1. Introduction
Moisture and salinity stress are two of the most limiting factors in crop production. Both are significant factors influencing crop growth and final productivity in arid and semi-arid climates. In addition, climate change and global warming contribute to high rates of evaporation and, thus, a shortage of fresh water supplies related to drought, which is a challenge to global food security [
1,
2,
3]. Subsequently, soil moisture and salinity stress-induced disruption in plant function leads to a significant decrease in many morpho-physiological plant characteristics, such as chlorophyll content, water status, photosynthetic performance, stomatal conductance and biomass accumulation, which ultimately causes significant losses in the quantity of grain yield [
4,
5,
6,
7]. Therefore, the timely detection of water deficiency and salinity stress effects on large scales and the application of the right amount of water at the right time is important for assuring high yields and minimizing water loses.
Estimates of crop biophysical and biochemical traits, as well as other related properties, from remotely sensed data are critical for avoiding crop yield losses [
7,
8]. Satellite platforms of high spatial and spectral resolution and radiometric ground-based data capabilities are suitable for estimating crop traits and many studies have demonstrated the potential of remotely sensed data in mapping vegetation [
3,
7,
8,
9]. As monitoring plant status using the sample-point technique at a regional scale is tedious, laborious, and expensive, several studies have demonstrated the potential of remotely sensed data to assess plant traits. Therefore, remote sensing can be a non-destructive technique that is simple, fast, and inexpensive to perform [
9,
10,
11].
Abiotic stress causes significant changes in the biophysical and biochemical properties of vegetation canopies. The spectral signatures reflected from the canopy at particular wavelengths in the visible (VIS) and near infrared (NIR) regions of the spectrum change dramatically as a result of these effects [
12,
13,
14,
15,
16,
17].
In the developed world, airborne imagery is used to track various aspects of the Earth’s surface, especially vegetation. Some studies have shown the ability of airborne measurements to track vegetation and crop health [
11,
18,
19]. However, obtaining airborne images is extremely expensive per unit area of ground cover. When comparing coverage areas, airborne images cover a smaller area than various satellite sensors. With the availability of free medium satellite images such as sentinel 1 and 2, the cost of monitoring agricultural crops will be far cheaper in comparison to airborne missions [
6]. Another downside of airborne missions is that they are usually one-time operations, while satellite missions allow for continuous monitoring of the Earth. Using this type of imagery is difficult due to the high cost of continuous crop monitoring and the problems associated with geometric correction using airborne remote sensing in management applications [
11].
Satellites with high spatial and spectral resolution have been increasingly used in precision farming in recent years. Higher-resolution satellite sensor technology (e.g., QuickBird and GeoEye1 sensors) can provide a reliable tool in site-specific management. The spatial resolution gap between satellite and airborne images has shrunk dramatically due to these types of satellite sensors [
18]. The low revisit duration (1–3 days) is one of the key advantages of these satellites, as it is difficult to achieve with many other satellite systems [
20]. Previous studies used QuickBird satellite images to estimate plant traits such as biochemical and biophysical parameters in crops. These include for instance estimating chlorophyll content [
21], the estimation of grain yield [
22,
23], measuring leaf area index [
24,
25], the identification of nitrogen status [
26], estimating above-ground biomass [
10,
27], the detection of crop disease [
28,
29], estimating crop evapotranspiration [
30] and estimating dry matter [
31,
32].
