Analysis of Temperature-Jump Boundary Conditions on Heat Transfer for Heterogeneous Microfluidic Immunosensors
Abstract
:1. Introduction
2. Device Geometry and Mathematical Formulation
2.1. Device Geometry
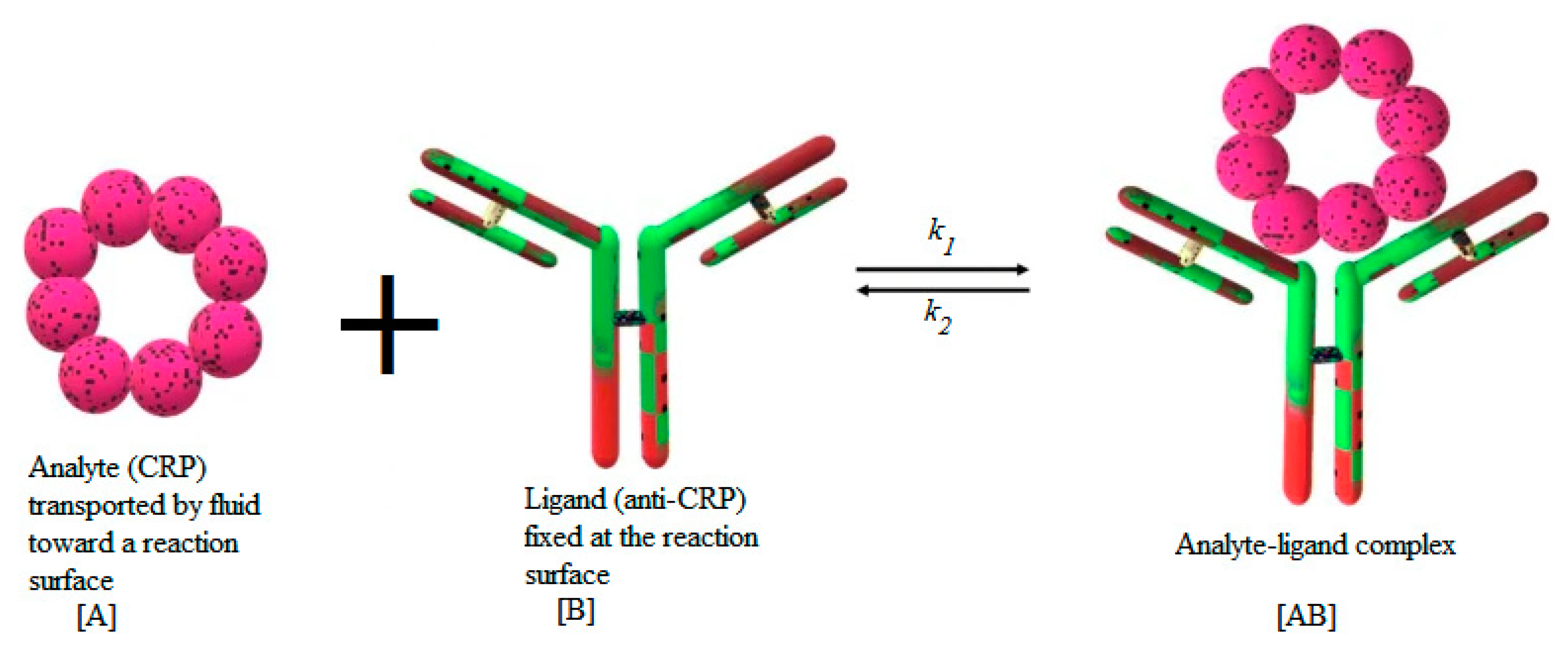
2.2. Transport Equations and Adsorption Model
2.3. Boundary Conditions
- At the inlet, a parabolic profile is adopted.
- At the channel exit, a fully developed flow condition is adopted.
- At lateral walls, a no-slip condition is adopted.
2.4. Numerical Method and Algorithm
- Solve the Poisson–Laplace equation to obtain the voltage and the electrical field, .
- Simultaneously solve Navier–Stokes and energy equations to deduce the dynamic and thermal fields, i.e., and .
- Solve the antigen transport equation and the Langmuir model to obtain the temporal evolution of the concentrations, and . These equations are time dependent.
