Low-Cost Reliable Corrosion Sensors Using ZnO-PVDF Nanocomposite Textiles
Abstract
1. Introduction
2. Materials and Methods
Thermal Cycling Method
3. Results and Discussion
3.1. EIS Results
3.2. Effect of Cyclic Temperature Exposure
3.3. Temperature Dependence of EIS Impedance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Augustyniak, A.; Tsavalas, J.; Ming, W. Early Detection of Steel Corrosion via “Turn-On” Fluorescence in Smart Epoxy Coatings. ACS Appl. Mater. Interfaces 2009, 1, 2618–2623. [Google Scholar] [CrossRef]
- Bierwagen, G.P.; Allahar, K.N.; Su, Q.; Gelling, V.J. Electrochemically characterizing the ac-dc-ac accelerated test method using embedded electrodes. Corros. Sci. 2009, 51, 95–101. [Google Scholar] [CrossRef]
- Bierwagen, G.; Wang, X.; Tallman, D. In situ study of coatings using embedded electrodes for ENM measurements. Prog. Org. Coat. 2003, 46, 163–175. [Google Scholar] [CrossRef]
- Kittel, J.; Celati, N.; Keddam, M.; Takenouti, H. New methods for the study of organic coatings by EIS: New insights into attached and free films. Prog. Org. Coat. 2001, 41, 93–98. [Google Scholar] [CrossRef]
- Kittel, J.; Celati, N.; Keddam, M.; Takenouti, H. Influence of the coating-substrate interactions on the corrosion protection: Characterisation by impedance spectroscopy of the inner and outer parts of a coating. Prog. Org. Coat. 2003, 46, 135–147. [Google Scholar] [CrossRef]
- Allahar, K.N.; Hinderliter, B.R.; Bierwagen, G.P.; Tallman, D.E. Army Vehicle Primer Properties During Wet-Dry Cycling. Corrosion 2009, 65, 126–135. [Google Scholar] [CrossRef]
