minas Gerais; CFSEMG/UFV: Viçosa, Brazil, 1999. (In Portuguese) [Google Scholar]
- Blaylock, A.D.; Belden, K.; Hough, H.W. Guide to Wyoming Fertilizer Recommendations; University of Wyoming: Laramie, WY, USA, 1996. [Google Scholar]
- Lucena, J.J. Methods of diagnosis of mineral nutrition of plants a critical review. Acta Hortic. 1997, 448, 179–192. [Google Scholar] [CrossRef]
- Cantarutti, R.B.; Barros, N.F.; Martinez, H.E.P.; Novais, R.F. Avaliação da fertilidade do solo e recomendação de fertilizantes. In Fertilidade do Solo; Novais, R.F., Alvarez, V., De Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; Socidade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007; pp. 769–850. (In Portuguese) [Google Scholar]
- Munson, R.D. Principles of plant analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; Taylor & Francis Group: New York, NY, USA, 1998; pp. 1–24. [Google Scholar] [CrossRef]
- Sturgeon, R.E. Current practice and recent developments in analytical methodology for trace element analysis of soils, plants, and water. Commun. Soil Sci. Plant Anal. 2000, 31, 1479–1512. [Google Scholar] [CrossRef]
- Elwali, A.M.O.; Gascho, G.J. Soil Testing, Foliar Analysis, and DRIS as Guides for Sugarcane Fertilization. Agron. J. 1984, 76, 466–470. [Google Scholar] [CrossRef]
- Creste, J.E.; Echer, F.R. Establishing standards for the integrated recommendation and diagnosis system (DRIS) for irrigated bean crops. Commun. Soil Sci. Plant Anal. 2010, 41, 1921–1933. [Google Scholar] [CrossRef]
- Maia, C.E. Fertilization response likelihood for the interpretation of leaf analyses. Rev. Bras. Cienc. Solo 2012, 36, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Miller, R. Nitric-Perchloric Acid Wet Digestion in an Open Vessel. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 57–61. [Google Scholar]
- Isaac, R.A.; Johnson, W.C., Jr. Elemental determination by inductively coupled plasma atomic emission spectroscopy. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 165–170. [Google Scholar]
- Weindorf, D.C.; Bakr, N.; Zhu, Y. Advances in portable X-ray fluorescence (PXRF) for environmental, pedological, and agronomic applications. Adv. Agron. 2014, 128, 1–45. [Google Scholar] [CrossRef]
- Ribeiro, B.T.; Silva, S.H.G.; Silva, E.A.; Guilherme, L.R.G. Portable X-ray fluorescence (pXRF) applications in tropical Soil Science. Cienc. Agrotec. 2017, 41, 245–254. [Google Scholar] [CrossRef] [Green Version]
- US EPA: Method 6200-Field Portable X-ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soil and Sediment. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/6200.pdf (accessed on 20 January 2020).
- Soil Survey Staff. Soil Survey Field and Laboratory Methods Manual. Soil Survey Investigations. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb1244466.pdf (accessed on 20 January 2020).
