Infrared Sensor Detection and Actuator Treatment Applied during Hemodialysis
Abstract
:1. Introduction
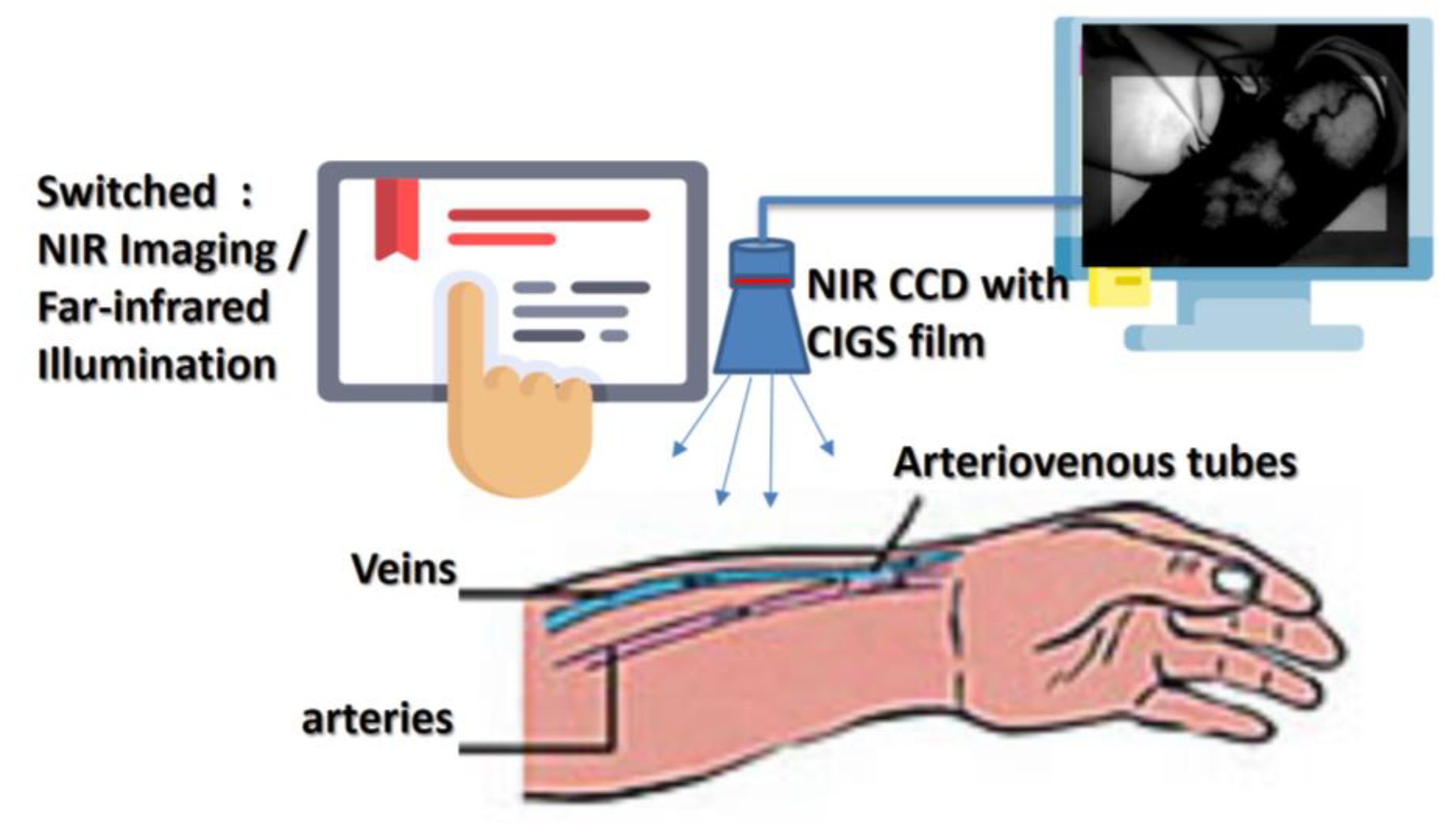
2. Infrared Treatment
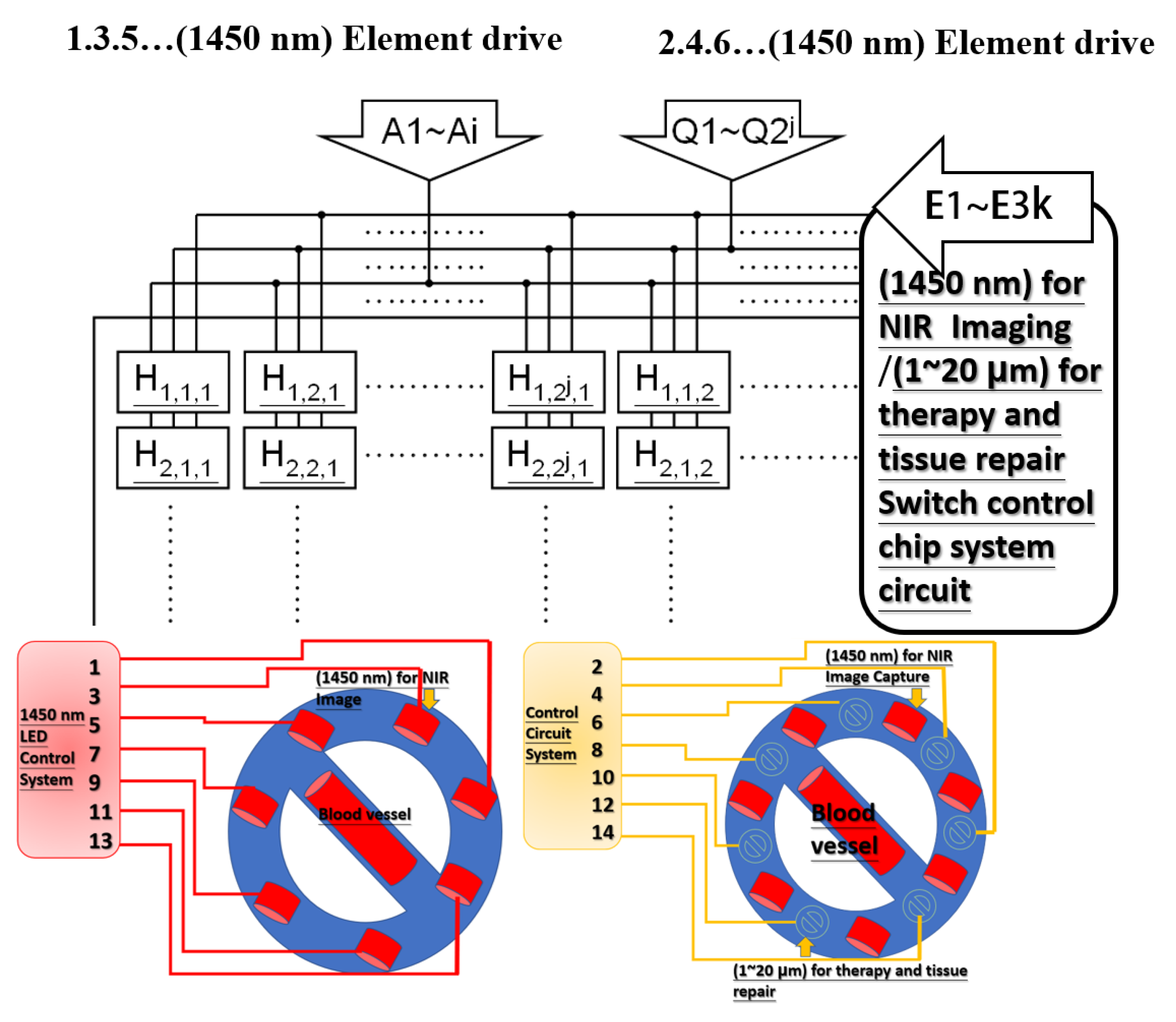
3. Design Architecture and Simulation
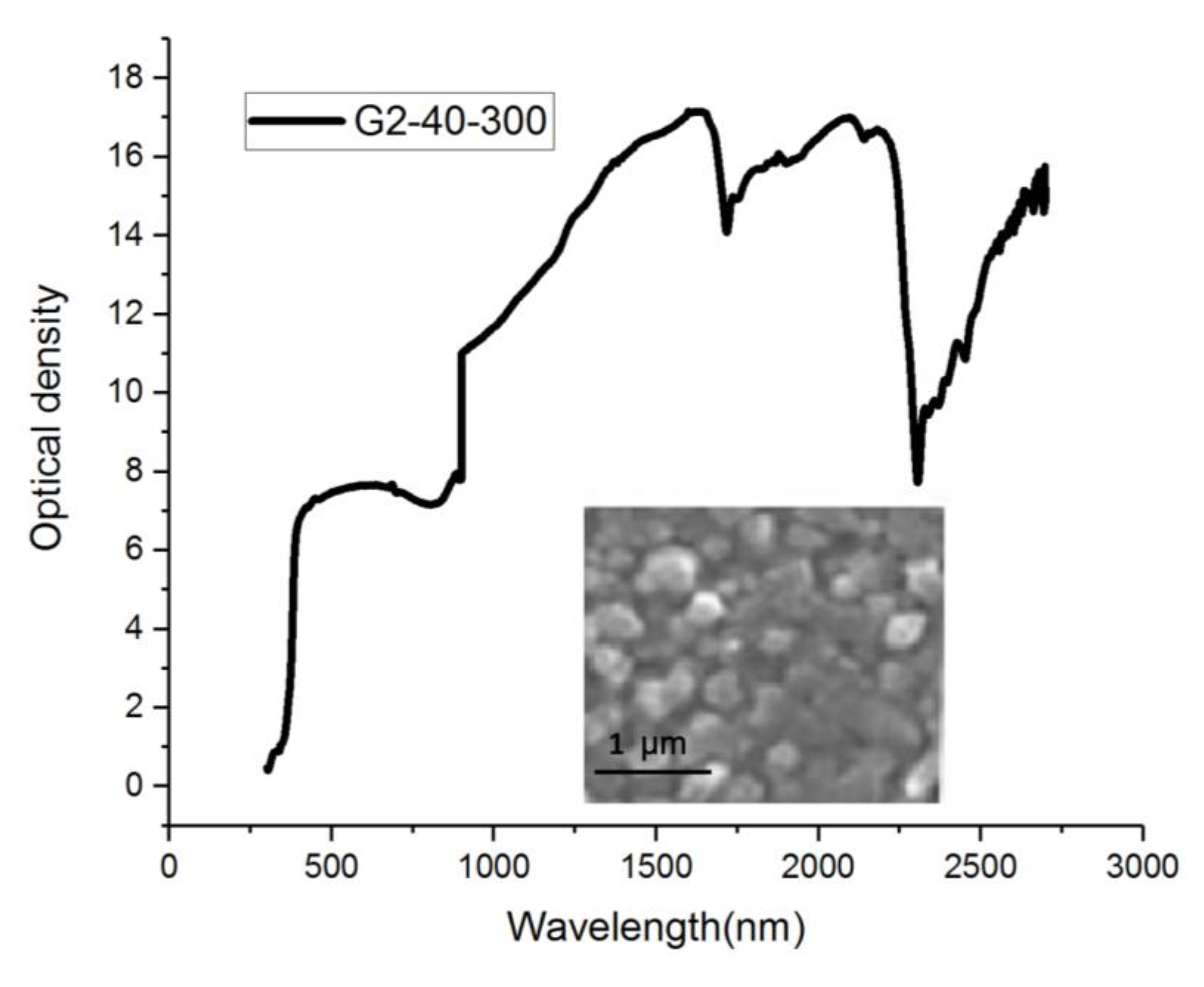
4. Experiment and Results
4.1. Light Source
4.2. Far-Infrared Implementation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Awazu, K.; Ishii, K.; Hazama, H. Novel laser therapy and diagnosis using mid-infrared laser. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 2–6 September 2009; pp. 4150–4153. [Google Scholar]
- Widanti, N.; Sumanto, B.; Rosa, P.; Miftahudin, M.F. Stress level detection using heart rate, blood pressure, and GSR and stress therapy by utilizing infrared. In Proceedings of the 2015 International Conference on Industrial Instrumentation and Control (ICIC), Pune, India, 28–30 May 2015; pp. 275–279. [Google Scholar]
- Li, W.W.; Head, J.F. Infrared imaging in the detection and evaluation of tumor angiogenesis. In Proceedings of the 22nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (Cat. No. 00CH37143), Chicago, IL, USA, 23–28 July 2000; Volume 3, pp. 1931–1932. [Google Scholar]
- Rao, W.; Sun, Z.Q.; Zhou, Y.X.; Liu, J. Thermal infrared image to quantify nano particles enhanced laser deposition during malignant tissue ablation. In Proceedings of the 2008 3rd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Sanya, China, 6–9 January 2008; pp. 890–894. [Google Scholar]