- Allahar, K.N.; Su, Q.; Bierwagen, G.P.; Lee, D.H. Monitoring of the AC-DC-AC Degradation of Organic Coatings Using Embedded Electrodes. Corrosion 2008, 64, 773–787. [Google Scholar] [CrossRef]
- Su, Q.; Allahar, K.N.; Bierwagen, G.P. Application of embedded sensors in the thermal cycling of organic coatings. Corros. Sci. 2008, 50, 2381–2389. [Google Scholar] [CrossRef]
- Su, Q.; Allahar, K.; Bierwagen, G. Embedded electrode electrochemical noise monitoring of the corrosion beneath organic coatings induced by ac-dc-ac conditions. Electrochim. Acta 2008, 53, 2825–2830. [Google Scholar] [CrossRef]
- Allahar, K.N.; Upadhyay, V.; Bierwagen, G.P.; Gelling, V.J. Monitoring of a military vehicle coating under Prohesion exposure by embedded sensors. Prog. Org. Coat. 2009, 65, 142–151. [Google Scholar] [CrossRef]
- Miszczyk, A.; Schauer, T. Electrochemical approach to evaluate the interlayer adhesion of organic coatings. Prog. Org. Coat. 2005, 52, 298–305. [Google Scholar] [CrossRef]
- Chowdhury, T.; D’Souza, N.; Ho, Y.H.; Dahotre, N.; Mahbub, I. Embedded Corrosion Sensing with ZnO-PVDF Sensor Textiles. Sensors 2020, 20, 3053. [Google Scholar] [CrossRef]
- Wright, R.F.; Lu, P.; Devkota, J.; Lu, F.; Ziomek-Moroz, M.; Ohodnicki, P.R., Jr. Corrosion Sensors for Structural Health Monitoring of Oil and Natural Gas Infrastructure: A Review. Sensors 2019, 19, 3964. [Google Scholar] [CrossRef]
- Baer, A. In The Effective Use of Epoxy Insulative Coatings in the Oil & Gas Industry. In Proceedings of the NACE International Corrosion Conference, Phoenix, Arizona, 15–19 April 2018. [Google Scholar]