- Weindorf, D.C.; Chakraborty, S. Portable X-ray Fluorescence Spectrometry Analysis of Soils. In Methods of Soil Analysis; Hirmas, D., Madison, W.I., Eds.; Soil Science Society of America: Madison, WI, USA, 2016; pp. 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.; Weindorf, D.C.; Zhang, W. Characterizing soils using a portable X-ray fluorescence spectrometer: 1. Soil texture. Geoderma 2011, 167–168, 167–177. [Google Scholar] [CrossRef]
- Sharma, A.; Weindorf, D.C.; Wang, D.D.; Chakraborty, S. Characterizing soils via portable X-ray fluorescence spectrometer: 4. Cation exchange capacity (CEC). Geoderma 2015, 239, 130–134. [Google Scholar] [CrossRef]
- de Lima, T.M.; Weindorf, D.C.; Curi, N.; Guilherme, L.R.G.; Lana, R.M.Q.; Ribeiro, B.T. Elemental analysis of Cerrado agricultural soils via portable X-ray fluorescence spectrometry: Inferences for soil fertility assessment. Geoderma 2019, 353, 264–272. [Google Scholar] [CrossRef]
- Andrade, R.; Faria, W.M.; Silva, S.H.G.; Chakraborty, S.; Weindorf, D.C.; Mesquita, L.F.; Guilherme, L.R.G.; Curi, N. Prediction of soil fertility via portable X-ray fluorescence (pXRF) spectrometry and soil texture in the Brazilian Coastal Plains. Geoderma 2020, 357, 113960. [Google Scholar] [CrossRef]
- Andrade, R.; Silva, S.H.G.; Weindorf, D.C.; Chakraborty, S.; Faria, W.M.; Mesquita, L.F.; Guilherme, L.R.G.; Curi, N. Assessing models for prediction of some soil chemical properties from portable X-ray fluorescence (pXRF) spectrometry data in Brazilian Coastal Plains. Geoderma 2020, 357, 113957. [Google Scholar] [CrossRef]
- Silva, F.M.; Weindorf, D.C.; Silva, S.H.G.; Silva, E.A.; Ribeiro, B.T.; Guilherme, L.R.G.; Curi, N. Tropical Soil Toposequence Characterization via pXRF Spectrometry. Pedology 2019, 83. [Google Scholar] [CrossRef]
- dos Santos Teixeira, A.F.; Pelegrino, M.H.P.; Faria, W.M.; Silva, S.H.G.; Gonçalves, M.G.M.; Acerbi, F.W., Jr.; Gomide, L.R.; Pádua, A.L., Jr.; de Souza, I.A.; Chakraborty, S.; et al. Tropical soil pH and sorption complex prediction via portable X-ray fluorescence spectrometry. Geoderma 2020, 361, 114132. [Google Scholar] [CrossRef]
- Bachiega, P.; de Almeida, E.; Salgado, J.M.; Arruda, M.A.Z.; Lehmann, E.L.; Morzelle, M.C.; de Carvalho, H.W.P. Benchtop and Handheld Energy-Dispersive X-Ray Fluorescence (EDXRF) as Alternative for Selenium Concentration Measurement in Biofortified Broccoli Seedling. Food Anal. Method. 2019, 12, 1520–1527. [Google Scholar] [CrossRef]
