Plasmonic Sensing Studies of a Gas-Phase Cystic Fibrosis Marker in Moisture Laden Air
Abstract
:1. Introduction
2. Detection Principle
3. Materials and Methods
3.1. Device Fabrication
3.2. System Setup
3.3. Experimental Measurements and Analysis Methods
4. Results and Discussion
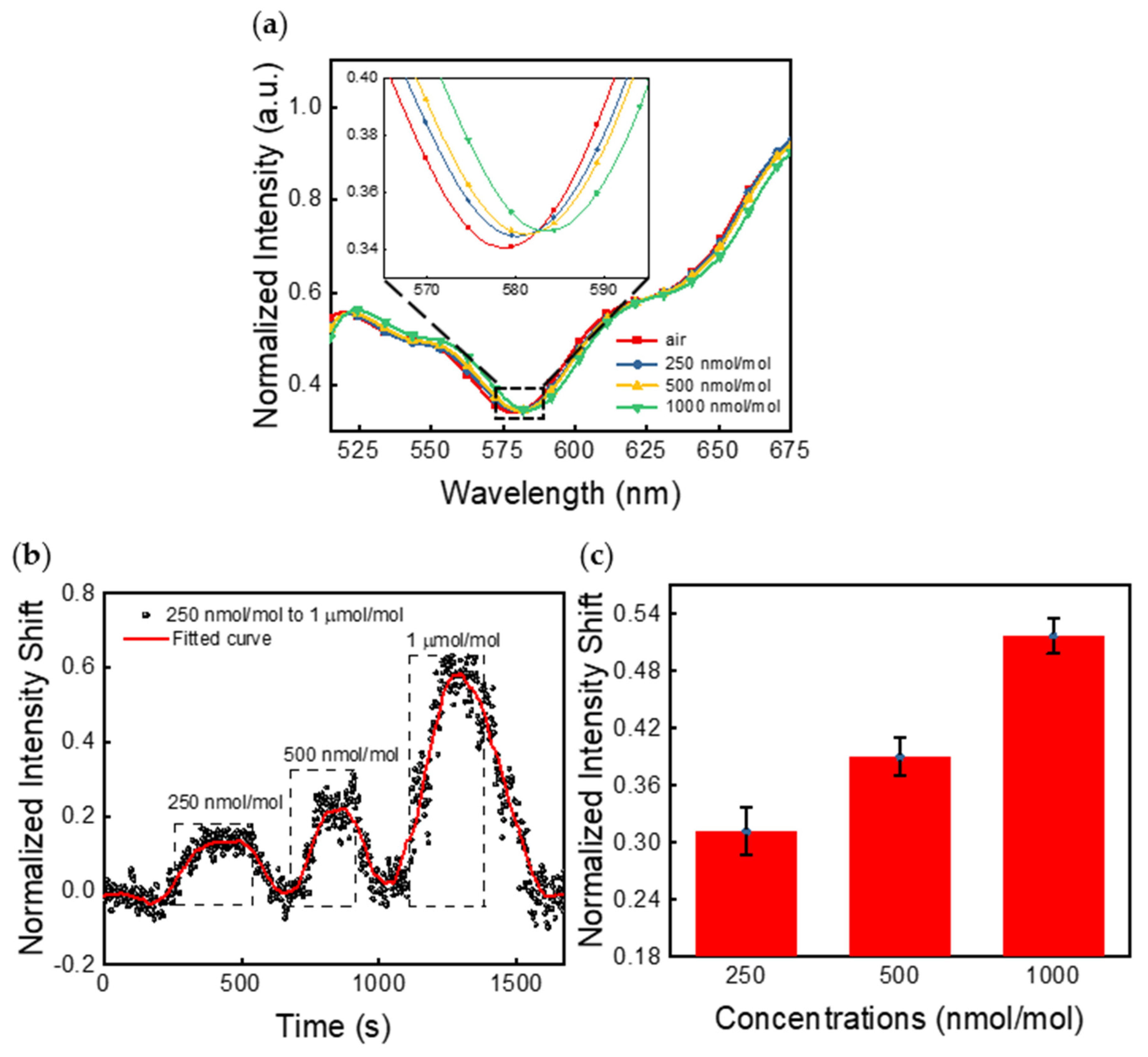
4.1. Spectrometer Measurements
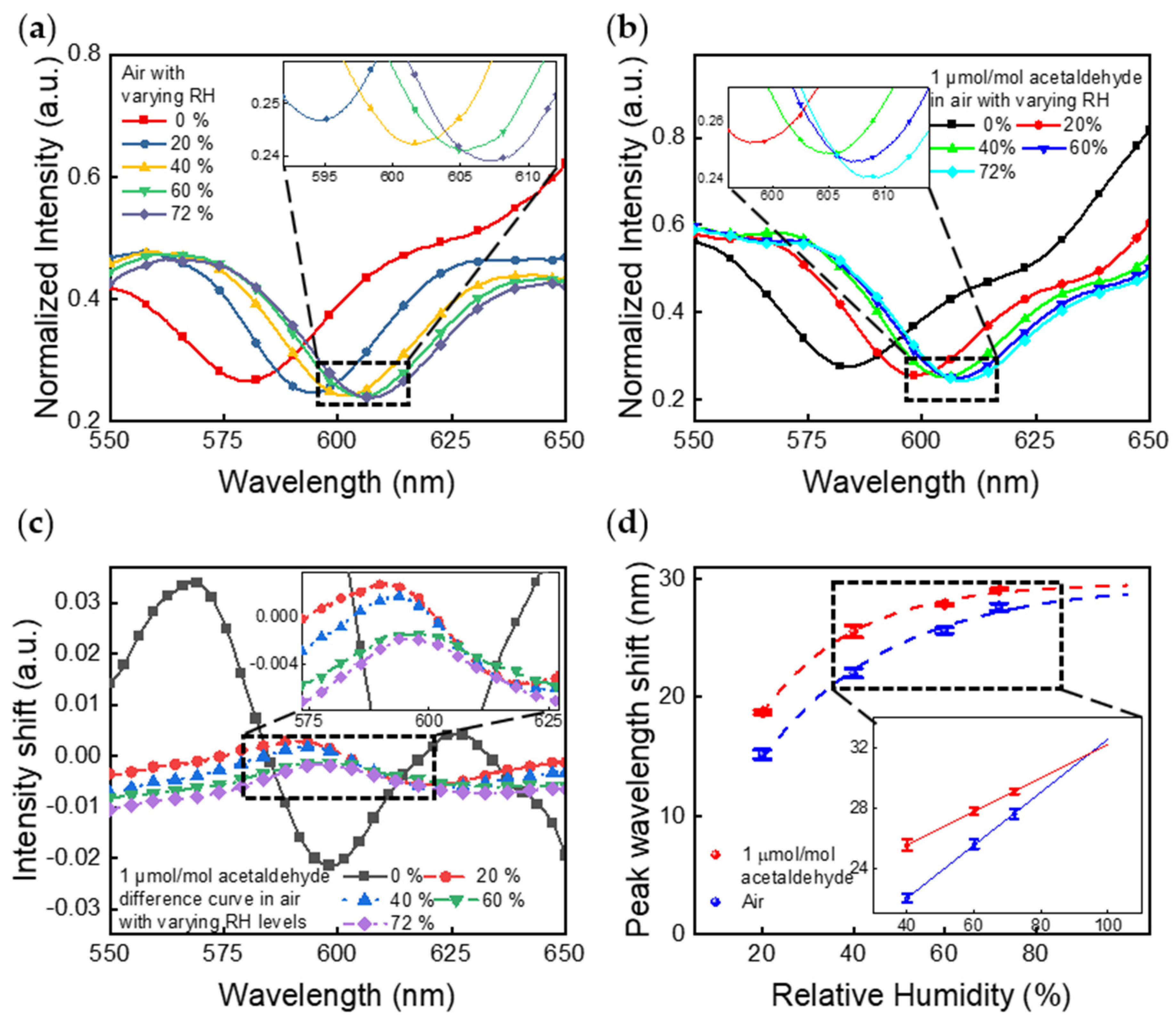
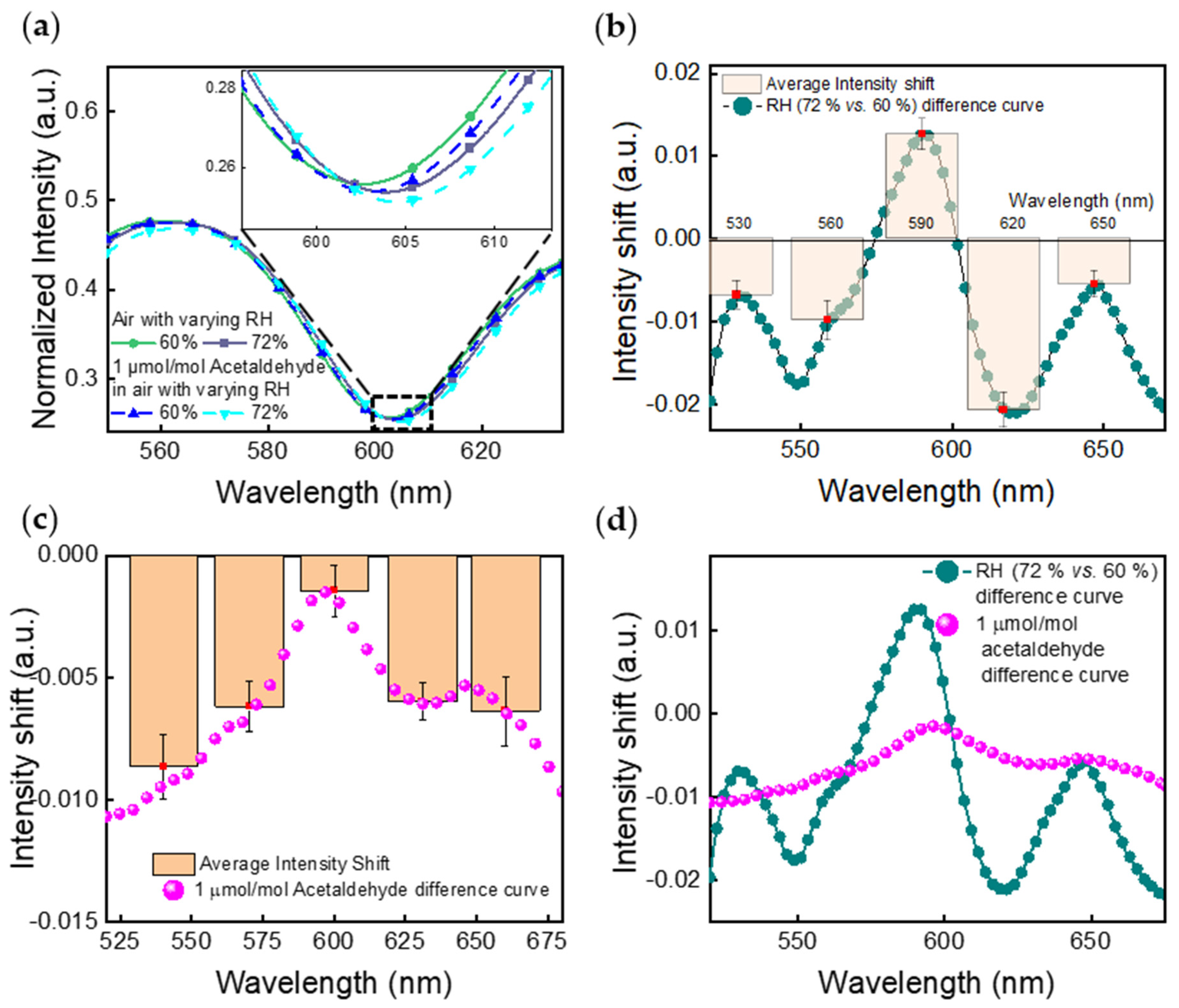
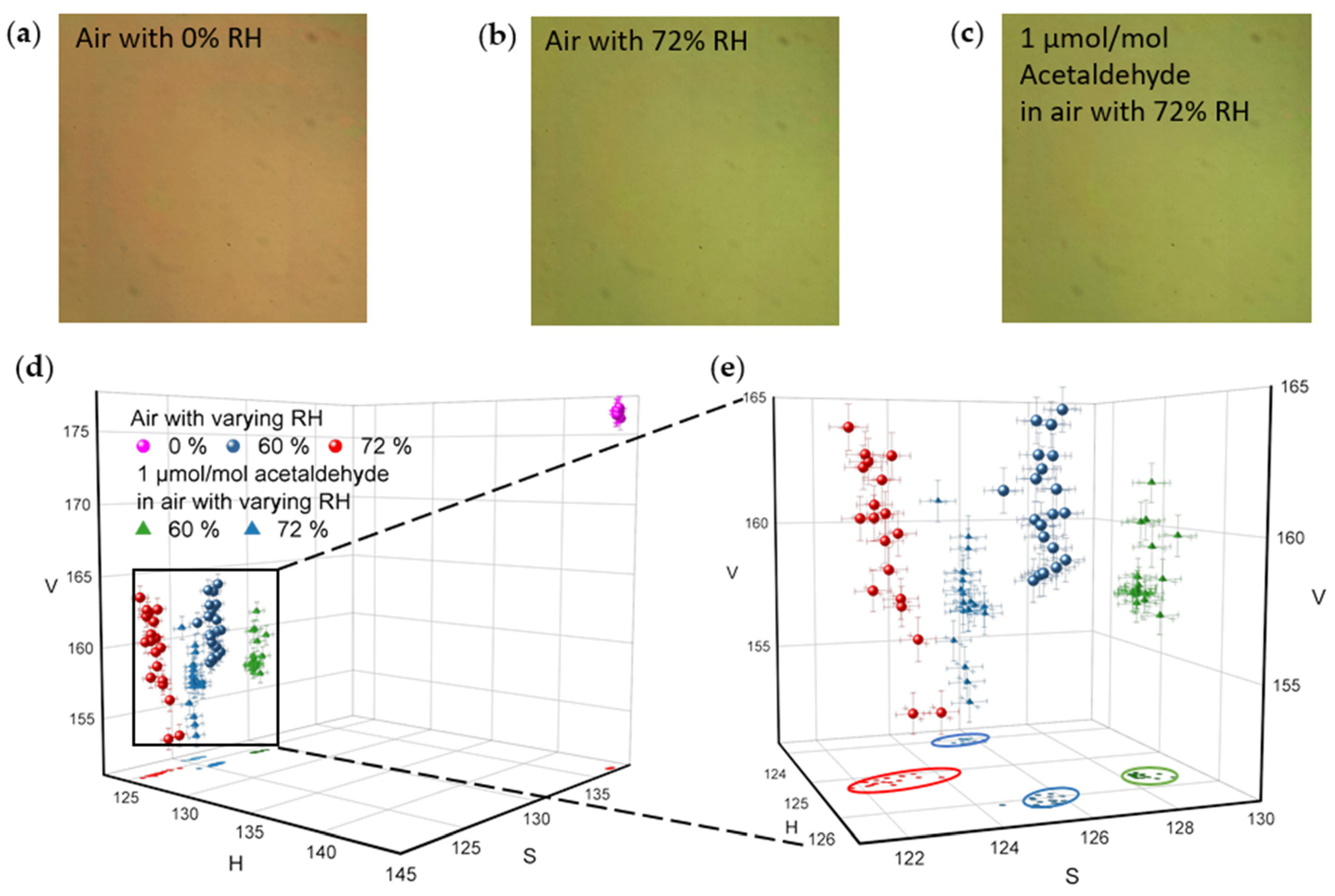
4.2. Camera Imaging
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- Keogh, R.; Seaman, S.; Barrett, J.; Taylor-Robinson, D.; Szczesniak, R. Dynamic Prediction of Survival in Cystic Fibrosis: A Landmarking Analysis using UK Patient Registry Data. Epidemiology 2019, 30, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Hamblett, N.; Boyle, M.; VanDevanter, D. Advancing Clinical Development Pathways for New CFTR Modulators in Cystic Fibrosis. Thorax 2016, 71, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Strug, L.J.; Stephenson, A.L.; Panjwani, N.; Harris, A. Recent Advances in Developing Therapeutics for Cystic Fibrosis. Hum. Mol. Genet. 