- Merino, S.F.; Caprari, J.J.; Torres, L.V.; Ramos, L.F.; Girola, A.H. Inhibitive action of tara tannin in rust converter formulation. Anti-Corros. Methods Mater. 2017, 64, 136–147. [Google Scholar] [CrossRef]
- Bower, K.; Murray, S.; Reinhart, A.; Nieto, A. Corrosion resistance of selective laser melted Ti–6Al–4V alloy in salt fog environment. Results Mater. 2020, 8, 100122. [Google Scholar] [CrossRef]
- Bierwagen, G.; Tallman, D.; Li, J.; He, L.; Jeffcoate, C. EIS studies of coated metals in accelerated exposure. Prog. Org. Coat. 2003, 46, 149–158. [Google Scholar] [CrossRef]
- Allahar, K.N.; Su, Q.; Bierwagen, G.P. In Situ Monitoring of Organic Coatings Under QUV/Prohesion Exposure by Embedded Sensors. Corrosion 2008, 64, 860–870. [Google Scholar] [CrossRef]
- Touzain, S.; Le Thu, Q.; Bonnet, G. Evaluation of thick organic coatings degradation in seawater using cathodic protection and thermally accelerated tests. Prog. Org. Coat. 2005, 52, 311–319. [Google Scholar] [CrossRef]
- Bierwagen, G.P.; He, L.; Li, J.; Ellingson, L.; Tallman, D. Studies of a new accelerated evaluation method for coating corrosion resistance—Thermal cycling testing. Prog. Org. Coat. 2000, 39, 67–78. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Accelerated ageing of organic coating systems by thermal treatment. Corros. Sci. 2001, 43, 1337–1343. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Effect of environmental temperature variations on protective properties of organic coatings. Prog. Org. Coat. 2003, 46, 49–54. [Google Scholar] [CrossRef]