- Telenkov, S.; Tanenbaum, B.; Goodman, D.; Nelson, J.; Milner, T. In vivo infrared tomographic imaging of laser-heated blood vessels. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.; Guofeng, S.; Jingfeng, B.; Yazhu, C. A method of estimating ultrasound fields at full power using infrared and hydrophone system. In Proceedings of the 2011 4th International Conference on Biomedical Engineering and Informatics (BMEI), Shanghai, China, 15–17 October 2011; Volume 3, pp. 1456–1458. [Google Scholar]
- Liu, Y.; Polo, A.; Zequera, M.; Harba, R.; Canals, R.; Vilcahuamán, L.; Bello, Y. Detection of diabetic foot hyperthermia by using a regionalization method, based on the plantar angiosomes, on infrared images. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 1389–1392. [Google Scholar]
- Harding, J.R.; Wertheim, D.F.; Williams, R.J.; Melhuish, J.M.; Banerjee, D.; Harding, K.G. Infrared imaging in diabetic foot ulceration. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vol.20 Biomedical Engineering towards the Year 2000 and Beyond (Cat. No.98CH36286), Hong Kong, China, 1 November 1998; Volume 2, pp. 916–918. [Google Scholar]
- Vilcahuaman, L.; Harba, R.; Canals, R.; Zequera, M.; Wilches, C.; Arista, M.T.; Torres, L.; Arbañil, H. Detection of diabetic foot hyperthermia by infrared imaging. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4831–4834. [Google Scholar]
- Luther, D.G.; Davidson, J.E.; Cromer, R.W.; Head, J.F. A head mounted infrared imager for treating the wounded on the battlefield. In Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Magnificent Milestones and Emerging Opportunities in Medical Engineering (Cat. No.97CH36136), Chicago, IL, USA, 30 October–2 November 1997; Volume 2, pp. 722–724. [Google Scholar]