- Costa, G.T., Jr.; Nunes, L.C.; Gomes, M.H.F.; de Almeida, E.; de Carvalho, H.W.P. Direct determination of mineral nutrients in soybean leaves under vivo conditions by portable X-ray fluorescence spectroscopy. X-ray Spectrom. 2019, 49, 1–10. [Google Scholar] [CrossRef]
- Melquiades, F.L.; Bortoleto, G.G.; Marchiori, L.F.S.; Bueno, M.I.M.S. Direct determination of sugar cane quality parameters by X-ray spectrometry and multivariate analysis. J. Agric. Food Chem 2012, 60, 10755–10761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaren, T.I.; Guppy, C.N.; Tighe, M.K. A Rapid and Nondestructive Plant Nutrient Analysis using Portable X-Ray Fluorescence. Soil Sci. Soc. Am. J. 2012, 76, 1446–1453. [Google Scholar] [CrossRef]
- Paltridge, N.G.; Milham, P.J.; Ortiz-Monasterio, J.I.; Velu, G.; Yasmin, Z.; Palmer, L.J.; Guild, G.E.; Stangoulis, J.C.R. Energy-dispersive X-ray fluorescence spectrometry as a tool for zinc, iron and selenium analysis in whole grain wheat. Plant Soil. 2012, 361, 261–269. [Google Scholar] [CrossRef]
- Paltridge, N.G.; Palmer, L.J.; Milham, P.J.; Guild, G.E.; Stangoulis, J.C.R. Energy-dispersive X-ray fluorescence analysis of zinc and iron concentration in rice and pearl millet grain. Plant Soil. 2012, 361, 251–260. [Google Scholar] [CrossRef]
- Towett, E.K.; Shepherd, K.D.; Drake, B.L. Plant elemental composition and portable X-ray fluorescence (pXRF) spectroscopy: Quantification under different analytical parameters. X-ray Spectrom. 2016, 45, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Sacristán, D.; Viscarra Rossel, R.A.; Recatalá, L. Proximal sensing of Cu in soil and lettuce using portable X-ray fluorescence spectrometry. Geoderma 2016, 265, 6–11. [Google Scholar] [CrossRef]
- McGladdery, C.; Weindorf, D.C.; Chakraborty, S.; Li, B.; Paulette, L.; Podar, D.; Pearson, D.; Kusi, N.Y.O.; Duda, B. Elemental assessment of vegetation via portable X-ray fluorescence (PXRF) spectrometry. J. Environ. Manag. 2018, 210, 210–225. [Google Scholar] [CrossRef]
- Montanha, G.S.; Rodrigues, E.S.; Marques, J.P.R.; Almeida, E.; Reis, A.R.; Carvalho, H.W.P. X-ray fluorescence spectroscopy (XRF) applied to plant science: Challenges towards in vivo analysis of plants. Metallomics 2019, 12, 183–192. [Google Scholar] [CrossRef]
- Gutiérrez-Ginés, M.J.; Pastor, J.; Hernández, A.J. Assessment of field portable X-ray fluorescence spectrometry for the in situ determination of heavy metals in soils and plants. Environ. Sci. Proc. Imp. 2013, 15, 1545–1552. [Google Scholar] [CrossRef] [Green Version]
