Real-Time High-Level Acute Pain Detection Using a Smartphone and a Wrist-Worn Electrodermal Activity Sensor
Abstract
:1. Introduction
2. Methods
2.1. EDA Features
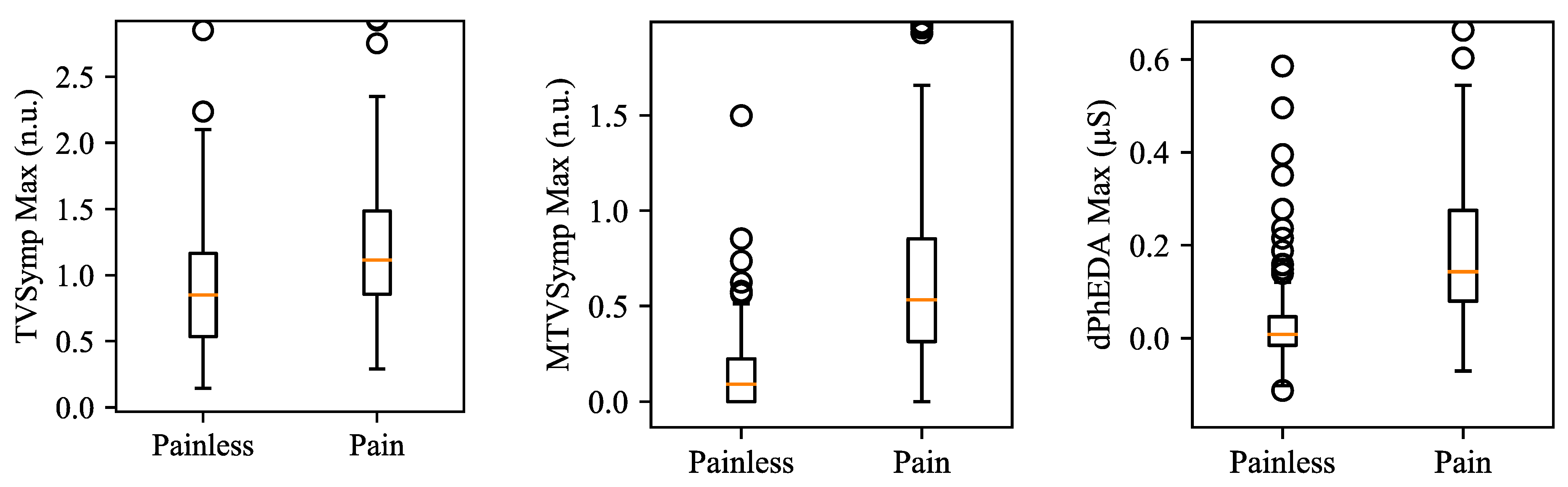
2.1.1. Time-Varying Index of Sympathetic Activity (TVSymp) and Modified TVSymp (MTVSymp)
2.1.2. Derivative of Phasic Component of EDA (dPhEDA)
2.2. Smartphone Application Development
2.3. Experiments
2.3.1. Thermal Grill Experiment
2.3.2. Electrical Pulse Experiment
2.4. Statistics
2.5. Machine Learning
3. Results
3.1. Electrical Pulse
3.2. Thermal Grill
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radnovich, R.; Chapman, C.R.; Gudin, J.A.; Panchal, S.J.; Webster, L.R.; Pergolizzi, J.V., Jr. Acute pain: Effective management requires comprehensive assessment. Postgrad. Med. 2014, 126, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Dunwoody, C.J.; Krenzischek, D.A.; Pasero, C.; Rathmell, J.P.; Polomano, R.C. Assessment, physiological monitoring, and consequences of inadequately treated acute pain. Pain Manag. Nurs. 2008, 9, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.; Tripp, H.; Halksworth-Smith, G. Assessment and management of acute pain in older people: Barriers and facilitators to nursing practice. Aust. J. Adv. Nurs. 2017, 35, 48. [Google Scholar]
- Chen, Q.; Larochelle, M.R.; Weaver, D.T.; Lietz, A.P.; Mueller, P.P.; Mercaldo, S.; Wakeman, S.E.; Freedberg, K.A.; Raphel, T.J.; Knudsen, A.B. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw. Open 2019, 2, e187621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Compton, W.M.; Blanco, C.; Jones, C.M. Correlates of prescription opioid use, misuse, use disorders, and motivations for misuse among US adults. J. Clin. Psychiatry 2018, 79, 79. [Google Scholar] [CrossRef]
- Volkow, N.D.; Jones, E.B.; Einstein, E.B.; Wargo, E.M. Prevention and treatment of opioid misuse and addiction: A review. JAMA Psychiatry 2019, 76, 208–216. [Google Scholar] [CrossRef]
- Wilson, N. Drug and opioid-involved overdose deaths—United States, 2017–2018. Mmwr. Morb. Mortal. Wkly. Rep. 2020, 69, 290–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florence, C.; Luo, F.; Xu, L.; Zhou, C. The Economic burden of prescription opioid overdose, abuse and dependence in the United States, 2013. Med. Care 2016, 54, 901–906. [Google Scholar] [CrossRef]
- Adibuzzaman, M.; Ostberg, C.; Ahamed, S.; Povinelli, R.; Sindhu, B.; Love, R.; Kawsar, F.; Ahsan, G.M.T. Assessment of pain using facial pictures taken with a smartphone. In Proceedings of the 2015 IEEE 39th Annual Computer Software and Applications Conference, Taichung, Taiwan, 1–5 July 2015; Volume 2, pp. 726–731. [Google Scholar]