- Valentinelli, L.; Vogelsang, J.; Ochs, H.; Fedrizzi, L. Evaluation of barrier coatings by cycling testing. Prog. Org. Coat. 2002, 45, 405–413. [Google Scholar] [CrossRef]
- Richards, B.T.; Begley, M.R.; Wadley, H.N. Mechanisms of Ytterbium Monosilicate/Mullite/Silicon Coating Failure During Thermal Cycling in Water Vapor. J. Am. Ceram. Soc. 2015, 98, 4066–4075. [Google Scholar] [CrossRef]
- Zhang, X.; Si, Y.; Mo, J.; Guo, Z. Robust micro-nanoscale flowerlike ZnO/epoxy resin superhydrophobic coating with rapid healing ability. Chem. Eng. J. 2017, 313, 1152–1159. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Chafidz, A.; Al-Zahrani, S.M.; Alharthi, N.H. Enhancing mechanical properties of epoxy/polyaniline coating with addition of ZnO nanoparticles: Nanoindentation characterization. Prog. Org. Coat. 2018, 119, 109–115. [Google Scholar] [CrossRef]
- Hu, C.; Li, Y.; Kong, Y.; Ding, Y. Preparation of poly(o-toluidine)/nano ZnO/epoxy composite coating and evaluation of its corrosion resistance properties. Synth. Met. 2016, 214, 62–70. [Google Scholar] [CrossRef]
- Amirudin, A.; Thieny, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Alsamuraee, A.M.A.; Jaafer, H.I. Electrochemical impedance spectroscopic evaluation of corrosion protection properties of polyurethane /polyvinyl chloride blend coatings on steel. Am. J. Sci. Ind. Res. 2011, 2, 761–768. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wilke, B.M.; Li, W.; Ning, C.; Chowdhury, T. Corrosion Behavior of Microarc Oxidized Mg Alloy in Earle’s Balance Salt Solution. Surf. Innov. 2017, 5, 1–35. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M. Studying the corrosion resistance and hydrolytic degradation of an epoxy coating containing ZnO nanoparticles. Mater. Chem. Phys. 2011, 130, 1208–1219. [Google Scholar] [CrossRef]