3. Results
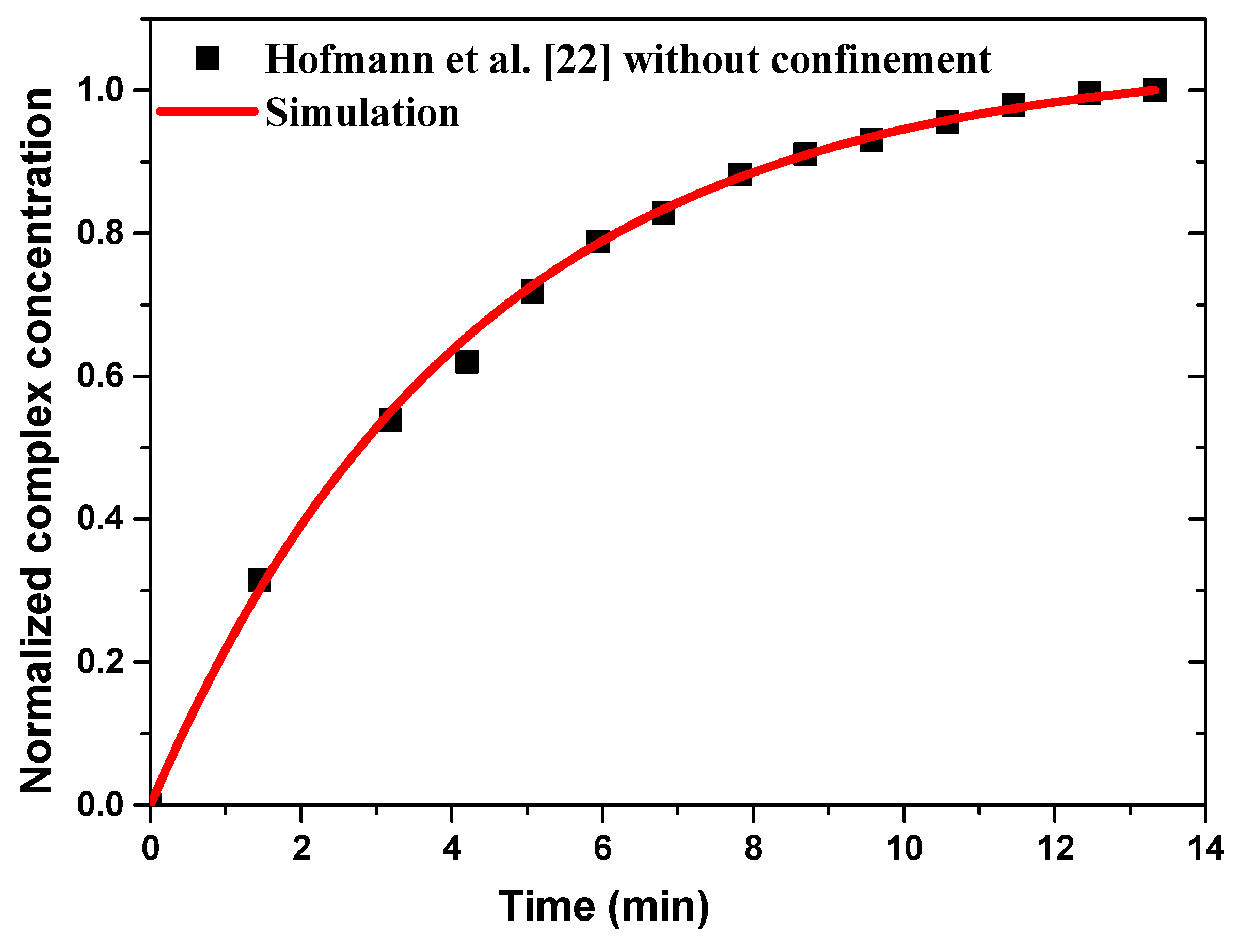
3.1. Model Validation
3.2. Effect of Surface Reaction Shape
3.3. Effect of Thermal Boundary Conditions
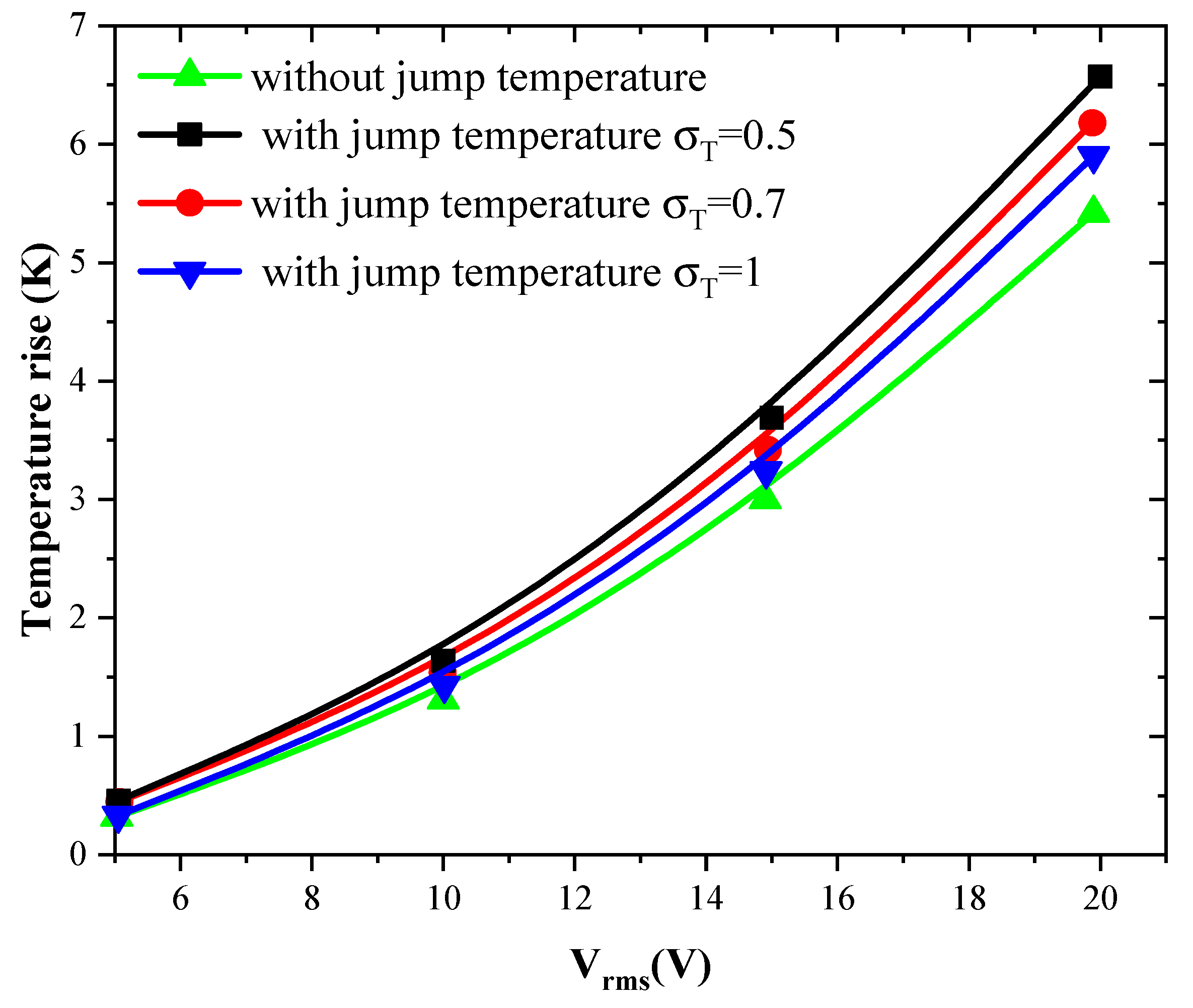
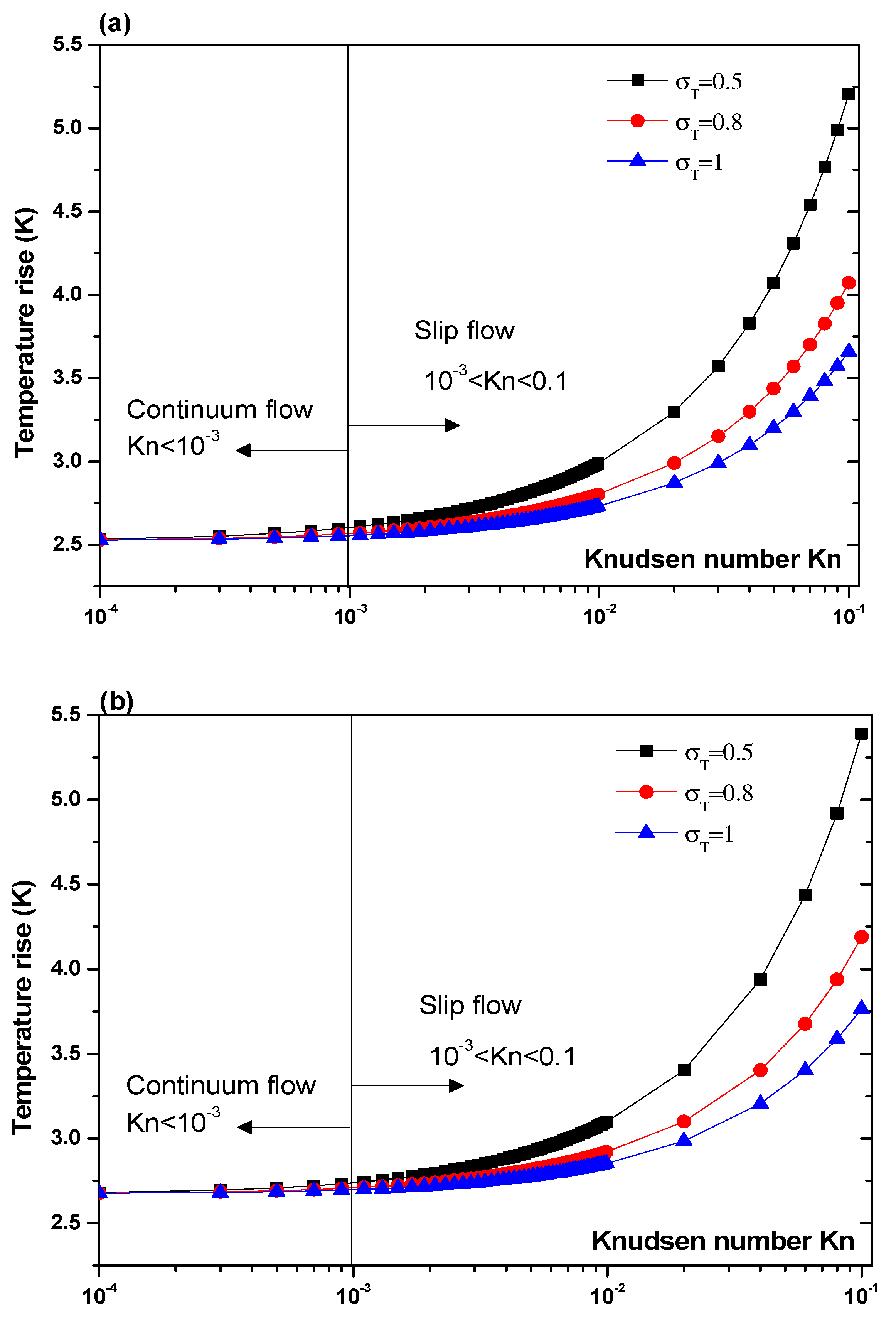
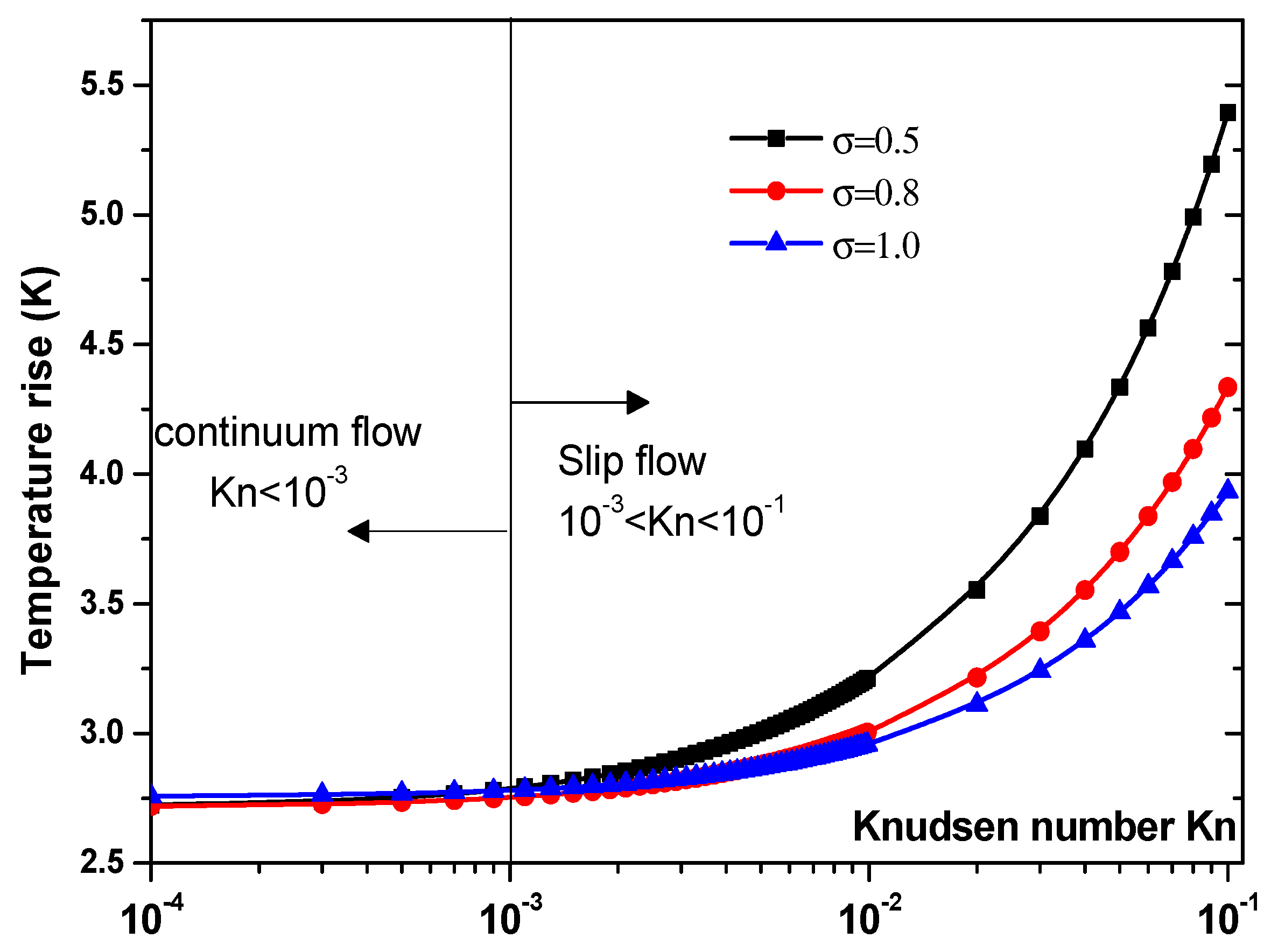
3.3.1. Effect of Jump Temperature on Temperature Rise
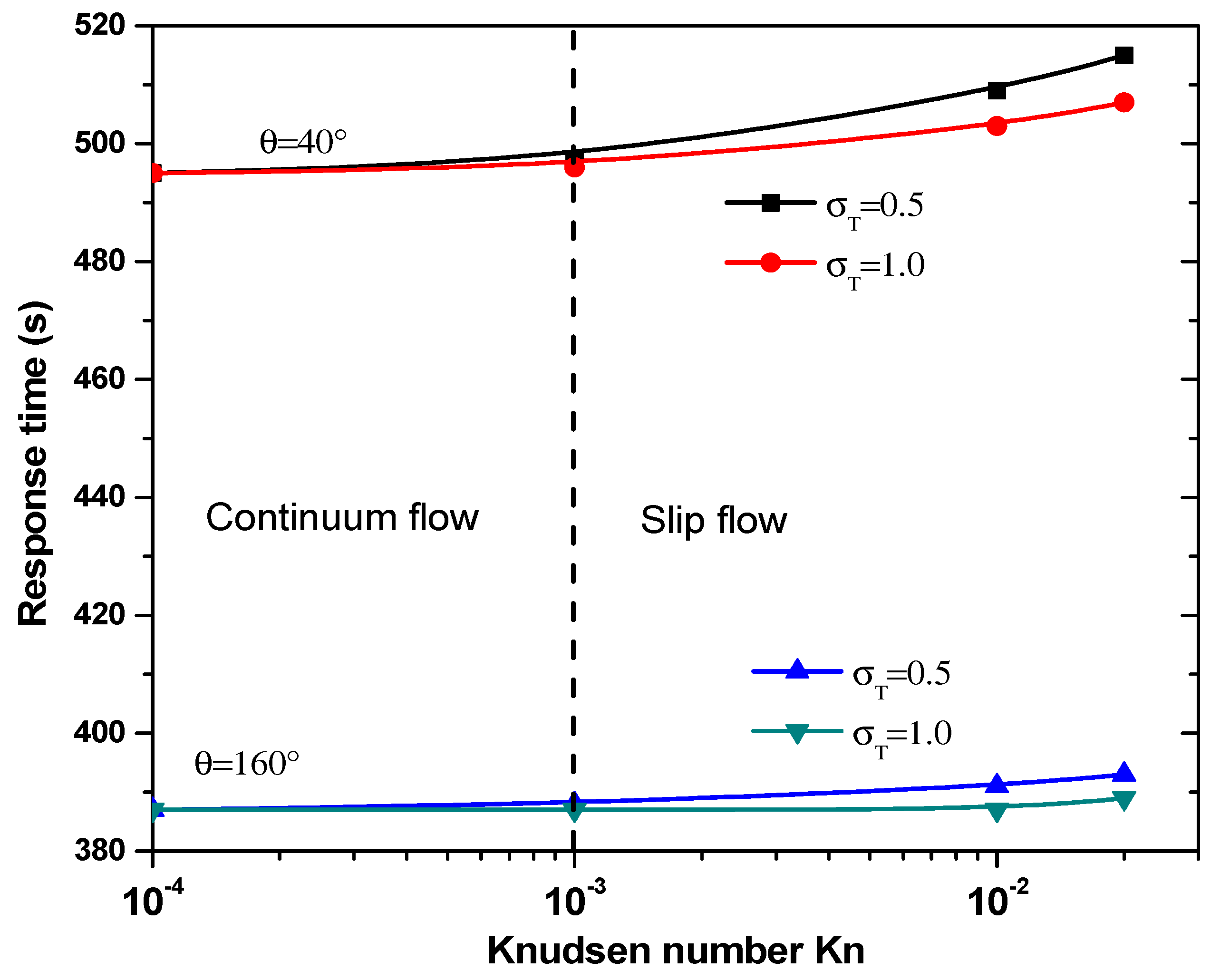
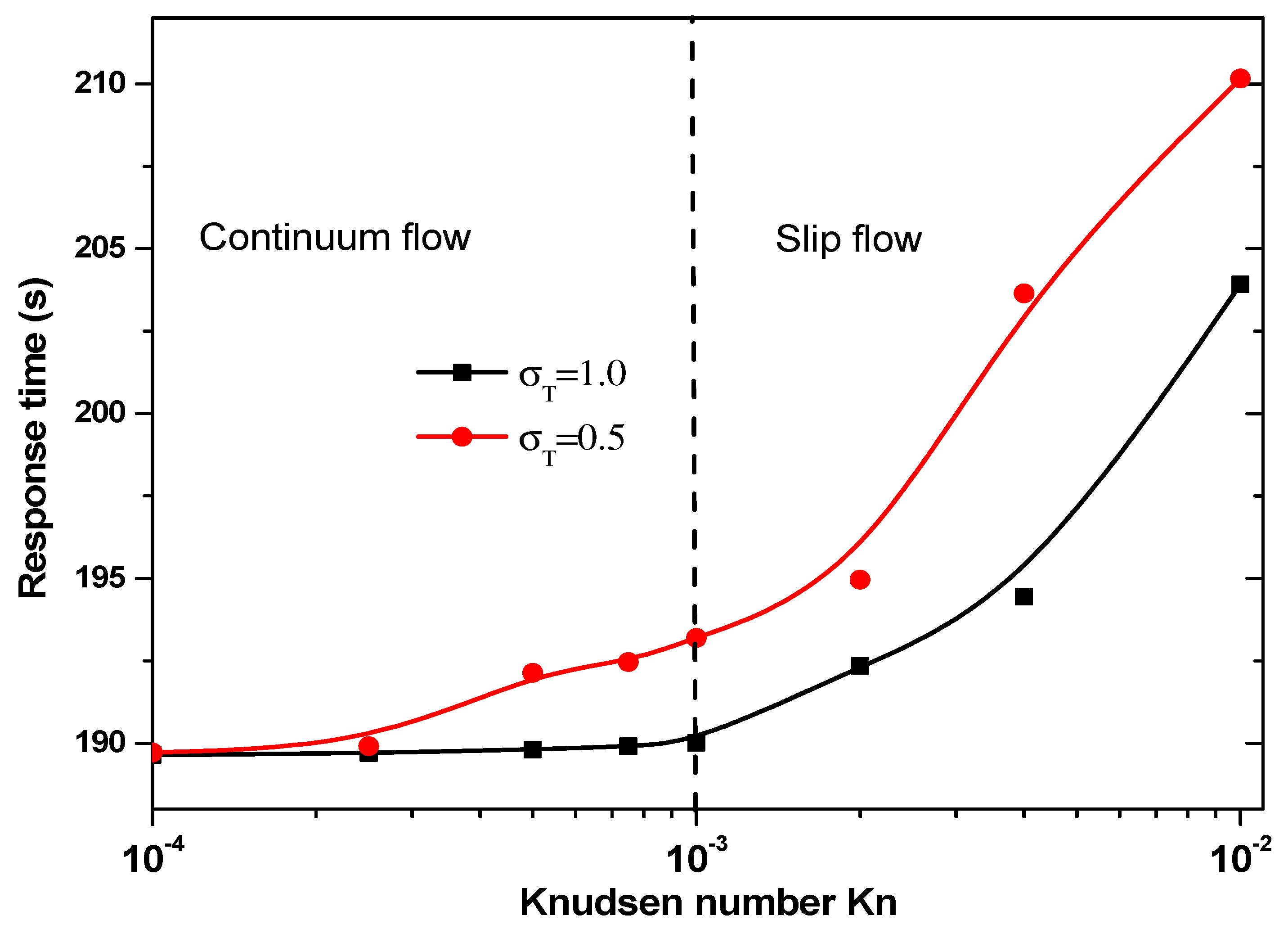
3.3.2. Effect of Jump Temperature on Response Time
4. Conclusions
- The performance of the microfluidic biosensor can be further enhanced by using the second design of the sensing area (circular ring) coupled with the electrothermal force.
- Taking into account the temperature jump in the vicinity of the wall of the microchannel is very important, especially in the slip flow regime (Kn > 10−3).
- Neglecting the temperature jump induces to overestimate temperature rise for biomedical applications and response time for microfluidic biosensors.
- The effect of thermal accommodation coefficient appears in slip flow.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salari, A.; Navi, M.; Lijnse, T.; Dalton, C. AC Electrothermal Effect in Microfluidics: A Review. Micromachines 2019, 10, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, L.A.; Cheung, Y.; Kinghorn, A.B.; Guo, W.; Shiu, S.C.; Jinata, C.; Liu, M.; Bhuyan, S.; Nan, L.; Shum, H.C.; et al. Microfluidic Technology for Nucleic Acid Aptamer Evolution and Application. Adv. Biosyst. 2019, 3, e1900012. [Google Scholar] [CrossRef]
- Ge, Z.Y.; Yan, H.; Liu, W.Y.; Song, C.L.; Xue, R.; Ren, Y.K. A Numerical Investigation of Enhancing Microfluidic Heterogeneous Immunoassay on Bipolar Electrodes Driven by Induced-Charge Electroosmosis in Rotating Electric Fields. Micromachines 2020, 11, 739. [Google Scholar] [CrossRef]