2018, 27, R173–R186. [Google Scholar] [CrossRef]
- Layeghifard, M.; Li, H.; Wang, P.W.; Donaldson, S.L.; Coburn, B.; Clark, S.T.; Caballero, J.D.; Zhang, Y.; Tullis, D.E.; Yau, Y.C.W.; et al. Microbiome Networks and Change-Point Analysis Reveal Key Community Changes Associated with Cystic Fibrosis Pulmonary Exacerbations. NPJ Biofilms Microbiomes 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.H.; Gregory, R.J.; Marshall, J.; Paul, S.; Souza, D.W.; White, G.A.; O’Riordan, C.R.; Smith, A.E. Defective Intracellular Transport and Processing of CFTR is the Molecular Basis of most Cystic Fibrosis. Cell 1990, 63, 827–834. [Google Scholar] [CrossRef]
- De Boeck, K.; Wilschanski, M.; Castellani, C.; Taylor, C.; Cuppens, H.; Dodge, J.; Sinaasappel, M. Cystic Fibrosis: Terminology and Diagnostic Algorithms. Thorax 2006, 61, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPhail, G.L.; Clancy, J.P. Ivacaftor: The First Therapy Acting on the Primary Cause of Cystic Fibrosis. Drugs Today 2013, 49, 253–260. [Google Scholar] [CrossRef]
- Kramer, R.; Sauer-Heilborn, A.; Welte, T.; Guzman, C.A.; Höfle, M.G.; Abraham, W. A Rapid Method for Breath Analysis in Cystic Fibrosis Patients. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 745–751. [Google Scholar] [CrossRef]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society Technical Standard: Exhaled Biomarkers in Lung Disease. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef] [Green Version]
- Ozoemena, K.I.; Musa, S.; Modise, R.; Ipadeola, A.K.; Gaolatlhe, L.; Peteni, S.; Kabongo, G. Fuel Cell-Based Breath-Alcohol Sensors: Innovation-Hungry Old Electrochemistry. Curr. Opin. Electrochem. 2018, 10, 82–87. [Google Scholar] [CrossRef]
- Lau, H.; Yu, J.; Lee, H.; Huh, J.; Lim, J. Investigation of Exhaled Breath Samples from Patients with Alzheimer’s Disease using Gas Chromatography-Mass