- Rosser, B.A.; Eccleston, C. Smartphone applications for pain management. J. Telemed. Telecare 2011, 17, 308–312. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahsan, G.M.T.; Ahamed, S.I.; Love, R.; Salim, R. Pain level detection from facial image captured by smartphone. J. Inf. Process. 2016, 24, 598–608. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Mieronkoski, R.; Syrjälä, E.; Anzanpour, A.; Terävä, V.; Rahmani, A.M.; Salanterä, S.; Aantaa, R.; Hagelberg, N.; Liljeberg, P. Acute pain intensity monitoring with the classification of multiple physiological parameters. J. Clin. Monit. Comput. 2019, 33, 493–507. [Google Scholar] [CrossRef] [Green Version]
- Posada-Quintero, H.F.; Chon, K.H. Innovations in electrodermal activity data collection and signal processing: A systematic review. Sensors 2020, 20, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, Á.D.; Chon, K.H. Highly sensitive index of sympathetic activity based on time-frequency spectral analysis of electrodermal activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R582–R591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Y.; Posada-Quintero, H.F.; Chon, K.H. Pain detection using a smartphone in real time. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, EMBS Virtual Academy, Montreal, QC, Canada, 20–24 July 2020; pp. 4526–4529. [Google Scholar]
- Xu, X.; Susam, B.T.; Nezamfar, H.; Diaz, D.; Craig, K.D.; Goodwin, M.S.; Akcakaya, M.; Huang, J.S.; De Sa, V.R. Towards automated pain detection in children using facial and electrodermal activity. In Proceedings of the International Workshop on Artificial Intelligence in Health, Stockholm, Sweden, 13–14 July 2018; pp. 181–189. [Google Scholar]
- Susam, B.T.; Akcakaya, M.; Nezamfar, H.; Diaz, D.; Xu, X.; De Sa, V.R.; Craig, K.D.; Huang, J.S.; Goodwin, M.S. Automated pain assessment using electrodermal activity data and machine learning. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Honolulu, HI, USA, 18–21 July 2018; pp. 372–375. [Google Scholar]
- Bari, D.S.; Aldosky, H.Y.Y.; Tronstad, C.; Kalvøy, H.; Martinsen, Ø.G. Electrodermal activity responses for quantitative assessment of felt pain. J. Electr. Bioimpedance 2018, 9, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Aqajari, S.A.H.; Cao, R.; Naeini, E.K.; Calderon, M.-D.; Zheng, K.; Dutt, N.; Liljeberg, P.; Salanterä, S.; Nelson, A.M.; Rahmani, A.M. Pain assessment tool with electrodermal activity for postoperative patients: Method validation study. JMIR Mhealth Uhealth 2021, 9, e25258. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Golshan, H.M.; Mahoor, M.H. A wavelet-based approach to emotion classification using EDA signals. Expert Syst. Appl. 2018, 112, 77–86. [Google Scholar] [CrossRef]
- Sharma, V.; Prakash, N.R.; Kalra, P. EDA wavelet features as social anxiety disorder (SAD) estimator in adolescent females. In Proceedings of the 2016 International Conference on Advances in Computing, Communications and Informatics, Jaipur, India, 21–24 September 2016; pp. 1843–1846. [Google Scholar]