- Aneja, K.S.; Böhm, H.M.; Khanna, A.; Böhm, S. Functionalised graphene as a barrier against corrosion. FlatChem 2017, 1, 11–19. [Google Scholar] [CrossRef]
- Li, J.; Jeffcoate, C.S.; Bierwagen, G.P.; Mills, D.J.; Tallman, D.E. Thermal Transition Effects and Electrochemical Properties in Organic Coatings: Part 1—Initial Studies on Corrosion Protective Organic Coatings. Corrosion 1998, 54, 763–771. [Google Scholar] [CrossRef]
- Sykes, J.M.; Whyte, E.P.; Yu, X.; Sahir, Z.S. Does “coating resistance” control corrosion? Prog. Org. Coat. 2017, 102, 82–87. [Google Scholar] [CrossRef]


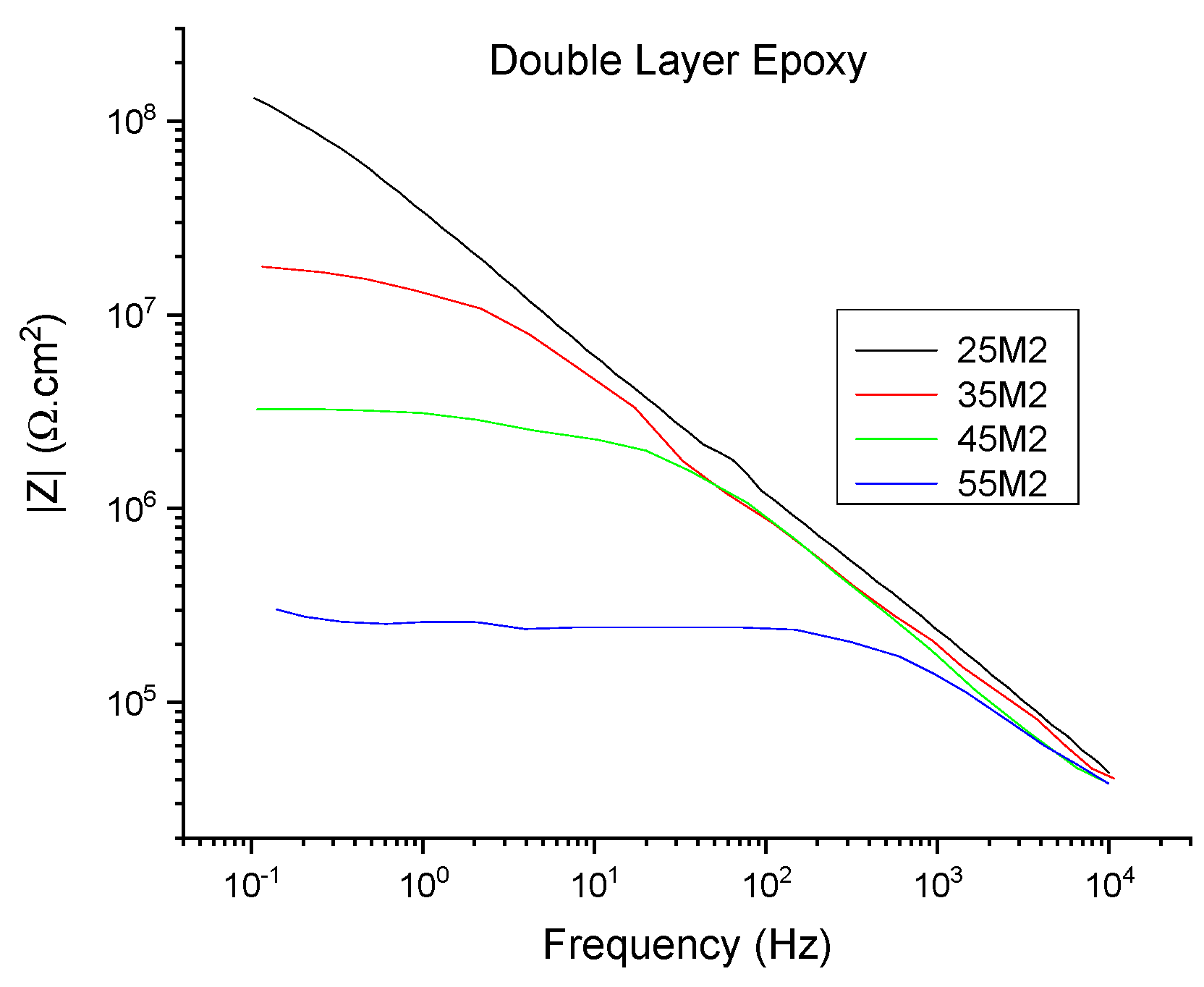
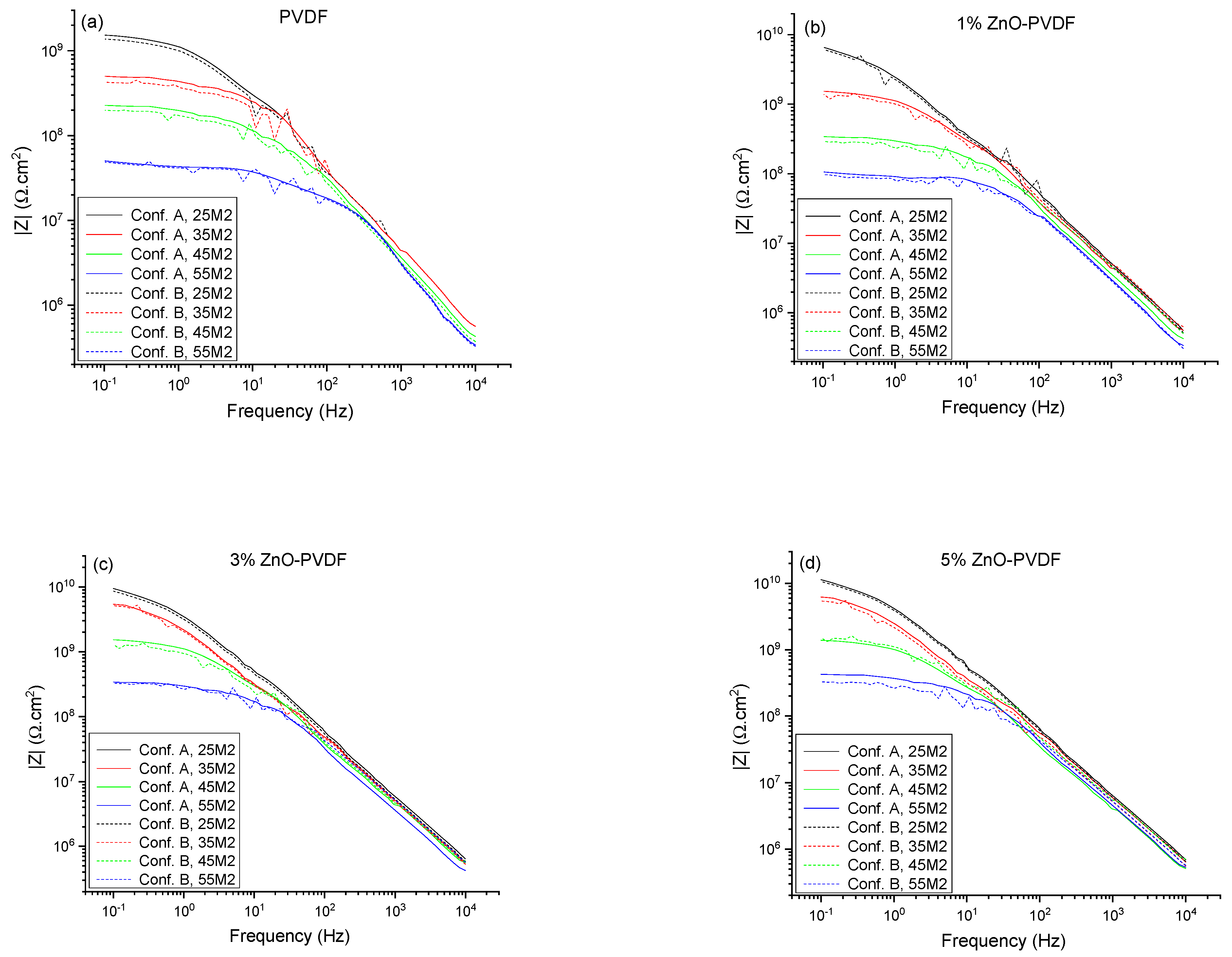

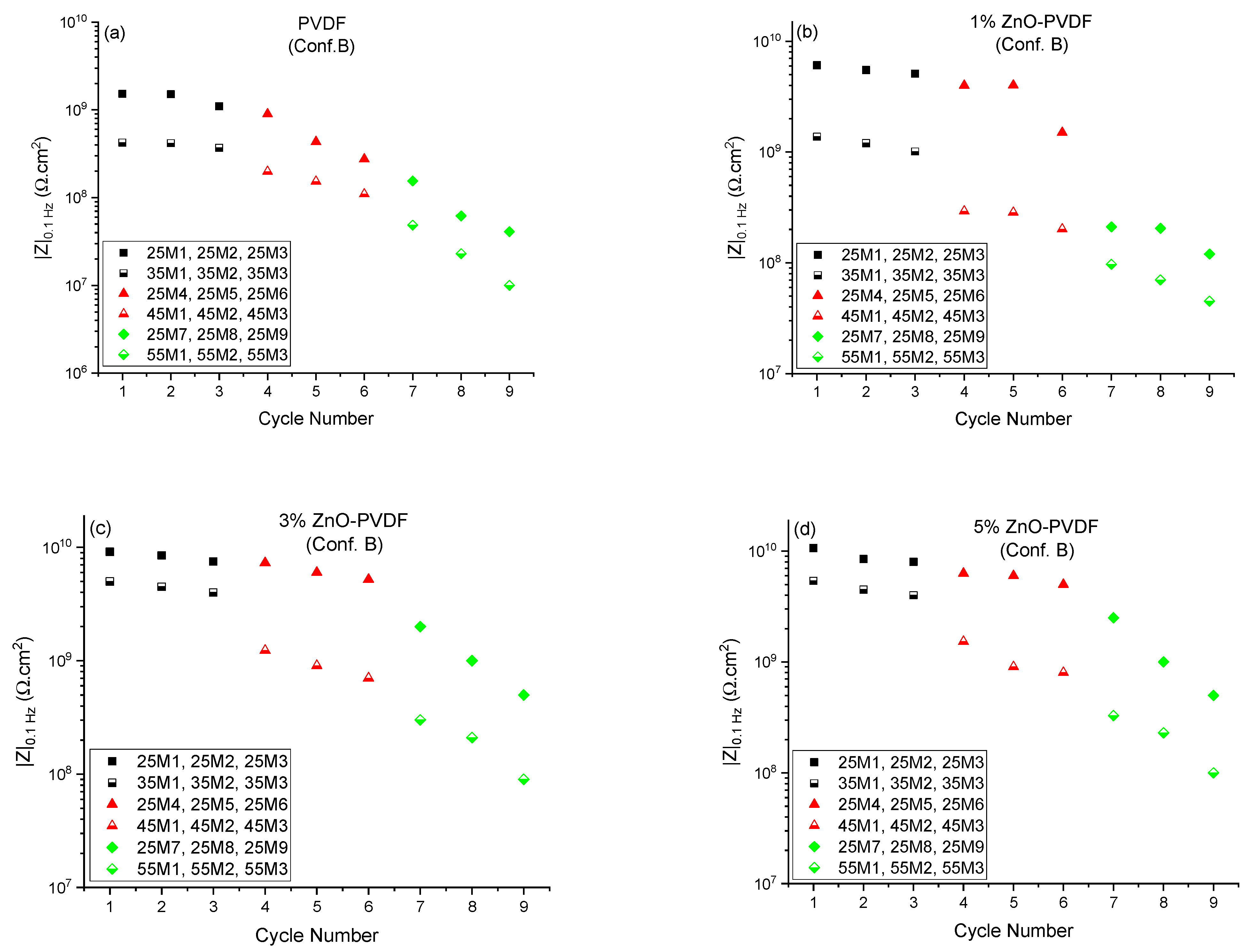
| Index | Meaning |
|---|---|
| 25M1, 25M2, 25M3 | EIS measurements at room temperature when set temperature is 35 °C (Tg −20 °C) |
| 25M4, 25M5, 25M6 | EIS measurements at room temperature when set temperature is 45 °C (Tg −10 °C) |
| 25M7, 25M8, 25M9 | EIS measurements at room temperature when set temperature is 55 °C (Tg) |
| 35M1, 35M2, 35M3 | EIS measurements at set temperature of 35 °C (Tg −20 °C) |
| 45M1, 45M2, 45M3 | EIS measurements at set temperature of 45 °C (Tg −10 °C) |
| 55M1, 55M2, 55M3 | EIS measurements at set temperature of 55 °C (Tg) |
