Magnetoimpedance Thin Film Sensor for Detecting of Stray Fields of Magnetic Particles in Blood Vessel
Abstract
1. Introduction
2. Materials and Methods
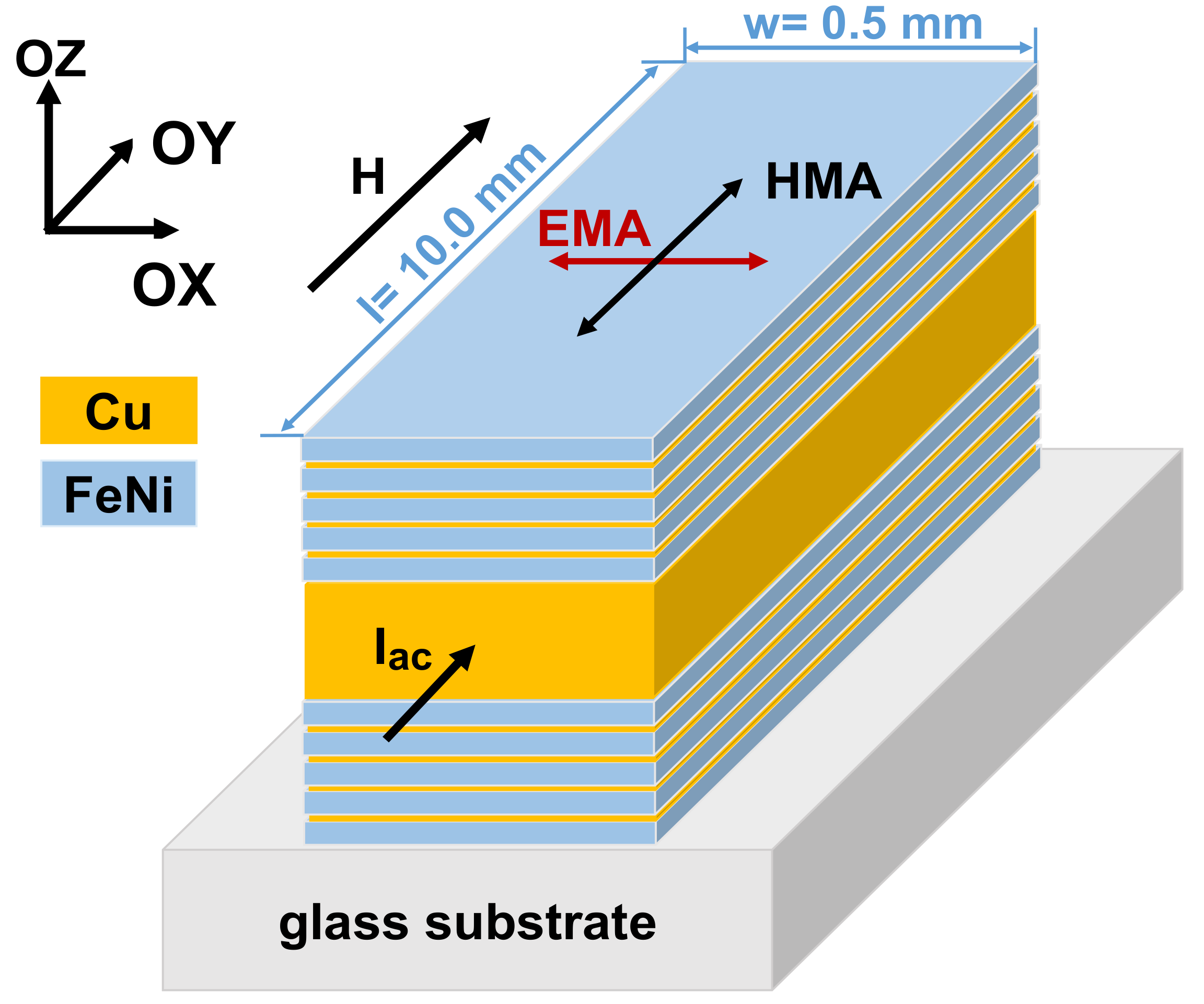
2.1. Materials
2.2. Experimental Methods
2.3. Magnetic Stray Field Distribution Modeling
3. Results
3.1. Structure and Magnetic Properties
3.2. Detection by MI Sensitive Element
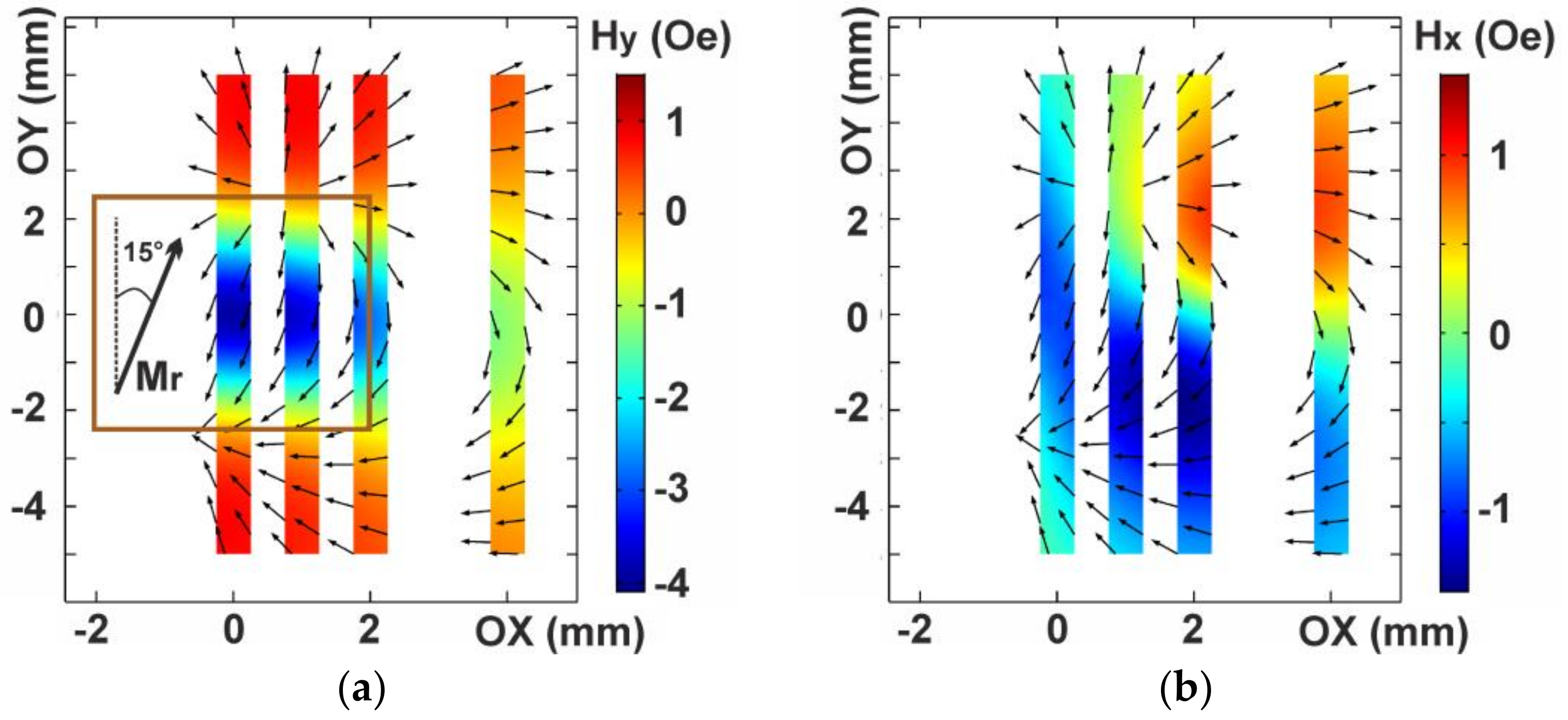
3.3. Magnetic Modeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prinz, G.A. Magnetoelectronics applications. J. Magn. Magn. Mater. 1999, 200, 57–68. [Google Scholar] [CrossRef]
- Zamani Kouhpanji, M.R.; Stadler, B.J.H. A guideline for Effectively Synthesizing and Characterizing Magnetic Nanoparticles for Advancing Nanobiotechnology: A Review. Sensors 2020, 20, 2554. [Google Scholar] [CrossRef]
- Bukreev, D.A.; Moiseev, A.A.; Derevyanko, M.S.; Semirov, A.V. High-Frequency Electric Properties of Amorphous Soft Magnetic Cobalt-Based Alloys in the Region of Transition to the Paramagnetic State. Russ. Phys. J. 2015, 58, 141–145. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Camp, P.J. Effects of interactions on magnetization relaxation dynamics in ferrofluids. Phys. Review E 2020, 102, 032610. [Google Scholar] [CrossRef]
- Wang, L.; Yan, Y.; Wang, M.; Yang, H.; Zhou, Z.; Peng, C.; Yang, S. An integrated nanoplatform for theranostics via multifunctional core-shell ferrite nanocubes. J. Mater. Chem. B 2016, 4, 1908–1914. [Google Scholar] [CrossRef]
- Um, J.; Zhang, Y.; Zhou, W.; Zamani Kouhpanji, M.R.; Radu, C.; Franklin, R.R.; Stadler, B.J.H. Magnetic Nanowire Biolabels Using Ferromagnetic Resonance Identification. ACS Appl. Nano Mater. 2021, 4, 3557–3564. [Google Scholar] [CrossRef]
- Svalov, A.V.; Arkhipov, A.V.; Andreev, S.V.; Neznakhin, D.S.; Larrañaga, A.; Kurlyandskaya, G.V. Modified field dependence of the magnetocaloric effect in Gd powder obtained by ball milling. Mater. Lett. 2021, 284, 128921. [Google Scholar] [CrossRef]
- Sciancalepore, C.; Gualtieri, A.F.; Scardi, P.; Flor, A.; Allia, P.; Tiberto, P.; Barrera, G.; Messori, M.; Bondioli, F. Structural characterization and functional correlation of Fe3O4 nanocrystals obtained using 2-ethyl-1,3-hexanediol as innovative reactive solvent in non-hydrolytic sol-gel synthesis. Mater. Chem. Phys. 2018, 207, 337–349. [Google Scholar] [CrossRef]