- Sapkota, Y.; McDonald, L.M.; Griggs, T.C.; Basden, T.J.; Drake, B.L. Portable X-Ray fluorescence spectroscopy for rapid and cost-effective determination of elemental composition of ground forage. Front. Plant Sci. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, M.B.B.; de Almeida, E.; Carvalho, G.G.A.; Souza, P.F.; Nunes, L.C.; Santos, D., Jr.; Krug, F.J. Comparison of analytical performance of benchtop and handheld energy dispersive X-ray fluorescence systems for the direct analysis of plant materials. J. Anal. Atom. Spectrom. 2014, 29, 1667–1674. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Associação Brasileira para pesquisa da Potassa e do Fosfato: Piracicaba, Brazil, 1997. (In Portuguese) [Google Scholar]
- Byers, H.L.; McHenry, L.J.; Grundl, T.J. XRF techniques to quantify heavy metals in vegetables at low detection limits. Food Chem. 2019, 1, 100001. [Google Scholar] [CrossRef]
- Lavilla, I.; Filgueiras, A.V.; Bendicho, C. Comparison of digestion methods for determination of trace and minor metals in plant samples. J. Agric. Food Chem. 1999, 47, 5072–5077. [Google Scholar] [CrossRef]
- Carneiro, C.; Reissmann, C.B.; Marques, R. Comparação de métodos de análise química de K, Ca, Mg e Al, em folhas de erva-mate (Ilex paraguariensis St. Hil.). Cerne 2006, 12, 113–122. [Google Scholar]
- Bernardi, A.C.C.; Oka, S.H.; de Souza, G.B. Comparação de técnicas analíticas para a extração de potássio de amostras de tecido vegetal com água e soluções ácidas concentrada e diluída. Eclet. Quim. 2010, 35, 45–49. [Google Scholar] [CrossRef]
- Kalnicky, D.J.; Singhvi, R. Field portable XRF analysis of environmental samples. J. Hazard. Mater. 2001, 83, 93–122. [Google Scholar] [CrossRef] [Green Version]
- Marguí, E.; Queralt, I.; Hidalgo, M. Application of X-ray fluorescence spectrometry to determination and quantitation of metals in vegetal material. TrAC Trend. Anal. Chem. 2009, 28, 362–372. [Google Scholar] [CrossRef]
- Omote, J.; Kohno, H.; Toda, K. X-Ray fluorescence analysis utilizing the fundamental parameter method for the determination of the elemental composition in plant samples. Anal. Chim. Acta 1995, 307, 117–126. [Google Scholar] [CrossRef]
- Kirkby, E. Introduction, Definition and Classification of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 3–14. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates Publishers: Sunderland, MA, USA, 2005. [Google Scholar]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.A.; Teles, A.P.B.; Herrera, W.F.B. Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef]