Several satellite-based vegetation spectral indices (vegetation-SRIs) have been used to determine crop growth and final grain yield. For instance, the normalized difference vegetation index (NDVI) is often used to evaluate crop growth and yield. Leaf area index (LAI) and biomass fresh weight (BFW) were found to be responsive to the simple ratio (SR) and the green NDVI (GNDVI) [
31,
33]. The red edge NDVI (NDVIre) and red edge dependent vegetation indices like the red edge triangle vegetation index (RTVI) have been found to be responsive to LAI and above-ground biomass [
24]. Therefore, using high-resolution satellite images such as QuickBird to measure these indices can be a reliable method for mapping within-field variability at large scales. The application of remote hyperspectral sensing, using these indices which include spectra band from the VIS and NIR, offers an easy, fast, and non-destructive approach for assessing various related plant traits compared to classical methods [
15,
16,
17,
18,
19,
20]. The indirect effects, which are manifested by changes in reflectance in the VIS and NIR ranges, are linked to leaf and canopy properties such as leaf pigments, leaf structure, and scattering, which change as a result of stress factors [
16,
17].
Weather conditions in many regions worldwide may restrict the acquisition of satellite images especially cloudy regions such as European countries which can be affected in the performance of spectral indices when estimating the crop traits [
20,
21,
22,
23,
24]. Also, it is difficult sometimes to have both in situ measurements and satellite data on the same days which lead to little difference of the relationship between extracted SRIs from both platforms. However, such satellite imagery may offer farmers with better understanding the change in crop traits in other countries like Egypt since the changes in solar illumination are not that great around noon time [
6].
Furthermore, in recent years, proximal remote-sensing technologies based on spectral reflectance have been used to accurately track crops during their growing period to promote good agricultural decision-making [
34,
35,
36,
37,
38,
39]. The advantage of proximal remote sensing is that the spectral measurements are taken close to the plant canopy to eliminate the effect of environmental conditions like the clouds and can be used as references for the satellite measurements [
40,
41]. In this study, the performance of vegetation-SRIs extracted from radiometric ground-based data and QuickBird satellite imagery were evaluated in full irrigation, water and salinity stress conditions.
Multivariate regression models, such as the partial least square regression (PLSR) and multiple linear regression (MLR), have substantially increased the efficiency of predicting the plant traits based on spectrum data. The PLSR and MLR have been proposed to resolve the strong multi-collinear and noisy variables in SRIs or spectral band data and to efficiently assess the measured traits [
36,
42,
43,
44]. Both methods combine a large number of SRIs or spectral bands into a single index to enhance the prediction of measured traits. Therefore, using these methods, the measured trait of maize can be simultaneously estimated through several vegetation-SRIs. PLSR can reduce a large number of calculated collinear spectral factors to a few non-correlated latent factors, avoiding over-fitting or under-fitting the data, and avoiding redundant data [
45].
There is little information available to evaluate the PLSR and MLR methods based on the combination vegetation-SRIs derived from both radiometric ground-based data and QuickBird satellite imagery to predict the LAI, BFW and Chlm of maize. Therefore, the purpose of this work was to (i) assess the effects of different stress conditions on three measured traits (LAI, BFW and Chlm) of maize; (ii) evaluate the performance of classification algorithms to map different crop types within the study area; (iii) evaluate the efficiency of radiometric ground-based data and QuickBird satellite imagery based on vegetation-SRIs to assess the three measured traits of maize across full irrigation, water stress and salinity stress conditions; and (v) evaluate the performance of PLSR and MLR models based on vegetation-SRI-derived radiometric ground-based data and QuickBird satellite imagery and their combination to predict the three measured traits of maize.