- Shahbazi, F.; Jabbari, M.; Esfahani, M.N.; Keshmiri, A. A computational simulation platform for designing real-time monitoring systems with application to COVID-19. Biosens. Bioelectron. 2021, 171, 112716. [Google Scholar] [CrossRef] [PubMed]
- Saad, Y.; Selmi, M.; Gazzah, M.H.; Belmabrouk, H. Improvement of Mass Transport at the Surface of an SPR Biosensor Applied in Microfluidics. In Proceedings of the International Conference Design and Modeling of Mechanical Systems, Hammamet, Tunisia, 18–20 March 2019; Springer: Berlin/Heidelberg, Germany, 2019; pp. 145–154. [Google Scholar]
- Fan, Z.H.; Ogunwobi, O.O.; Chen, T.; Zhang, J.; George, T.J.; Liu, C.; Fan, Z.H. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip 2014, 14, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.; Lisak, G. Sponge-based microfluidic sampling for potentiometric ion sensing. Anal. Chim. Acta 2019, 1091, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, M.; Shtepliuk, I.; Filippini, D.; Ivanov, I.G.; Yakimova, R.; Eriksson, J. Real-time sensing of lead with epitaxial gra-phene-integrated microfluidic devices. Sens. Actuators B Chem. 2019, 288, 425–431. [Google Scholar] [CrossRef]
- Lindholm-Sethson, B.; Nyström, J.; Geladi, P.; Koeppe, R.; Nelson, A.; Whitehouse, C. Are biosensor arrays in one membrane pos-sible? A combination of multifrequency impedance measurements and chemometrics. Anal. Bioanal. Chem. 2003, 377, 478–485. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, L.; Li, L.; Zong, S.; Wang, Z.; Cui, Y. Simultaneous and highly sensitive detection of multiple breast cancer bi-omarkers in real samples using a SERS microfluidic chip. Talanta 2018, 188, 507–515. [Google Scholar] [CrossRef]