- Kong, Y.; Posada-Quintero, H.; Chon, K. Sensitive physiological indices of pain based on differential characteristics of electrodermal activity. IEEE Trans. Biomed. Eng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Siu, K.; Ju, K.; Chon, K.H. A high resolution approach to estimating time-frequency spectra and their amplitudes. Ann. Biomed. Eng. 2006, 34, 326–338. [Google Scholar] [CrossRef]
- Greco, A.; Valenza, G.; Lanata, A.; Scilingo, E.P.; Citi, L. CvxEDA: A convex optimization approach to electrodermal activity processing. IEEE Trans. Biomed. Eng. 2015, 63, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Sauer, T. Numerical Analysis, 3rd ed.; Pearson: London, UK, 2013; ISBN 978-0-13-469645-4. [Google Scholar]
- Burns, A.; Greene, B.R.; McGrath, M.J.; O’Shea, T.J.; Kuris, B.; Ayer, S.M.; Stroiescu, F.; Cionca, V. SHIMMERTM–A wireless sensor platform for noninvasive biomedical research. IEEE Sens. J. 2010, 10, 1527–1534. [Google Scholar] [CrossRef]
- Eigen V3. Available online: https://eigen.tuxfamily.org (accessed on 7 June 2021).
- Thunberg, T. Förnimmelserna vid till samma ställe lokaliserad, samtidigt p\aag\aaende köld-och värmeretning. Upps. Läkfören Förh 1896, 2, 489–495. [Google Scholar]
- Craig, A.D.; Bushnell, M.C. The thermal grill illusion: Unmasking the burn of cold pain. Science 1994, 265, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Defrin, R.; Benstein-Sheraizin, A.; Bezalel, A.; Mantzur, O.; Arendt-Nielsen, L. The spatial characteristics of the painful thermal grill illusion. Pain 2008, 138, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G. Temperature perception and nociception. J. Neurobiol. 2004, 61, 13–29. [Google Scholar] [CrossRef]
- Lindstedt, F.; Johansson, B.; Martinsen, S.; Kosek, E.; Fransson, P.; Ingvar, M. Evidence for thalamic involvement in the thermal grill illusion: An FMRI study. PLoS ONE 2011, 6, e27075. [Google Scholar] [CrossRef]
- Craig, A.D. Can the basis for central neuropathic pain be identified by using a thermal grill? Pain 2008, 135, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Posada-Quintero, H.F.; Kong, Y.; Nguyen, K.; Tran, C.; Beardslee, L.; Chen, L.; Guo, T.; Cong, X.; Feng, B.; Chon, K.H. Using electrodermal activity to validate multilevel pain stimulation in healthy volunteers evoked by thermal grills. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R366–R375. [Google Scholar] [CrossRef] [PubMed]
- Ciortan, M. Overview of feature selection methods. Towards Data Science. 26 July 2019. Available online: https://towardsdatascience.com/overview-of-feature-selection-methods-a2d115c7a8f7 (accessed on 18 July 2020).