2. Materials and Methods
2.1. Study Site Description
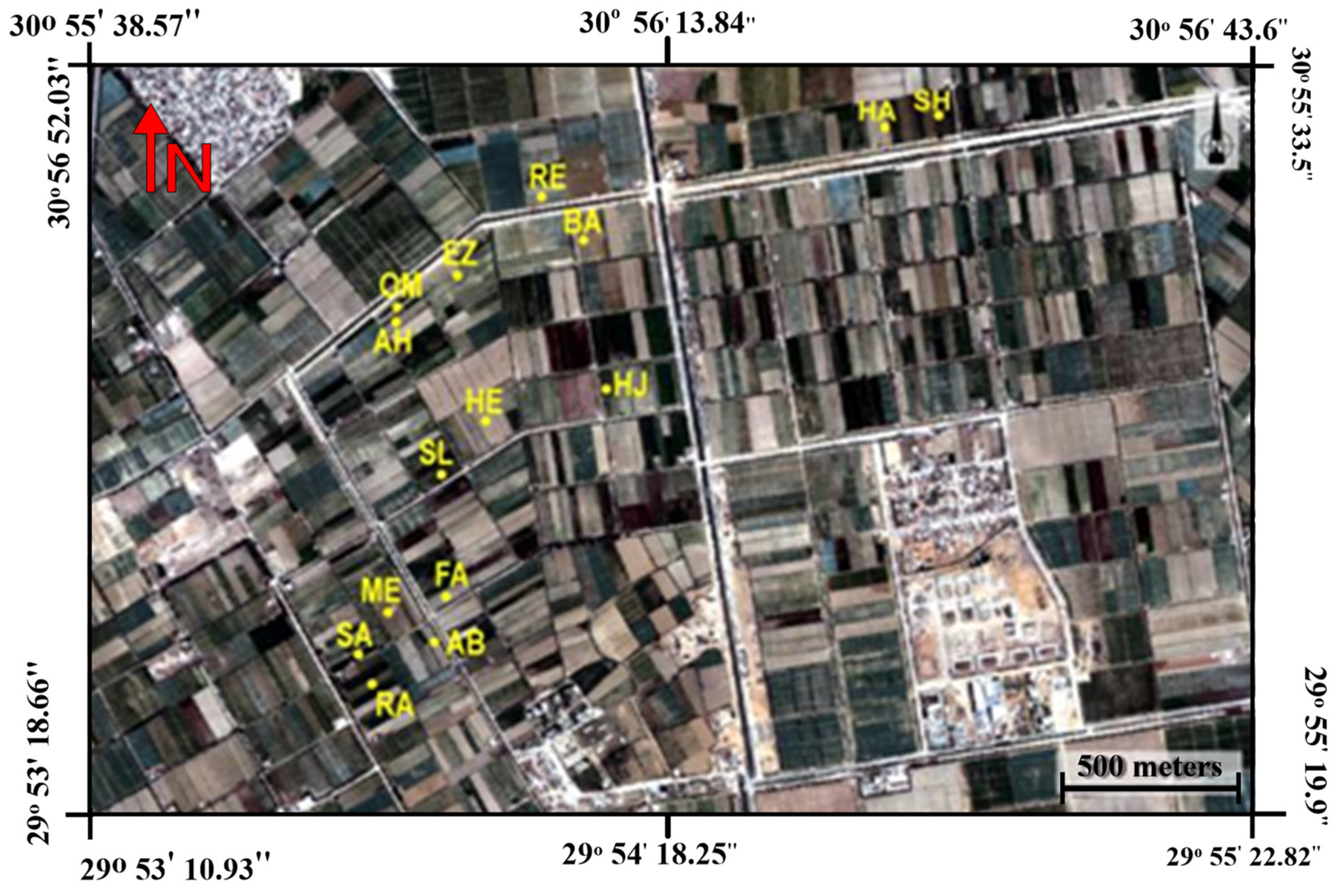
The study area is located in south-west Alexandria, Egypt (latitude of 30°55′50″ and longitude of 29°53′35.6″). To have different growing conditions for maize such as full irrigation, water and salinity stress, 15 fields were chosen (
Figure 1). The weather in this region is characterized by hot summers and mild winters. The detailed climatic parameters of the study area during the experimental periods are shown in
Table 1. During field visits, soil samples were collected from random fields to identify some soil chemical and physical properties. The soil at these sites is mainly a sandy loam, as these sites have been recently reclaimed from the Eastern Desert. Flood irrigation is the main irrigation system used within the study area. In this region and with the system of traditional irrigation fields at the end of an irrigation canal network, many fields suffer from water scarcity. Farmers at the end of the irrigation network are forced to use agricultural drainage water with high salinity levels as an alternative to fresh water to irrigate their crops, even with the risk of salinity stress. Other farmers wait until fresh water is available to irrigate their crops and, therefore, subject them to water stress, which leads to reductions in crop growth traits. In the 15 different fields, the maize was sown in the first week of May and was harvested in the last week of September.