- Roy, E.D.; Willig, E.; Richards, P.D.; Martinelli, L.A.; Vazquez, F.F.; Pegorini, L.; Spera, S.A.; Porder, S. Soil phosphorus sorption capacity after three decades of intensive fertilization in Mato Grosso, Brazil. Agric. Ecosyst. Environ. 2017, 249, 206–214. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 191–248. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 135–189. [Google Scholar] [CrossRef]
- Clarkson, D.T. The Uptake and Translocation of Manganese by Plant Roots. In Manganese in Soils and Plants; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 101–111. [Google Scholar] [CrossRef]
- de Varennes, A.; Carneiro, J.P.; Goss, M.J. Characterization of manganese toxicity in two species of annual medics. J. Plant Nutr. 2001, 24, 1947–1955. [Google Scholar] [CrossRef]
- Gates, W.P. Chapter 12.3 X-ray Absorption Spectroscopy. In Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: London, UK, 2006; pp. 789–864. [Google Scholar] [CrossRef]
- Reidinger, S.; Ramsey, M.H.; Hartley, S.E. Rapid and accurate analyses of silicon and phosphorus in plants using a portable X-ray fluorescence spectrometer. New Phytol. 2012, 195, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Tighe, M.; Forster, N. Rapid, Nondestructive Elemental Analysis of Tree and Shrub Litter. Commun. Soil Sci. Plant Anal. 2014, 45, 53–60. [Google Scholar] [CrossRef]
- Melo, L.C.A.; Silva, C.A. Influência de métodos de digestão e massa de amostra na recuperação de nutrientes em resíduos orgânicos. Quim. Nova 2008, 31, 556–561. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho, A.M.; Dantas, R.D.A.; Coelho, M.C.; Lima, W.M.; Souza, J.P.S.P.; Fonseca, O.P.; Guimarães, R., Jr. Teores de Hemiceluloses, Celulose e Lignina em Plantas de Cobertura com Potencial para Sistema Plantio Direto no Cerrado; Embrapa Cerrados: Planaltina, Brazil, 2010; pp. 1–15. [Google Scholar]
- Kagiliery, J.; Chakraborty, S.; Acree, A.; Weindorf, D.C.; Brevik, E.C.; Jelinski, N.A.; Li, B.; Jordan, C. Rapid quantification of lignite sulfur content: Combining optical and X-ray approaches. Int. J. Coal Geol. 2019, 216, 103336. [Google Scholar] [CrossRef]
- Weindorf, D.C.; Chakraborty, S.; Herrero, J.; Li, B.; Castañeda, C.; Choudhury, A. Simultaneous assessment of key properties of arid soil by combined PXRF and Vis-NIR data. Eur. J. Soil Sci. 2016, 67, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Kalcsits, L.A. Non-destructive measurement of calcium and potassium in apple and pear using handheld X-ray fluorescence. Front. Plant Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