- Hergt, R.; Hiergeist, R.; Zeisberger, M.; Schüler, D.; Heyen, U.; Hilger, I.; Kaiser, W.A. Magnetic properties of bacterial magnetosomes as potential diagnostic and therapeutic tools. J. Magn. Magn. Mater. 2005, 293, 80–86. [Google Scholar] [CrossRef]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in cancer medicine. Phys. Today 2012, 65, 38–42. [Google Scholar] [CrossRef]
- Safronov, A.P.; Beketov, I.V.; Komogortsev, S.V.; Kurlyandskaya, G.V.; Medvedev, A.I.; Leiman, D.V.; Larranaga, A.; Bhagat, S.M. Spherical magnetic nanoparticles fabricated by laser target evaporation. AIP Adv. 2013, 3, 052135. [Google Scholar] [CrossRef]
- Khawja Ansari, S.A.M.; Ficiara, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials 2019, 12, 465. [Google Scholar] [CrossRef]
- Agra, K.; Mori, T.J.A.; Dorneles, L.S.; Escobar, V.M.; Silva, U.C.; Chesmana, C.; Bohna, F.; Corrêa, M.A. Dynamic magnetic behavior in non-magnetostrictive multilayered films grown on glass and flexible substrates. J. Magn. Magn. Mater. 2014, 355, 136–141. [Google Scholar] [CrossRef]
- Lee, W.; Joo, S.; Kim, S.U.; Rhie, K.; Hong, J.; Shin, K.-H.; Kim, K.H. Magnetic bead counter using a micro-Hall sensor for biological applications. Appl. Phys. Lett. 2009, 94, 153903. [Google Scholar] [CrossRef]
- Yuvchenko, A.A.; Lepalovskii, V.N.; Vas’kovskii, V.O.; Safronov, A.P.; Volchkov, S.O.; Kurlyandskaya, G.V. Magnetic impedance of structured film meanders in the presence of magnetic micro- and nanoparticles. Tech. Phys. 2014, 59, 230–236. [Google Scholar] [CrossRef]
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar] [CrossRef]
- Cardoso, S.; Leitao, D.C.; Dias, T.M.; Valadeiro, J.; Silva, M.D.; Chicharo, A.; Silverio, V.; Gaspar, J.; Freitas, P.P. Challenges and trends in magnetic sensor integration with microfluidics for biomedical applications. J. Phys. D Appl. Phys. 2017, 50, 213001. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Portnov, D.S.; Beketov, I.V.; Larrañaga, A.; Safronov, A.P.; Orue, I.; Medvedev, A.I.; Chlenova, A.A.; Sanchez-Ilarduya, M.B.; Martinez-Amesti, A.; et al. Nanostructured materials for magnetic biosensing Nanostructured materials for magnetic biosensing. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Nishibe, Y.; Yamadera, H.; Nonomura, Y.; Takeuchi, M.; Taga, Y. Giant magneto-impedance effect in layered thin films. IEEE Trans. Magn. 1997, 33, 4367–4372. [Google Scholar] [CrossRef]