- Aranda, P.R.; Messina, G.A.; Bertolino, F.A.; Pereira, S.V.; Baldo, M.A.F.; Raba, J. Nanomaterials in fluorescent laser-based im-munosensors: Review and applications. Microchem. J. 2018, 141, 308–323. [Google Scholar] [CrossRef]
- Bange, A.; Halsall, H.B.; Heineman, W.R. Microfluidic immunosensor systems. Biosens. Bioelectron. 2005, 20, 2488–2503. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Electrochemical immunosensors for the detection of cytokine tumor necrosis factor alpha: A review. Talanta 2020, 211, 120758. [Google Scholar] [CrossRef] [PubMed]
- Henares, T.G.; Mizutani, F.; Hisamoto, H. Current development in microfluidic immunosensing chip. Anal. Chim. Acta 2008, 611, 17–30. [Google Scholar] [CrossRef]
- Jeong, S.; Park, M.-J.; Song, W.; Kim, H.S. Current immunoassay methods and their applications to clinically used biomarkers of breast cancer. Clin. Biochem. 2020, 78, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niessner, R.; Tang, D.; Knopp, D. Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal. Chim. Acta 2016, 912, 10–23. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Heterogeneous and homogeneous immunoassays for drug analysis. Bioanalysis 2014, 6, 2877–2896. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.H.C.; Uddayasankar, U.; Wheeler, A.R. Immunoassays in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, K.; Mafe, S.; Stroeve, P. Convection, diffusion and reaction in a surface-based biosensor: Modeling of cooperativity and binding site competition on the surface and in the hydrogel. J. Colloid Interface Sci. 2006, 296, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, D.B.; Gooding, J.J.; Erokhin, P. Kinetics of irreversible adsorption with diffusion: Application to biomolecule immobi-lization. Langmuir 2002, 18, 1770–1776. [Google Scholar] [CrossRef]