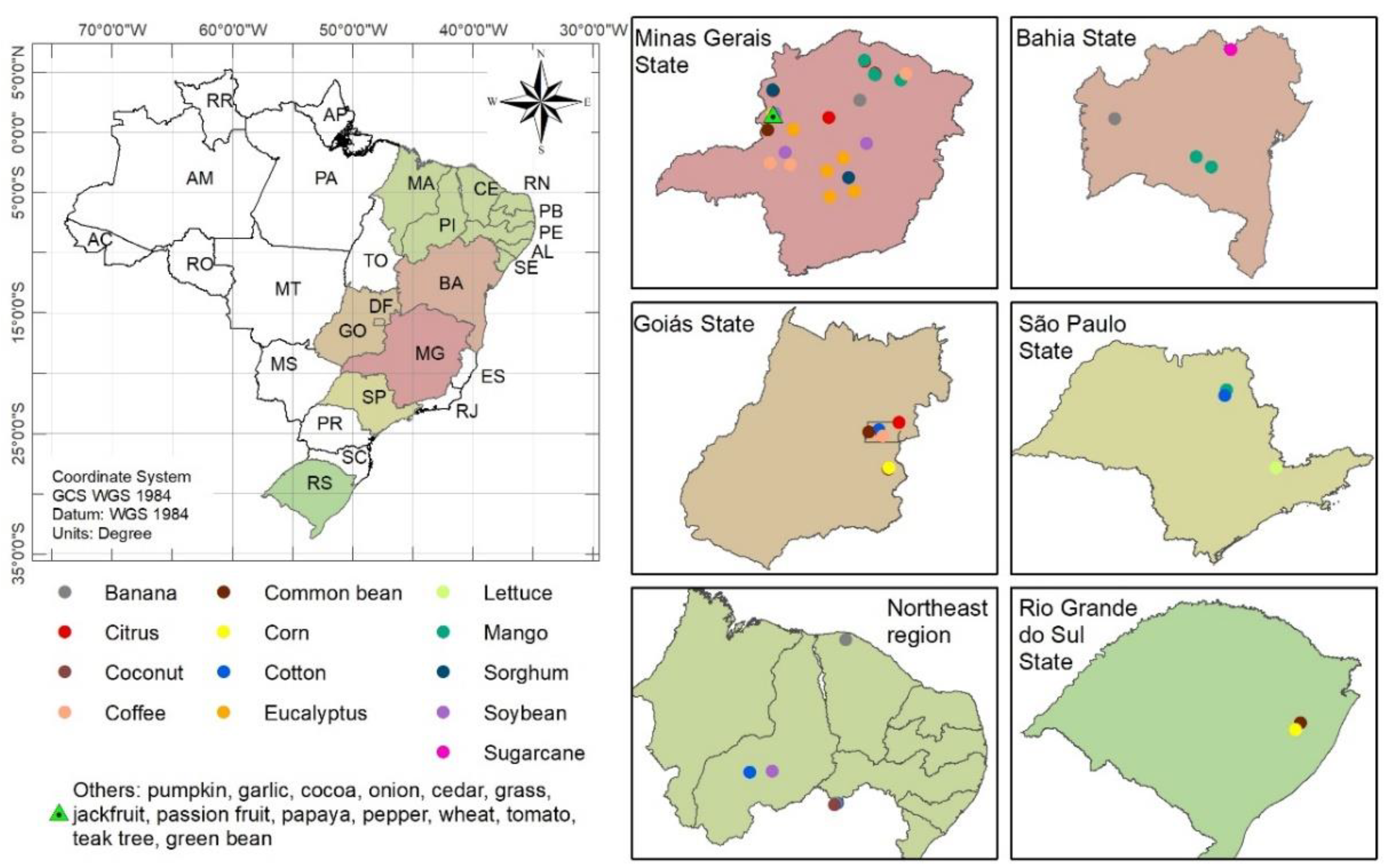



| Crop | Number of Samples (n) | |
|---|---|---|
| Cereals and Oilseeds (n = 157) | ||
| Bean | Phaseolus vulgaris | 45 |
| Corn | Zea mays | 14 |
| Soybean | Glycine max | 11 |
| Sorghum | Sorghum bicolor L. | 24 |
| Wheat | Triticum spp | 1 |
| Cotton | Gossypium hirsutum L. | 62 |
| Fruits (n = 186) | ||
| Banana | Musa spp. | 96 |
| Coconut | Cocos nucifera L. | 53 |
| Jackfruit | Artocarpus heterophyllus | 1 |
| Mango | Mangifera indica | 26 |
| Passion fruit | Passiflora edulis | 2 |
| Papaya | Carica papaya | 8 |
| Vegetables (n = 28) | ||
| Garlic | Allium sativum | 2 |
| Green bean | Phaseolus vulgaris L. | 1 |
| Onion | Allium cepa | 2 |
| Tomato | Solanum lycopersicum | 1 |
| Lettuce | Lactuca sativa | 14 |
| Pumpkin | Cucurbita spp | 7 |
| Pepper | Capsicum annuum | 1 |
| Citrus (n = 46) | ||
| Orange | Citrus sinensisL. Osbeck | 7 |
| Lemon | Citrus limon | 39 |
| Forest trees (n = 84) | ||
| Cedar | Cedrela fissilis | 5 |
| Eucalyptus | Eucalyptus globulus Labill | 78 |
| Teak trees | Tectona grandis L.f. | 1 |
| Perennials and semi-perennials (n = 113) | ||
| Coffee | Coffea ssp. | 96 |
| Cocoa | Theobroma cacao | 1 |
| Sugarcane | Saccharum officinarum L. | 12 |
| Grass | Poaceae | 4 |
| Nutrient | Minimum | Maximum | Median | Mean | s.d. | MACP | % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pXRF | AD | pXRF | AD | pXRF | AD | pXRF | AD | pXRF | AD | |||
| P (g kg−1) | 0.52 | 0.32 | 8.99 | 10.96 | 1.67 | 1.64 | 2.46 | 2.23 | 1.75 | 1.71 | 2 | 38 |
| K (g kg−1) | 2.56 | 0.83 | 91.68 | 49.18 | 21.27 | 17.56 | 24.20 | 18.12 | 15.41 | 9.48 | 10 | 83 |
| Ca (g kg−1) | 2.08 | 0.48 | 55.49 | 58.74 | 13.96 | 10.80 | 13.64 | 14.15 | 9.94 | 10.96 | 5 | 81 |
| Mg (g kg−1)** | 4.34 | 0.79 | 19.34 | 15.09 | 8.36 | 3.89 | 8.62 | 4.30 | 2.35 | 2.34 | 2 | 90 |
| S (g kg−1) | 0.65 | 0.51 | 14.96 | 15.46 | 2.53 | 1.86 | 2.95 | 2.24 | 1.68 | 1.61 | 1 | 86 |
| Fe (mg kg−1) | 43.00 | 6.90 | 792.06 | 687.13 | 143.62 | 104.30 | 194.14 | 131.59 | 121.76 | 93.49 | 100 | 53 |
| Cu (mg kg−1) | 0.00 | 0.18 | 795.84 | 719.40 | 8.16 | 7.39 | 20.76 | 18.03 | 61.55 | 54.53 | 6 | 63 |
| Mn (mg kg−1) | 21.73 | 0.34 | 4170.04 | 3273.00 | 183.33 | 97.50 | 282.58 | 220.76 | 440.31 | 355.79 | 50 | 61 |
| Zn (mg kg−1) | 7.65 | 2.38 | 376.89 | 345.58 | 24.48 | 23.79 | 35.25 | 31.32 | 35.70 | 33.32 | 20 | 67 |
| Plant-Nutrient | Equation | R | R² |
|---|---|---|---|
| P | AD = 0.80pXRF + 0.27 * | 0.84 | 0.70 |
| K | AD = 0.49pXRF + 6.23 * | 0.81 | 0.66 |
| Ca | AD = 1.01pXRF − 2.24 * | 0.92 | 0.84 |