| Sample | Conf. | Rpore (MΩ) | Rct (GΩ) | Cpore (pF.sn−1.cm−2) | Cdl (µF.sn−1.cm−2) | Error for Rpore (%) | Error for Rct (%) | Error for Cpore (%) | Error for Cct (%) |
|---|---|---|---|---|---|---|---|---|---|
| Pure_Epoxy_25°C | - | 0.23 | 0.12 | 5.77 | 0.35 | - | - | - | - |
| Pure_Epoxy_35°C | - | 0.19 | 0.08 | 5.93 | 1.66 | - | - | - | - |
| Pure_Epoxy_45°C | - | 0.16 | 0.02 | 6.75 | 2.12 | - | - | - | - |
| Pure_Epoxy_55°C | - | 0.12 | 0.001 | 7.23 | 2.43 | - | - | - | - |
| PVDF_25°C | A | 2.47 ± 0.17 | 1.40 ± 0.11 | 5.92 ± 0.12 | 2.74 ± 0.16 | 5.34 | 6.23 | 1.08 | 5.12 |
| B | 2.34 ± 0.13 | 1.31 ± 0.14 | 5.92 ± 0.20 | 2.60 ± 0.22 | |||||
| PVDF_35°C | A | 1.09 ± 0.29 | 0.41 ± 0.12 | 6.62 ± 0.27 | 2.98 ± 0.30 | 9.33 | 10.75 | 8.56 | 6.37 |
| B | 1.01 ± 0.37 | 0.37 ± 0.25 | 6.28 ± 0.38 | 2.79 ± 0.43 | |||||
| PVDF_45°C | A | 0.28 ± 0.79 | 0.11 ± 0.87 | 6.91 ± 0.51 | 3.15 ± 0.55 | 17.71 | 19.09 | 9.11 | 9.40 |
| B | 0.23 ± 0.68 | 0.09 ± 0.76 | 6.28 ± 0.68 | 2.90 ± 0.82 | |||||
| PVDF_55°C | A | 0.02 ± 1.05 | 0.03 ± 1.06 | 7.11 ± 1.82 | 3.32 ± 1.49 | 27.12 | 22.22 | 10.13 | 12.65 |
| B | 0.01 ± 1.11 | 0.01 ± 1.01 | 6.39 ± 1.79 | 2.90 ± 1.95 | |||||
| 1%_ZnO_25°C | A | 5.21 ± 0.25 | 5.67 ± 0.23 | 5.21 ± 0.05 | 5.45 ± 0.08 | 4.35 | 3.27 | 1.88 | 2.83 |
| B | 4.98 ± 0.39 | 5.48 ± 0.21 | 5.49 ± 0.12 | 5.29 ± 0.15 | |||||
| 1%_ZnO_35°C | A | 2.42 ± 0.16 | 1.35 ± 0.28 | 6.01 ± 0.16 | 5.82 ± 0.02 | 7.85 | 6.67 | 4.16 | 2.58 |
| B | 2.23 ± 0.27 | 1.26 ± 0.14 | 5.76 ± 0.08 | 5.67 ± 0.57 | |||||
| 1%_ZnO_45°C | A | 1.23 ± 0.59 | 0.25 ± 0.55 | 6.38 ± 0.81 | 5.97 ± 0.72 | 7.57 | 9.70 | 8.62 | 4.02 |
| B | 1.10 ± 0.45 | 0.22 ± 0.68 | 6.93 ± 0.49 | 5.73 ± 0.54 | |||||