2.2. Measured Plant Traits of Maize
Three samples from each field of study were collected immediately following reflectance measurements from maize canopies to quantify different measured traits (LAI, BFW and Chlm). Following the collection of the spectral data, a square area of 1 m length from the same locations of spectra acquisition was sampled at the ground level, and its above-ground biomass fresh weight was immediately identified. This was repeated three times and the average values were used to keep variations at a minimum. LAI was calculated as the ratio between total leaf area of a certain number of harvested plants and the area occupied by these plants. After calculating the leaf area for each sample point, the LAI was calculated by dividing the total leaf area for each sample by the area occupied by these plants according to Elmetwalli [
6] as follows:
where LAI is the leaf area index, LA is the leaf area per sample, and OA is the occupied land area.
Chlorophyll was measured during field work visits using a hand-held SPAD (Soil Plant Analysis Development)-502 m (Minolta, Osaka, Japan). Apical leaves were chosen to measure Chlm. To reduce variability, Chlm was measured at three different locations on each leaf from the leaf tip to the leaf base and the average of these three readings was calculated.
2.3. Ground-Based Remote-Sensing Measurements
For maize, radiometric ground-based measurements were undertaken throughout 15 fields in the study area during the summer growing season of 2007, concurrent with the acquisition of QuickBird satellite imagery. From June 28 to 30, spectra were obtained from random fields, taking into account the scale of the field and the stress status of these fields. The reflectance of crop canopies was measured using an ASD FieldSpec handheld spectroradiometer. The ASD FieldSpec has two units, one connected to a diffuser to measure the light radiation as a reference signal and the second unit captures the spectral reflectance from the canopy at 300 and 1000 nm and is interpolated to a final spectral resolution of 0.5 nm. The instrument was pointed at the same angle (nadir position) to minimize changes. To keep illumination difference at a minimum, the canopy reflectance was calculated by using a calibration factor obtained from a white reference standard which was a custom built to correct readings from the spectrometer unit. This was done prior collecting reflectance at every location. Finally, the spectral reflectance was smoothed to eliminate noise at the beginning and end of the electromagnetic spectrum by passing it through a 5 nm running mean filter over the whole spectrum. Spectral reflectance from the visible (VIS) and near infrared (NIR) was used to calculate the selected spectral indices. To keep consistent illumination over field work visits, spectra measurements were restricted at noon to keep the variation at a minimum and to minimize the influence of changes in solar zenith angle. During spectra collection, an iron stand of 3 m height was used to keep the spectrometer at a consistent distance from the soil surface.
2.4. Remote-Sensing Image Acquisition, Processing and Analysis
QuickBird high spatial resolution images of the maize crop within the study area were acquired. The QuickBird satellite is a high spatial resolution satellite comprising four multi spectral bands (blue, green, red and near infrared) of 2.4 m spatial resolution. The QuickBird satellite image of maize fields was acquired at 09:13 h GMT on 29 June 2007. The image was radiometrically corrected by the supplier of the image (Infoterra group based in the UK). The images were geo-corrected using an image to image technique using ground control points (GCP) collected for fixed points during various field visits covering the entire study site. The images were atmospherically corrected using the FLAASH (Fast Line-of-sight Atmospheric Analysis of Hypercubes) module technique in ENVI v4.9. FLAASH is considered to be more accurate than non-physics-based models such as QUAC (QUick Atmospheric Correction) and is a remarkably established atmospheric compensation algorithm and it is mainly supportive for the majority of multi and hyper spectral remote-sensing platforms. Images were also classified using both unsupervised classification (k-means) and supervised classification (MLC; Maximum Likelihood Classification) to identify different crops in each image. K-means unsupervised and maximum likelihood supervised algorithms were used to identify different crops in the study site. K-means is one of the commonly used unsupervised classification algorithms and during the calculation of overall classification efficiency and Kappa coefficient. We tried both k-means and iso data algorithms and k-means produced higher efficiencies. These two algorithms were performed on QuickBird using the ENVI v4.9 package. The advantage of the k-means is that no additional ground reference points are required to perform the classification. Unlike the k-means technique, MLC requires a reference dataset during imagery acquisition to perform this algorithm. A confusion matrix was derived for both k-means and MLC classifications of the QuickBird satellite imagery. To perform the supervised algorithm, a validation dataset, which was independent from the training dataset, was created manually. The validation dataset included at least 2000 pixels for each class to avoid interference between various classes.