Spectrometry and an Exhaled Breath Sensor System. Sensors 2017, 17, 1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, N.; Jamalabadi, H.; Tavoli, F. Breath Acetone Sensors as Non-Invasive Health Monitoring Systems: A Review. JSEN 2020, 20, 5–31. [Google Scholar] [CrossRef]
- Wang, C.; Sahay, P. Breath Analysis using Laser Spectroscopic Techniques: Breath Biomarkers, Spectral Fingerprints, and Detection Limits. Sensors 2009, 9, 8230–8262. [Google Scholar] [CrossRef]
- Wojtas, J.; Bielecki, Z.; Stacewicz, T.; Mikołajczyk, J.; Nowakowski, M. Ultrasensitive Laser Spectroscopy for Breath Analysis. Opto-Electron. Rev. 2012, 20, 26–39. [Google Scholar] [CrossRef]
- Whiteson, K.L.; Meinardi, S.; Lim, Y.W.; Schmieder, R.; Maughan, H.; Quinn, R.; Blake, D.R.; Conrad, D.; Rohwer, F. Breath Gas Metabolites and Bacterial Metagenomes from Cystic Fibrosis Airways Indicate Active pH Neutral 2,3-Butanedione Fermentation. ISME J. 2014, 8, 1247–1258. [Google Scholar] [CrossRef]