- Ma, T.; Schajer, G.; Inagaki, T.; Pirouz, Z.; Tsuchikawa, S. Optical characteristics of Douglas fir at various densities, grain directions and thicknesses investigated by near-infrared spatially resolved spectroscopy (NIR-SRS). Holzforschung 2018, 72, 789–796. [Google Scholar] [CrossRef]
- Renfu, L. Quality Evaluation of Fruit by Hyperspectral Imaging; Elsevier: Amsterdam, The Netherlands, 2008; pp. 319–348. [Google Scholar]
- Papayan, G.; Akopov, A.; Petrishchev, N. Experimental and Clinical Application of Near-Infrared Fluorescence Diagnostics and Photodynamic Therapy. In Proceedings of the 2018 International Conference Laser Optics (ICLO), St. Petersburg, Russia, 4–8 June 2018; p. 581. [Google Scholar]
- Hassan, M.; Hattery, D.; Vogel, A.; Cheraomordik, V.; Demos, S.; Aleman, K.; Little, R.; Yarchoan, R.; Gandjbakhche, A.H. Noninvasive infrared imaging for quantitative assessment of tumor vasculature and response to therapy. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 1200–1202. [Google Scholar]
- Yu, B. Quantitative optical spectroscopy and imaging for cancer diagnosis and treatment monitoring. In Proceedings of the 2016 Progress in Electromagnetic Research Symposium (PIERS), Shanghai, China, 8–11 August 2016; p. 2322. [Google Scholar]
- Keith F., N.; Kong, R.; Pryia, A.; Bhargava, R. Data Processing for Tissue Histopathology Using Fourier Transform Infrared Spectral Data. In Proceedings of the 2006 Fortieth Asilomar Conference on Signals, Systems and Computers, Pacific Grove, CA, USA, 29 October–1 November 2006; pp. 71–75. [Google Scholar]