2.5. Calculating Vegetation Spectral Indices
Ten commonly used broad band vegetation-SRIs were derived from both radiometric ground-based data and QuickBird satellite imagery to assess the ability of remote sensing to detect maize traits.
Table 2 summarizes the formulae of varying vegetation-SRIs along with references. The vegetation-SRIs were selected based on their sensitivity to changes in biomass, leaf pigmentation, leaf/tissue structure and plant water content.
2.6. Partial Least Squares Regression
Partial least squares regression (PLSR) is a versatile tool that can easily manage data when the number of input variables is much greater than the number of target variables, and the input variables have a lot of collinearity and noise [
55,
56]. In this study, to link the input variables (vegetation-SRIs listed in
Table 1, which are derived from both radiometric ground-based data and QuickBird satellite imagery) to the output variables (three destructively measured traits), PLSR was combined with leave-one-out cross-validation (LOOCV). An important step in PLSR analysis is to select the optimal number of latent variables (LVs) in order to represent the calibration data without over-fitting or under-fitting. LVs parameter was calculated using the (LOOCV) according to the lowest value of the root means squared error (RMSE). Random 10-fold cross-validation was applied on the datasets to increase the robustness of the results as indicated by the software program Unscrambler X software version 10.2 (CAMO Software AS, Oslo). The performance of PLSR models based on SRIs derived from two methods and their combination were evaluated to predict the four measured traits of maize and wheat. The best model for both calibration (Cal.) and validation (Val.) was chosen depending on the lowest value of RMSE and mean absolute deviations (MAD) as well as the highest value for R
2.
2.7. Multiple Linear Regression
Multiple linear regression (MLR) is a regression technique that analyzes a dependent parameter (destructively measured traits) using two or more independent parameters (SRIs are listed in
Table 1, which were derived from both radiometric ground-based data and QuickBird satellite imagery). MLR attempts to model the linear relationship between the independent and the response (dependent) variable [
36,
37,
38]. In addition, MLR was used to predict three measured traits as PLSR. The best model for both Cal. and Val. was also chosen depending on the lowest value of RMSE and mean absolute deviations (MAD) as well as the highest value for R
2. The least squares approach was used to calculate the parameters using the regression equation, which minimizes the sum of the errors squared. The formula of MLR is:
where, for
i =
n observations, Y
i = dependent variable, xi = explanatory variables, β
0 = y-intercept (constant term), β
p = slope coefficients for each explanatory variable, ϵ = the model’s error term (also known as the residuals).
2.8. Statistical Analysis
This statistical analysis was performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Data were checked for normality using the Anderson–Darling method with 95% significance level. Additionally, a simple linear regression of the relationship between the selected SRIs and the three plant traits was performed using SigmaPlot version 11.0 (Systat Software Inc., San Jose, CA, USA) to identify optimal SRIs based on the highest value for R2. The significance level of the coefficients of determination (R2) for these relationships was set at the 0.05 probability level.