- Bos, L.D.J.; Meinardi, S.; Blake, D.; Whiteson, K. Bacteria in the Airways of Patients with Cystic Fibrosis are Genetically Capable of Producing VOCs in Breath. J. Breath Res. 2016, 10, 047103. [Google Scholar] [CrossRef]
- Tardif, R. The Determination of Acetaldehyde in Exhaled Breath. Novartis Found. Symp. 2007, 285, 125. [Google Scholar] [PubMed]
- Zhao, Y.; Mukherjee, K.; Benkstein, K.D.; Sun, L.; Steffens, K.L.; Montgomery, C.B.; Guo, S.; Semancik, S.; Zaghloul, M.E. Miniaturized Nanohole Array Based Plasmonic Sensor for the Detection of Acetone and Ethanol with Insights into the Kinetics of Adsorptive Plasmonic Sensing. Nanoscale 2019, 11, 11922–11932. [Google Scholar] [CrossRef]
- Zhao, Y.; Zaghloul, M.; Lilach, Y.; Benkstein, K.; Semancik, S. Metal Organic Framework-Coated Optical VOC Gas Sensor. In Proceedings of the 2018 IEEE Photonics Conference (IPC), Reston, VA, USA, 30 September–4 October 2018; pp. 1–2. [Google Scholar]