- Bradley, A.P. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recognit. 1997, 30, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.G.; Smouse, P.E.; Scofield, D.G.; Sork, V.L. What seeds tell us about birds: A multi-year analysis of acorn woodpecker foraging movements. Mov. Ecol. 2014, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Lme4: Linear mixed-effects models using eigen and S4. R Package Version 1.1-7. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Massey, F.J., Jr. The Kolmogorov-Smirnov test for goodness of fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-Learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Royer, C.W.; O’Neill, M.; Wright, S.J. A Newton-CG Algorithm with complexity guarantees for smooth unconstrained optimization. Math. Program. 2020, 180, 451–488. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.C.; Nocedal, J. On the limited memory BFGS method for large scale optimization. Math. Program. 1989, 45, 503–528. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Le Roux, N.; Bach, F. Minimizing finite sums with the stochastic average gradient. arXiv 2013, arXiv:1309.2388. [Google Scholar]
- Defazio, A.; Bach, F.; Lacoste-Julien, S. SAGA: A fast incremental gradient method with support for non-strongly convex composite objectives. In Proceedings of the Advances in Neural Information Processing Systems, Montreal, QC, Canada, 8–13 December 2014; pp. 1646–1654. [Google Scholar]
- Jibb, L.A.; Stevens, B.J.; Nathan, P.C.; Seto, E.; Cafazzo, J.A.; Johnston, D.L.; Hum, V.; Stinson, J.N. Implementation and preliminary effectiveness of a real-time pain management smartphone app for adolescents with cancer: A multicenter pilot clinical study. Pediatric Blood Cancer 2017, 64, e26554. [Google Scholar] [CrossRef] [PubMed]
- Jibb, L.; Nathan, P.C.; Breakey, V.; Fernandez, C.; Johnston, D.; Lewis, V.; McKillop, S.; Patel, S.; Sabapathy, C.; Strahlendorf, C. Pain squad+ smartphone app to support real-time pain treatment for adolescents with cancer: Protocol for a randomised controlled trial. BMJ Open 2020, 10, e037251. [Google Scholar] [CrossRef] [Green Version]
- Thurnheer, S.E.; Gravestock, I.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Benefits of mobile apps in pain management: Systematic review. JMIR Mhealth Uhealth 2018, 6, e11231. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.L.; Fillingim, R.B.; Keefe, F. Race, ethnicity and pain. Pain 2001, 94, 133–137. [Google Scholar] [CrossRef]
- Woodrow, K.M.; Friedman, G.D.; Siegelaub, A.B.; Collen, M.F. Pain tolerance: Differences according to age, sex and race. Psychosom. Med. 1972, 34, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Kvachadze, I.; Tsagareli, M.G.; Dumbadze, Z. An overview of ethnic and gender differences in pain sensation. Georgian Med. News 2015, 102–108. [Google Scholar]
- Pillay, T.; Van Zyl, H.A.; Blackbeard, D. Chronic pain perception and cultural experience. Procedia Soc. Behav. Sci. 2014, 113, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Bari, D.S. Gender differences in tonic and phasic electrodermal activity components. Sci. J. Univ. Zakho 2020, 8, 29–33. [Google Scholar] [CrossRef]