| Mg | AD = 0.13pXRF + 3.26 ns | 0.12 | 0.01 |
| S | AD = 0.79pXRF − 0.08 * | 0.81 | 0.66 |
| Cu | AD = 0.87pXRF + 0.09 * | 0.98 | 0.97 |
| Fe | AD = 0.63pXRF + 7.76 * | 0.82 | 0.66 |
| Zn | AD = 0.59pXRF + 0.52 * | 0.92 | 0.85 |
| Mn | AD = 0.69pXRF + 18.73 * | 0.91 | 0.83 |
| Crop | P | K | Ca | Mg | S | Cu | Fe | Zn | Mn |
|---|---|---|---|---|---|---|---|---|---|
| ----------------------(g kg−1) ---------------------- | ------------------- (mg kg−1)--------------------- | ||||||||
| Banana | 0.19 | 6.3 | 0.75 | 4.41 | 0.49 | 1.56 | 41.28 | 1.72 | 106.07 |
| Citrus | 0.24 | 0.24 | 2.94 | 1.55 | 0.01 | 0.37 | 66.22 | 1.58 | 30.39 |
| Coconut | 0.65 | 5.94 | 1.99 | 1.84 | 1.27 | 1.92 | 50.83 | 2.23 | 54.39 |
| Coffee | 0.01 | 4.73 | 4.5 | 3.49 | 0.67 | 5.91 | 44.13 | 3.2 | 48.86 |
| Common bean | 0.45 | 2.68 | 6.45 | 1.71 | 0.74 | 2.98 | 80.16 | 7.72 | 33.7 |
| Corn | 1.29 | 18.41 | 4.61 | * | 2.12 | 7.43 | 102.52 | 23.91 | 52.75 |
| Cotton | 0.37 | 0.51 | 1.43 | 5.73 | 1.02 | 1.45 | 26.89 | 1.64 | 33.08 |
| Eucalyptus | 0.58 | 3.37 | 3.65 | 5.64 | 0.52 | 1.77 | 96.6 | 5.55 | 153.14 |
| Lettuce | 2.16 | 32.59 | 3.63 | 2.49 | 1.82 | 17.37 | 91.2 | 14.24 | 56.18 |
| Mango | 0.33 | 0.76 | 5.19 | * | 0.24 | 4.28 | 31.75 | 1.51 | 40.31 |
| Sorghum | 2.17 | 35.39 | 4.24 | 8.17 | 1.95 | 5.69 | 194.55 | 22.42 | 32.72 |
| Soybean | 0.81 | 3.5 | 6.05 | 0.9 | 0.88 | 15.78 | 99.18 | 10.27 | 55.21 |
| Sugarcane | 0.41 | 14.57 | 2.96 | * | 0.18 | 2.16 | 67.59 | 6.13 | 50.29 |
| Others | 0.42 | 6.85 | 0.7 | 6.39 | 0.45 | 3.34 | 47.64 | 1.14 | 30.79 |
| Mean | 0.72 | 9.70 | 3.51 | 3.85 | 0.88 | 5.14 | 74.32 | 7.38 | 55.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, C.S.; Weindorf, D.C.; Carvalho, G.S.; Guilherme, L.R.G.; Takayama, T.; Curi, N.; Lima, G.J.E.O.; Ribeiro, B.T. Foliar Elemental Analysis of Brazilian Crops via Portable X-ray Fluorescence Spectrometry. Sensors 2020, 20, 2509. https://doi.org/10.3390/s20092509
Borges CS, Weindorf DC, Carvalho GS, Guilherme LRG, Takayama T, Curi N, Lima GJEO, Ribeiro BT. Foliar Elemental Analysis of Brazilian Crops via Portable X-ray Fluorescence Spectrometry. Sensors. 2020; 20(9):2509. https://doi.org/10.3390/s20092509
Chicago/Turabian StyleBorges, Camila S., David C. Weindorf, Geila S. Carvalho, Luiz R. G. Guilherme, Thalita Takayama, Nilton Curi, Geraldo J. E. O. Lima, and Bruno T. Ribeiro. 2020. "Foliar Elemental Analysis of Brazilian Crops via Portable X-ray Fluorescence Spectrometry" Sensors 20, no. 9: 2509. https://doi.org/10.3390/s20092509