- Llandro, J.; Palfreyman, J.J.; Ionescu, A.; Barnes, C.H.W. Magnetic biosensor technologies for medical applications: A review. Med. Biol. Eng. Comput. 2010, 48, 977–998. [Google Scholar] [CrossRef] [PubMed]
- Buznikov, N.A.; Safronov, A.P.; Orue, I.; Golubeva, E.V.; Lepalovskij, V.N.; Svalov, A.V.; Chlenova, A.A.; Kurlyandskaya, G.V. Modelling of magnetoimpedance response of thin film sensitive element in the presence of ferrogel: Next step toward development of biosensor for in tissue embedded magnetic nanoparticles detection. Bios. Bioelectr. 2018, 117, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Prilepskii, A.Y.; Fakhardo, A.F.; Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Shtil, A.A.; Bel’tyukov, P.P.; Shibeko, A.M.; Koltsova, E.M.; Nechipurenko, D.Y.; et al. Urokinase-Conjugated Magnetite Nanoparticles as a Promising Drug Delivery System for Targeted Thrombolysis: Synthesis and Preclinical Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 36764–36775. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Elbaile, L.; Alves, F.; Ahamada, B.; Barrue, R.; Svalov, A.V.; Vas’kovskiy, V.O. Domain structure and magnetization process of a giant magnetoimpedance geometry FeNi/Cu/FeNi(Cu)FeNi/Cu/FeNi sensitive element. J. Phys. Condens. Matter 2004, 16, 6561–6568. [Google Scholar] [CrossRef]
- Cheng, S.F.; Lubitz, P.; Zheng, Y.; Edelstein, A.S. Effects of spacer layer on growth, stress and magnetic properties of sputtered permalloy film. J. Magn. Magn. Mater 2004, 282, 109–113. [Google Scholar] [CrossRef]
- Corrêa, M.A.; Viegas, A.D.C.; Da Silva, R.B.; De Andrade, A.M.H.; Sommer, R.L. Magnetoimpedance of single and multilayered FeCuNbSiB films in frequencies up to 1.8 GHz. J. Appl. Phys 2007, 101, 043905. [Google Scholar] [CrossRef]
- Saito, N.; Fujiwara, H.; Sugita, Y. A new type magnetic domain in negative magnetostriction Ni-Fe films. J. Phys. Soc. Jpn. 1964, 19, 1116–1125. [Google Scholar] [CrossRef]
- Svalov, A.V.; Kurlyandskaya, G.V.; Hammer, H.; Savin, P.A.; Tutynina, O.I. Modification of the “transcritical” state in NiFeCuMo films produced by RF sputtering. Tech. Phys. 2004, 49, 868–871. [Google Scholar] [CrossRef]
- Coïsson, M.; Vinal, F.; Tiberto, P.; Celegato, F. Magnetic properties of FeSiB thin films displaying stripe domains. J. Magn. Magn. Mater. 2009, 321, 806–809. [Google Scholar] [CrossRef]
- Buznikov, N.A.; Svalov, A.V.; Kurlyandskaya, G.V. Influence of the Parameters of Permalloy-Based Multilayer Film Structures on the Sensitivity of Magnetic Impedance Effect. Phys. Met. Metallogr. 2021, 122, 223–229. [Google Scholar] [CrossRef]