| Classifiers | Parameters | Values |
|---|---|---|
| Support Vector Machine | C | 1, 10, 100, 1000 |
| Gamma | 0.0001, 0.0001, 0.001, 0.1 | |
| Decision Tree and Random Forest | Criterion | Gini, Entropy |
| Multi-layer Perceptron | Hidden Layer | 1, 2, 3 (Hidden Unit: 100) |
| Activation | Logistic, tanh, rectifier linear unit | |
| Solver | Stochastic gradient descent, Adam, LBFGS | |
| Learning rate | 0.0001, 0.001, 0.01 | |
| Logistic Regression | Solver | Newton-CG, LBFGS, Lib Linear, SAG, SAGA |
| K-nearest neighbors | K | 3, 5, 7, 9 |
| Features | Fisher’s Ratio | AUROC | ||||
|---|---|---|---|---|---|---|
| Mean | Max | Mean | 95% CI of Mean | Max | 95% CI of Max | |
| TVSymp | 0.272 | 0.591 | 0.660 | 0.613–0.707 | 0.746 | 0.704–0.789 |
| MTVSymp | 0.810 | 0.954 | 0.852 | 0.819–0.885 | 0.877 | 0.847–0.908 |
| dPhEDA | 0.566 | 0.567 | 0.829 | 0.793–0.865 | 0.821 | 0.784–0.857 |
| Classifiers | Protocol | Accuracy (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| Support Vector Machine Linear (L-SVM) | 1 | 0.808 (0.751–0.862) | 0.752 (0.623–0.860) | 0.863 (0.803–0.918) |
| 2 | 0.795 (0.739–0.851) | 0.670 (0.578–0.762) | 0.920 (0.867–0.973) | |
| 3 | 0.818 (0.776–0.861) | 0.771 (0.705–0.836) | 0.866 (0.813–0.919) | |
| Support Vector Machine 3rd order Polynomial (P-SVM) | 1 | 0.774 (0.708–0.835) | 0.813 (0.687–0.921) | 0.736 (0.645–0.820) |
| 2 | 0.780 (0.723–0.837) | 0.670 (0.578–0.762) | 0.890 (0.829–0.951) | |
| 3 | 0.729 (0.680–0.778) | 0.873 (0.820–0.925) | 0.586 (0.509–0.663) | |
| Support Vector Machine Radial basis function (R-SVM) | 1 | 0.811 (0.750–0.870) | 0.755 (0.621–0.861) | 0.867 (0.805–0.923) |
| 2 | 0.795 (0.739–0.851) | 0.640 (0.546–0.734) | 0.950 (0.907–0.993) | |
| 3 | 0.815 (0.772–0.858) | 0.752 (0.684–0.819) | 0.879 (0.828–0.930) | |
| Decision Tree | 1 | 0.761 (0.706–0.812) | 0.733 (0.624–0.826) | 0.789 (0.728–0.849) |
| 2 | 0.625 (0.558–0.692) | 0.290 (0.238–0.422) | 0.960 (0.953–1.000) | |
| 3 | 0.764 (0.717–0.811) | 0.796 (0.733–0.859) | 0.732 (0.663–0.802) | |
| Random Forest | 1 | 0.815 (0.754–0.869) | 0.789 (0.662–0.900) | 0.842 (0.784–0.896) |
| 2 | 0.655 (0.589–0.721) | 0.330 (0.238–0.422) | 0.980 (0.953–1.000) | |
| 3 | 0.809 (0.765–0.852) | 0.796 (0.733–0.859) | 0.822 (0.762–0.882) | |
| Multi-layer Perceptron (MLP) | 1 | 0.796 (0.733–0.855) | 0.759 (0.617–0.873) | 0.833 (0.760–0.899) |
| 2 | 0.800 (0.745–0.855) | 0.660 (0.567–0.753) | 0.940 (0.893–0.987) | |
| 3 | 0.701 (0.650–0.751) | 0.904 (0.858–0.950) | 0.497 (0.419–0.575) | |
| Logistic Regression | 1 | 0.813 (0.757–0.869) | 0.754 (0.602–0.880) | 0.873 (0.816–0.926) |
| 2 | 0.800 (0.745–0.855) | 0.670 (0.578–0.762) | 0.930 (0.880–0.980) | |
| 3 | 0.831 (0.790–0.873) | 0.866 (0.813–0.919) | 0.796 (0.733–0.859) | |
| K-nearest Neighbors (KNN) | 1 | 0.780 (0.719–0.833) | 0.799 (0.678–0.894) | 0.760 (0.689–0.825) |
| 2 | 0.795 (0.739–0.851) | 0.670 (0.578–0.762) | 0.920 (0.880–0.980) | |
| 3 | 0.806 (0.762–0.849) | 0.803 (0.740–0.865) | 0.809 (0.747–0.870) |
| Features | Fisher’s Ratio | AUROC | ||||
|---|---|---|---|---|---|---|
| Mean | Max | Mean | 95% CI of Mean | Max | 95% CI of Max | |
| TVSymp | 0.142 | 0.495 | 0.576 | 0.513–0.639 | 0.698 | 0.640–0.756 |