- Huang, K.-R.; Chang, J.-S.; Der Chao, S.; Wu, K.-C.; Yang, C.-K.; Lai, C.-Y.; Chen, S.-H. Simulation on binding efficiency of immunoassay for a biosensor with applying electrothermal effect. J. Appl. Phys. 2008, 104, 064702. [Google Scholar] [CrossRef]
- Hofmann, O.T.; Voirin, G.; Niedermann, P.; Manz, A. Three-Dimensional Microfluidic Confinement for Efficient Sample Delivery to Biosensor Surfaces. Application to Immunoassays on Planar Optical Waveguides. Anal. Chem. 2002, 74, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.C.; Sigurdson, M.; Meinhart, C.D. AC electrothermal enhancement of heterogeneous assays in microfluidics. Lab Chip 2007, 7, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; Lec, R.; Noh, H. (Moses) Enhancement of heterogeneous immunoassays using AC electroosmosis. Sens. Actuators B Chem. 2010, 147, 366–375. [Google Scholar] [CrossRef]
- Liu, X.; Yang, K.; Wadhwa, A.; Eda, S.; Li, S.; Wu, J. Development of an AC electrokinetics-based immunoassay system for on-site serodiagnosis of infectious diseases. Sens. Actuators A Phys. 2011, 171, 406–413. [Google Scholar] [CrossRef]
- Mahmoodi, S.R.; Bayati, M.; Hosseinirad, S.; Foroumadi, A.; Gilani, K.; Rezayat, S.M. AC electrokinetic manipulation of selenium nanoparticles for potential nanosensor applications. Mater. Res. Bull. 2013, 48, 1262–1267. [Google Scholar] [CrossRef]
- Selmi, M.; Echouchene, F.; Gazzah, M.H.; Belmabrouk, H. Flow Confinement Enhancement of Heterogeneous Immunoassays in Microfluidics. IEEE Sens. J. 2015, 15, 7321–7328. [Google Scholar] [CrossRef]
- Selmi, M.; Khemiri, R.; Echouchene, F.; Belmabrouk, H. Enhancement of the Analyte Mass Transport in a Microfluidic Biosensor by Deformation of Fluid Flow and Electrothermal Force. J. Manuf. Sci. Eng. 2016, 138, 081011. [Google Scholar] [CrossRef]