- Sun, L.; Zhao, Y.; Dong, C.; Benkstein, K.D.; Semancik, S.; Zaghloul, M.E. Simulated and Experimental Studies of Size Effects on Plasmonic Gas Sensing with Nanohole Arrays. ECS Meet. Abstr. 2020, MA2020-01, 2360. [Google Scholar] [CrossRef]
- Zhao, Y.; Benkstein, K.D.; Semancik, S.; Zaghloul, M.E. Smart Sensing: A Plasmonic-Based Chemical Sensing System with Camera Imaging and Machine Learning Assisted Identification and Quantification. ECS Meet. Abstr. 2020, MA2020-01, 2258. [Google Scholar] [CrossRef]
- Genet, C.; Ebbesen, T.W. Light in Tiny Holes. Nature 2007, 445, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.A.; Atwater, H.A. Plasmonics: Localization and Guiding of Electromagnetic Energy in Metal/Dielectric Structures. J. Appl. Phys. 2005, 98, 11101. [Google Scholar] [CrossRef] [Green Version]
- Commercial equipment and materials are identified in order to adequately specify certain procedures. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
- Bradski, G.; Kaehler, A. OpenCV. Dr. Dobb’s J. Softw. Tools 2000, 3. [Google Scholar]

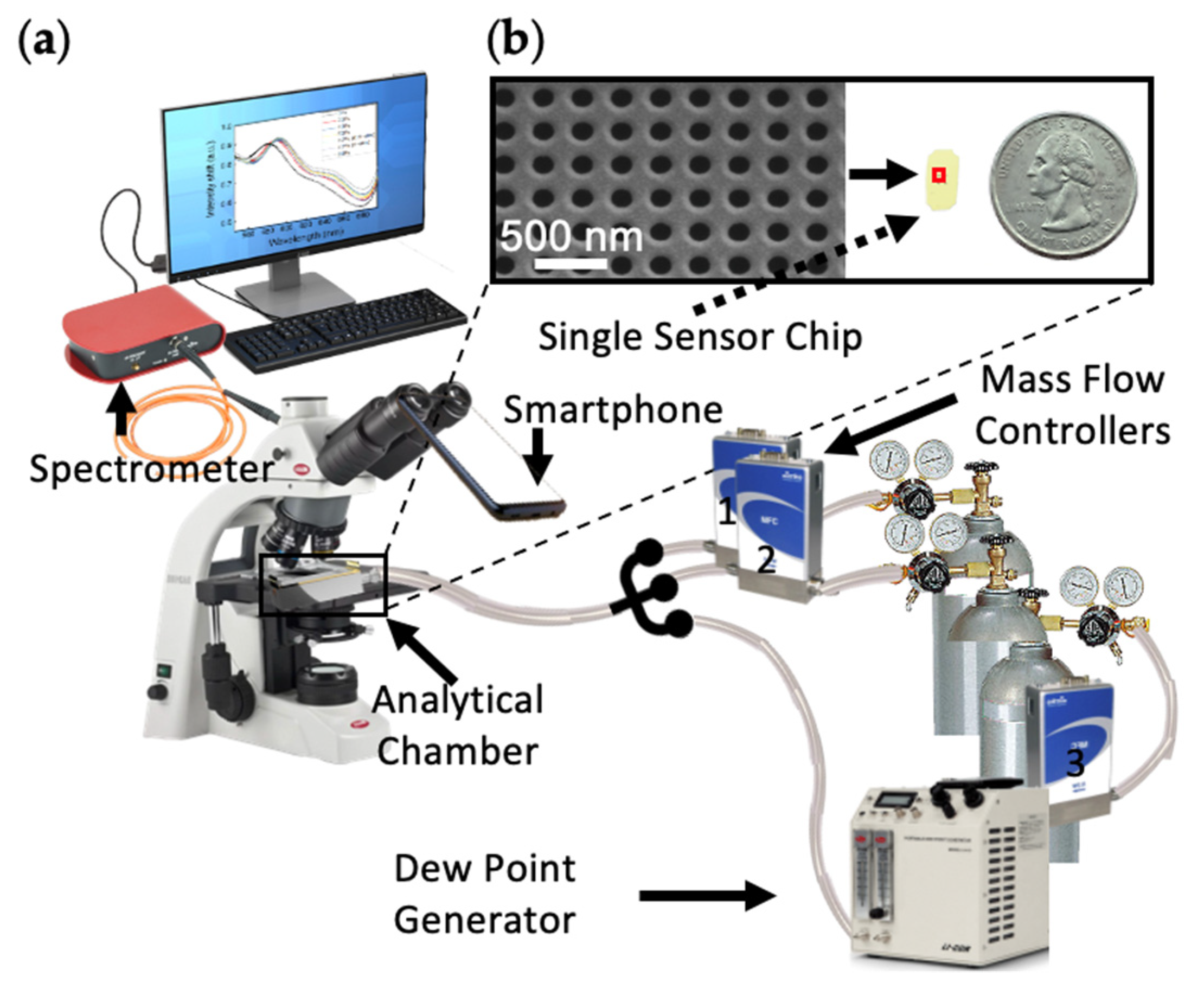

| MFC | Gas Varieties | Control Ranges 1 (sccm) |
|---|---|---|
| MFC1 | acetaldehyde | 1 to 50 |
| MFC2 | dry air | 20 to 2000 |
| MFC3 | moist air (90% RH) | 20 to 2000 |
| MFC4 2 | dry air | 1 to 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Conrad, D.; Hall, D.A.; Benkstein, K.D.; Semancik, S.; Zaghloul, M.E. Plasmonic Sensing Studies of a Gas-Phase Cystic Fibrosis Marker in Moisture Laden Air. Sensors 2021, 21, 3776. https://doi.org/10.3390/s21113776
Sun L, Conrad D, Hall DA, Benkstein KD, Semancik S, Zaghloul ME. Plasmonic Sensing Studies of a Gas-Phase Cystic Fibrosis Marker in Moisture Laden Air. Sensors. 2021; 21(11):3776. https://doi.org/10.3390/s21113776
Chicago/Turabian StyleSun, Libin, Douglas Conrad, Drew A. Hall, Kurt D. Benkstein, Steve Semancik, and Mona E. Zaghloul. 2021. "Plasmonic Sensing Studies of a Gas-Phase Cystic Fibrosis Marker in Moisture Laden Air" Sensors 21, no. 11: 3776. https://doi.org/10.3390/s21113776