- Fukumoto, I. Computer simulation of Purkinje-Sanson images for the biofeedback therapy of myopia. In Proceedings of the IEEE Region 10 Conference, TENCON 99, Multimedia Technology for Asia-Pacific Information Infrastructure (Cat. No.99CH37030), Cheju Island, Korea, 15–17 September 1999; Volume 2, pp. 1166–1169. [Google Scholar]
- Fantini, S.; Fabbri, F.; Nadgir, S.; Henry, M.E.; Renshaw, P.F.; Franceschini, M.A. Bilateral near-infrared monitoring of the cerebral concentration and oxygenation of hemoglobin during unilateral electro-convulsive therapy. In Proceedings of the Conference on Lasers and Electro-Optics. CLEO 03., Taipei, Taiwan, 15–19 December 2003; pp. 1–2. [Google Scholar]
- Gibson, P.L.; Havey, G.D.; Seifert, G.J.; Hoey, M.F.; Kalpin, S.L. Radiometric infrared microbolometer arrays for surgical ablation. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society, Engineering in Medicine and Biology, Houston, TX, USA, 23–16 October 2002; Volume 2, pp. 1151–1152. [Google Scholar]
- Wang, M.Q.; Xia, Q.L.; Wu, X.Y.; Wang, X.; Zheng, X.L.; Hou, W.S. Optical stimulation of primary motor cortex with 980 nm infrared neural stimulation. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 6143–6146. [Google Scholar]
- Hirsch, L.R.; West, J.L.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Price, R.E.; Hazle, N.J.; Halas, J.D. Nanoshell-mediated near infrared photothermal tumor therapy. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No.03CH37439), Cancun, Mexico, 17–21 September 2003; pp. 1230–1231. [Google Scholar]