- Selmi, M.; Gazzah, M.H.; Belmabrouk, H. Optimization of microfluidic biosensor efficiency by means of fluid flow engineering. Sci. Rep. 2017, 7, 5721. [Google Scholar] [CrossRef] [Green Version]
- Selmi, M.; Belmabrouk, H. 3D Numerical Simulation of Binding Efficiency of Immunoassay for a Biosensor with Involving a Cylinder. Sens. Lett. 2018, 16, 498–505. [Google Scholar] [CrossRef]
- Selmi, M.; Echouchene, F.; Belmabrouk, H.; Marwa, S.; Fraj, E.; Hafedh, B. Analysis of Microfluidic Biosensor Efficiency Using a Cylindrical Obstacle. Sens. Lett. 2016, 14, 26–31. [Google Scholar] [CrossRef]
- Selmi, M.; Khemiri, R.; Echouchene, F.; Belmabrouk, H. Electrothermal effect on the immunoassay in a microchannel of a bio-sensor with asymmetrical interdigitated electrodes. Appl. Therm. Eng. 2016, 105, 77–84. [Google Scholar] [CrossRef]
- Selmi, M.; Gazzah, M.H.; Belmabrouk, H. Numerical Study of the Electrothermal Effect on the Kinetic Reaction of Immunoas-says for a Microfluidic Biosensor. Langmuir 2016, 32, 13305–13312. [Google Scholar] [CrossRef] [PubMed]
- Selmi, M.; Belmabrouk, H. AC Electroosmosis Effect on Microfluidic Heterogeneous Immunoassay Efficiency. Micromachines 2020, 11, 342. [Google Scholar] [CrossRef] [Green Version]
- Echouchene, F.; Alshahrani, T.; Belmabrouk, H. Simulation of the Slip Velocity Effect in an AC Electrothermal Micropump. Micromachines 2020, 11, 825. [Google Scholar] [CrossRef]
- Green, N.G.; Ramos, A.; González, A.; Castellanos, A.; Morgan, H. Electrothermally induced fluid flow on microelectrodes. J. Electrost. 2001, 53, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Bouzid, M.; Sellaoui, L.; Khalfaoui, M.; Belmabrouk, H.; Ben Lamine, A. Adsorption of ethanol onto activated carbon: Modeling and consequent interpretations based on statistical physics treatment. Phys. A Stat. Mech. Appl. 2016, 444, 853–869. [Google Scholar] [CrossRef]