| MTVSymp | 0.755 | 0.948 | 0.852 | 0.809–0.894 | 0.893 | 0.857–0.930 |
| dPhEDA | 0.709 | 0.715 | 0.872 | 0.832–0.912 | 0.888 | 0.851–0.925 |
| Classifiers | Accuracy (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|
| Support Vector Machine Linear (L-SVM) | 0.819 (0.755–0.874) | 0.795 (0.668–0.890) | 0.844 (0.771–0.909) |
| Support Vector Machine 3rd order Polynomial (P-SVM) | 0.837 (0.776–0.889) | 0.909 (0.857–0.955) | 0.765 (0.661–0.865) |
| Support Vector Machine Radial basis function (R-SVM) | 0.820 (0.753–0.871) | 0.795 (0.663–0.888) | 0.844 (0.771–0.907) |
| Decision Tree | 0.759 (0.700–0.818) | 0.758 (0.660–0.843) | 0.760 (0.646–0.870) |
| Random Forest | 0.843 (0.804–0.882) | 0.877 (0.811–0.939) | 0.809 (0.713–0.902) |
| Multi-layer Perceptron (MLP) | 0.813 (0.737–0.880) | 0.822 (0.679–0.926) | 0.805 (0.681–0.906) |
| Logistic Regression | 0.815 (0.739–0.873) | 0.773 (0.625–0.880) | 0.857 (0.777–0.924) |
| K-nearest Neighbors (KNN) | 0.841 (0.778–0.900) | 0.887 (0.794–0.959) | 0.796 (0.706–0.874) |
| Features | Fisher’s Ratio | AUROC | ||||
|---|---|---|---|---|---|---|
| Mean | Max | Mean | 95% CI of Mean | Max | 95% CI of Max | |
| TVSymp | 0.693 | 0.991 | 0.849 | 0.795–0.903 | 0.859 | 0.806–0.911 |
| MTVSymp | 1.001 | 1.039 | 0.845 | 0.791–0.900 | 0.851 | 0.797–0.905 |
| dPhEDA | 0.365 | 0.387 | 0.872 | 0.832–0.912 | 0.888 | 0.851–0.925 |
| Classifiers | Accuracy (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|
| Support Vector Machine Linear (L-SVM) | 0.740 (0.625–0.855) | 0.590 (0.340–0.840) | 0.890 (0.780–0.980) |
| Support Vector Machine 3rd order Polynomial (P-SVM) | 0.765 (0.660–0.870) | 0.610 (0.360–0.830) | 0.920 (0.820–1.000) |
| Support Vector Machine Radial basis function (R-SVM) | 0.735 (0.620–0.845) | 0.550 (0.290–0.800) | 0.920 (0.810–0.990) |
| Decision Tree | 0.755 (0.640–0.860) | 0.690 (0.480–0.870) | 0.820 (0.720–0.920) |
| Random Forest | 0.755 (0.645–0.865) | 0.670 (0.400–0.900) | 0.840 (0.710–0.950) |
| Multi-layer Perceptron (MLP) | 0.750 (0.645–0.865) | 0.670 (0.400–0.890) | 0.830 (0.670–0.950) |
| Logistic Regression | 0.760 (0.640–0.870) | 0.640 (0.390–0.870) | 0.880 (0.760–0.980) |
| K-nearest Neighbors (KNN) | 0.735 (0.635–0.845) | 0.720 (0.490–0.920) | 0.750 (0.650–0.850) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Posada-Quintero, H.F.; Chon, K.H. Real-Time High-Level Acute Pain Detection Using a Smartphone and a Wrist-Worn Electrodermal Activity Sensor. Sensors 2021, 21, 3956. https://doi.org/10.3390/s21123956
Kong Y, Posada-Quintero HF, Chon KH. Real-Time High-Level Acute Pain Detection Using a Smartphone and a Wrist-Worn Electrodermal Activity Sensor. Sensors. 2021; 21(12):3956. https://doi.org/10.3390/s21123956
Chicago/Turabian StyleKong, Youngsun, Hugo F. Posada-Quintero, and Ki H. Chon. 2021. "Real-Time High-Level Acute Pain Detection Using a Smartphone and a Wrist-Worn Electrodermal Activity Sensor" Sensors 21, no. 12: 3956. https://doi.org/10.3390/s21123956
APA StyleKong, Y., Posada-Quintero, H. F., & Chon, K. H. (2021). Real-Time High-Level Acute Pain Detection Using a Smartphone and a Wrist-Worn Electrodermal Activity Sensor. Sensors, 21(12), 3956. https://doi.org/10.3390/s21123956