- Rothgang, E.; Gilson, W.D.; Strehl, W.; Pan, L.; Roland, J.; Lorenz, C.H.; Hornegger, J. Interventional MR-imaging for thermal ablation therapy. In Proceedings of the 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, IL, USA, 30 March–2 April 2011; pp. 1864–1868. [Google Scholar]
- Lei, T.; Manchanda, R.; Fernandez-Fernandez, A.; Huang, Y.C.; McGoron, A.J. Theranostic Nanoparticles for Imaging and Therapy and Cellular Response after Laser-induced Heating. In Proceedings of the 2013 29th Southern Biomedical Engineering Conference, Miami, FL, USA, 3–5 May 2013; pp. 21–22. [Google Scholar]
- Gono, K. Novel Multifunctional Endoscopic Imaging System for Support of Early Cancer Diagnosis. In Proceedings of the LEOS 2007-IEEE Lasers and Electro-Optics Society Annual Meeting, Lake Buena Vista, FL, USA, 21–25 October 2007; pp. 69–70. [Google Scholar]










| Patients (n = 35) | |
|---|---|
| Age, mean ± SD | 68 ± 12 |
| Gender, n (%) | |
| Male | 18/35 |
| Female | 17/35 |
| Problem: | |
| Poorly controlled hypertension | 32/35 |
| Aortic aneurysm | 16/35 |
| Detour | 28/35 |
| With Far-Infrared treatment | 35/35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liou, J.-C.; Hsiao, Y.-C.; Yang, C.-F. Infrared Sensor Detection and Actuator Treatment Applied during Hemodialysis. Sensors 2020, 20, 2521. https://doi.org/10.3390/s20092521
Liou J-C, Hsiao Y-C, Yang C-F. Infrared Sensor Detection and Actuator Treatment Applied during Hemodialysis. Sensors. 2020; 20(9):2521. https://doi.org/10.3390/s20092521
Chicago/Turabian StyleLiou, Jian-Chiun, Yu-Cheng Hsiao, and Cheng-Fu Yang. 2020. "Infrared Sensor Detection and Actuator Treatment Applied during Hemodialysis" Sensors 20, no. 9: 2521. https://doi.org/10.3390/s20092521