- Pang, X.; Bouzid, M.; Dos Santos, J.M.; Gazzah, M.H.; Dotto, G.L.; Belmabrouk, H.; Bajahzar, A.; Erto, A.; Li, Z. Theoretical study of indigotine blue dye adsorption on CoFe2O4/chitosan magnetic composite via analytical model. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589. [Google Scholar] [CrossRef]
- Chou, C.; Hsu, H.-Y.; Wu, H.-T.; Tseng, K.-Y.; Chiou, A.; Yu, C.-J.; Lee, Z.-Y.; Chan, T.-S. Fiber optic biosensor for the detection of C-reactive protein and the study of protein binding kinetics. J. Biomed. Opt. 2007, 12, 024025. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-R.; Chang, J.-S. Three dimensional simulation on binding efficiency of immunoassay for a biosensor with applying electrothermal effect. Heat Mass Transf. 2013, 49, 1647–1658. [Google Scholar] [CrossRef]
- Echouchene, F.; Belmabrouk, H. Effect of Temperature Jump on Nonequilibrium Entropy Generation in a MOSFET Transistor Using Dual-Phase-Lagging Model. J. Heat Transf. 2017, 139, 122007. [Google Scholar] [CrossRef]
- Sajadifar, S.A.; Karimipour, A.; Toghraie, D. Fluid flow and heat transfer of non-Newtonian nanofluid in a microtube consid-ering slip velocity and temperature jump boundary conditions. Eur. J. Mech. B Fluids 2017, 61, 25–32. [Google Scholar] [CrossRef]
- Shojaeian, M.; Yildiz, M.; Koşar, A. Heat transfer characteristics of plug flows with temperature-jump boundary conditions in parallel-plate channels and concentric annuli. Int. J. Therm. Sci. 2014, 84, 252–259. [Google Scholar] [CrossRef]