- Hubert, A.; Schäfer, R. Magnetic Domains; Springer: Berlin, Germany, 1998. [Google Scholar]
- Barabesi, L.; Greco, L. A Note on the exact computation of the Student t, Snedecor F and sample correlation coefficient distribution functions. J. R. Stat. Soc. Ser. D 2002, 51, 105–110. [Google Scholar]
- Rikken, R.S.M.; Engelkamp, H.; Nolte, R.J.M.; Maan, J.C.; van Hest, J.C.M.; Wilson, D.A.; Christianen, P.C.M. Shaping polymersomes into predictable morphologies via out-of-equilibrium self-assembly. Nat. Commun. 2016, 7, 12606. [Google Scholar] [CrossRef] [PubMed]
- Bender, P.; Günther, A.; Tschöpe, A.; Birringer, R. Synthesis and characterization of uniaxial ferrogels with Ni nanorods as magnetic phase. J. Magn. Magn. Mater. 2011, 323, 2055–2063. [Google Scholar] [CrossRef]
- Alzola, N.V.; Kurlyandskaya, G.V.; Larrañaga, A.; Svalov, A.V. Structural Peculiarities and Magnetic Properties of FeNi Films and FeNi/Ti-Based Magnetic Nanostructures. IEEE Trans. Magn. 2012, 48, 1605–1608. [Google Scholar] [CrossRef]
- O’Handley, R.C. Modern Magnetic Materials; John Wiley & Sons: New York, USA, 1972; p. 740. [Google Scholar]
- Vas’kovskii, V.O.; Savin, P.A.; Volchkov, S.O.; Lepalovskii, V.N.; Bukreev, D.A.; Buchkevich, A.A. Nanostructuring Effects in Soft Magnetic Films and Film Elements with Magnetic Impedance. Tech. Phys. 2013, 58, 105–110. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Bebenin, N.G.; Vas’kovskiy, V.O. Giant magnetic impedance of wires with a thin magnetic coating. Phys. Met. Metallogr. 2011, 111, 133–154. [Google Scholar] [CrossRef]
- Semirov, A.V.; Moiseev, A.A.; Bukreev, D.A.; Kovaleva, N.P.; Vasyukhno, N.V.; Nemirova, V.A. Asymmetric magnetoimpedance of a magnetically soft wire. Phys. Met. Metallogr. 2017, 118, 535–540. [Google Scholar] [CrossRef]
- Spizzo, F.; Sgarbossa, P.; Sieni, E.; Semenzato, A.; Dughiero, F.; Forzan, M.; Bertani, R.; Del Bianco, L. Synthesis of ferrofluids made of iron oxide nanoflowers: Interplay between carrier fluid and magnetic properties. Nanomaterials 2017, 7, 373. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Hornowski, T.; Dobosz, B.; Józefczak, A. Influence of magnetic nanoparticles on the focused ultrasound hyperthermia. Materials 2018, 11, 1607. [Google Scholar] [CrossRef]