- Torabi, M.; Zhang, Z.; Peterson, G. Interface entropy generation in micro porous channels with velocity slip and temperature jump. Appl. Therm. Eng. 2017, 111, 684–693. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Q.; Nakayama, A.; Qiu, T. Effect of temperature jump on forced convective transport of nanofluids in the con-tinuum flow and slip flow regimes. Chem. Eng. Sci. 2015, 137, 730–739. [Google Scholar] [CrossRef]



| Parameter | Unit | Value |
|---|---|---|
| 6.4 | ||
| 0.6 | ||
| 1000 | ||
| 4.184 | ||
| 80.2 |
| Vrms = 0 V | Vrms = 15 V | |
|---|---|---|
| First model (θ = 40°) | 3.64 | 4.39 |
| Second model | 4.53 | 17.4 |
| Huang et al. [40] (Type-4) | 1.48 | 4.51 |
| Applied Voltage (V) | 5 | 10 | 15 | 20 | |
|---|---|---|---|---|---|
| Temperature rise (K) [40] | 0.31 | 1.31 | 2.73 | 4.93 | |
| Temperature rise (K), first model (θ = 160°) | Isothermal | 0.301 | 1.373 | 3.089 | 5.492 |
| Jump temperature = 1 | 0.374 | 1.499 | 3.383 | 6.063 | |
| Temperature rise (K) second model | Isothermal | 0.28 | 1.375 | 3.15 | 5.65 |
| Jump temperature = 1 | 0.33 | 1.42 | 3.22 | 5.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echouchene, F.; Al-shahrani, T.; Belmabrouk, H. Analysis of Temperature-Jump Boundary Conditions on Heat Transfer for Heterogeneous Microfluidic Immunosensors. Sensors 2021, 21, 3502. https://doi.org/10.3390/s21103502
Echouchene F, Al-shahrani T, Belmabrouk H. Analysis of Temperature-Jump Boundary Conditions on Heat Transfer for Heterogeneous Microfluidic Immunosensors. Sensors. 2021; 21(10):3502. https://doi.org/10.3390/s21103502
Chicago/Turabian StyleEchouchene, Fraj, Thamraa Al-shahrani, and Hafedh Belmabrouk. 2021. "Analysis of Temperature-Jump Boundary Conditions on Heat Transfer for Heterogeneous Microfluidic Immunosensors" Sensors 21, no. 10: 3502. https://doi.org/10.3390/s21103502