- Pavlov, A.M.; De Geest, B.G.; Louage, B.; Lybaert, L.; De Koker, S.; Koudelka, Z.; Sapelkin, A.; Sukhorukov, G.B. Magnetically engineered microcapsules as intracellular anchors for remote control over cellular mobility. Adv. Mater. 2013, 25, 6945–6950. [Google Scholar] [CrossRef]
- Novoselova, I.P.; Safronov, A.P.; Samatov, O.M.; Beketov, I.V.; Medvedev, A.I.; Kurlyandskaya, G.V. Water based suspensions of iron oxide obtained by laser target evaporation for biomedical applications. J. Magn. Magn. Mater. 2016, 415, 35–38. [Google Scholar] [CrossRef]
- Vallejo-Fernandez, G.; Whear, O.; Roca, A.G.; Hussain, S.; Timmis, J.; Patel, V.; O’Grady, K. Mechanisms of hyperthermia in magnetic nanoparticles. J. Phys. D Appl. Phys. 2013, 46, 312001. [Google Scholar] [CrossRef]
- Barrera, G.; Coisson, M.; Celegato, F.; Martino, L.; Priyanka Tiwari, P.; Verma, R.; Kane, S.N.; Mazaleyrat, F.; Tiberto, P. Specific loss power of Co/Li/Zn-mixed ferrite powders for magnetic hyperthermia. Sensors 2020, 20, 2151. [Google Scholar] [CrossRef] [PubMed]
- Zverev, V.I.; Pyatakov, A.P.; Shtil, A.A.; Tishin, A.M. Novel applications of magnetic materials and technologies for medicine. J. Magn. Magn. Mater. 2018, 459, 182–186. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Matters 2013, 3, 660–672. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Novoselova, I.P.; Schupletsova, V.V.; Andrade, R.; Dunec, N.A.; Litvinova, L.S.; Safronov, A.P.; Yurova, K.A.; Kulesh, N.A.; Dzyuman, A.N.; et al. Nanoparticles for magnetic biosensing systems. J. Magn. Magn. Mater. 2017, 431, 249–254. [Google Scholar] [CrossRef]
- Bulk, M.; van der Weerd, L.; Breimer, W.; Lebedev, N.; Webb, A.; Goeman, J.J.; Ward, R.J.; Huber, M.; Oosterkamp, T.H.; Bossoni, L. Quantitative comparison of different iron forms in the temporal cortex of Alzheimer patients and control subjects. Sci. Rep. 2018, 8, 6898. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.; Henderson, D.; Schenck, J.; Zimmerman, E.A. Iron accumulation in the substantia nigra of patients with Alzheimer disease and Parkinsonism. Arch Neurol. 2009, 66, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Kurlyandskaya, G.V.; Sánchez, M.L.; Hernando, B.; Prida, V.M.; Gorria, P.; Tejedor, M. Giant-magnetoimpedance-based sensitive element as a model for biosensors. Appl. Phys. Lett. 2003, 82, 3053–3056. [Google Scholar]
- Blanc-Béguin, F.; Nabily, S.; Gieraltowski, J.; Turzo, A.; Querellou, S.; Salaun, P.Y. Cytotoxicity and GMI bio-sensor detection of maghemite nanoparticles internalized into cells. J. Magn. Magn. Mater. 2009, 321, 192–197. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.H.; Dong, X.W.; Yan, H.L.; Lei, C.; Luo, Y.S. Giant magnetoimpedance based immunoassay for cardiac biomarker myoglobin. Anal. Methods 2017, 9, 3636–3642. [Google Scholar] [CrossRef]
- Chlenova, A.A.; Buznikov, N.A.; Safronov, A.P.; Golubeva, E.V.; Lepalovskii, V.N.; Melnikov, G.Y.; Kurlyandskaya, G.V. Detecting the total stray fields of ferrogel nanoparticles using a prototype magnetoimpedance sensor: Modeling and experiment. Bull. Russ. Acad. Sci. Phys. 2019, 83, 906–908. [Google Scholar]
- Uchiyama, T.; Mohri, K.; Honkura, Y.; Panina, L.V. Recent advances of pico-Tesla resolution magneto-impedance sensor based on amorphous wire CMOS IC MI Sensor. IEEE Trans. Magn. 2012, 48, 3833–3839. [Google Scholar] [CrossRef]
- García-Arribas, A.; Fernández, E.; Svalov, A.; Kurlyandskaya, G.V.; Barandiaran, J.M. Thin-film magneto-impedance structures with very large sensitivity. J. Magn. Magn. Mater. 2016, 400, 321–326. [Google Scholar] [CrossRef]
- Buznikov, N.A.; Kurlyandskaya, G.V. Magnetoimpedance of Periodic Partly Profiled Multilayer Film Structures. Fizika Metallov i Metallovedenie 2021, 122, 755–760. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikov, G.Y.; Lepalovskij, V.N.; Svalov, A.V.; Safronov, A.P.; Kurlyandskaya, G.V. Magnetoimpedance Thin Film Sensor for Detecting of Stray Fields of Magnetic Particles in Blood Vessel. Sensors 2021, 21, 3621. https://doi.org/10.3390/s21113621
Melnikov GY, Lepalovskij VN, Svalov AV, Safronov AP, Kurlyandskaya GV. Magnetoimpedance Thin Film Sensor for Detecting of Stray Fields of Magnetic Particles in Blood Vessel. Sensors. 2021; 21(11):3621. https://doi.org/10.3390/s21113621
Chicago/Turabian StyleMelnikov, Grigory Yu., Vladimir N. Lepalovskij, Andrey V. Svalov, Alexander P. Safronov, and Galina V. Kurlyandskaya. 2021. "Magnetoimpedance Thin Film Sensor for Detecting of Stray Fields of Magnetic Particles in Blood Vessel" Sensors 21, no. 11: 3621. https://doi.org/10.3390/s21113621
APA StyleMelnikov, G. Y., Lepalovskij, V. N., Svalov, A. V., Safronov, A. P., & Kurlyandskaya, G. V. (2021). Magnetoimpedance Thin Film Sensor for Detecting of Stray Fields of Magnetic Particles in Blood Vessel. Sensors, 21(11), 3621. https://doi.org/10.3390/s21113621