| 1%_ZnO_55°C | A | 0.05 ± 0.75 | 0.09 ± 0.75 | 6.49 ± 0.74 | 6.25 ± 0.96 | 21.82 | 11.11 | 14.18 | 20.80 |
| B | 0.04 ± 0.79 | 0.08 ± 0.89 | 7.41 ± 0.49 | 4.95 ± 0.79 | |||||
| 3%_ZnO_25°C | A | 7.05 ± 0.29 | 8.02 ± 0.15 | 9.68 ± 0.08 | 5.08 ± 0.22 | 1.75 | 3.15 | 2.80 | 5.03 |
| B | 6.93 ± 0.17 | 7.76 ± 0.23 | 9.41 ± 0.10 | 4.82 ± 0.09 | |||||
| 3%_ZnO_35°C | A | 5.43 ± 0.28 | 5.23 ± 0.21 | 9.89 ± 0.17 | 5.45 ± 0.26 | 4.42 | 3.25 | 4.55 | 8.44 |
| B | 5.19 ± 0.37 | 5.06 ± 0.35 | 9.44 ± 0.15 | 4.26 ± 0.29 | |||||
| 3%_ZnO_45°C | A | 1.93 ± 0.19 | 1.37 ± 0.27 | 10.81 ± 0.34 | 6.69 ± 0.52 | 8.95 | 8.14 | 9.16 | 10.51 |
| B | 2.18 ± 0.23 | 1.19 ± 0.31 | 9.82 ± 0.49 | 5.92 ± 0.38 | |||||
| 3%_ZnO_55°C | A | 0.59 ± 0.74 | 0.27 ± 0.84 | 10.98 ± 0.91 | 7.28 ± 0.71 | 15.90 | 19.28 | 10.66 | 10.16 |
| B | 0.43 ± 0.87 | 0.21 ± 0.72 | 9.81 ± 0.69 | 6.54 ± 0.65 | |||||
| 5%_ZnO_25°C | A | 8.34 ± 0.16 | 10.27 ± 0.19 | 10.97 ± 0.24 | 6.33 ± 0.03 | 3.93 | 4.82 | 3.20 | 5.96 |
| B | 8.01 ± 0.10 | 9.78 ± 0.26 | 10.62 ± 0.20 | 6.08 ± 0.14 | |||||
| 5%_ZnO_35°C | A | 5.86 ± 0.25 | 6.09 ± 0.23 | 11.16 ± 0.16 | 6.65 ± 0.31 | 4.78 | 2.79 | 1.34 | 5.71 |
| B | 5.58 ± 0.22 | 5.92 ± 0.17 | 11.01 ± 0.28 | 6.27 ± 0.20 | |||||
| 5%_ZnO_45°C | A | 2.28 ± 0.36 | 2.01 ± 0.41 | 12.62 ± 0.54 | 7.24 ± 0.47 | 9.84 | 9.95 | 8.56 | 6.35 |
| B | 2.01 ± 0.47 | 1.79 ± 0.51 | 11.54 ± 0.62 | 6.78 ± 0.23 | |||||
| 5%_ZnO_55°C | A | 1.21 ± 0.92 | 0.59 ± 0.98 | 12.89 ± 0.79 | 7.54 ± 0.81 | 15.70 | 18.64 | 9.46 | 13.93 |
| B | 1.02 ± 0.89 | 0.48 ± 0.91 | 11.67 ± 0.65 | 6.49 ± 0.77 |
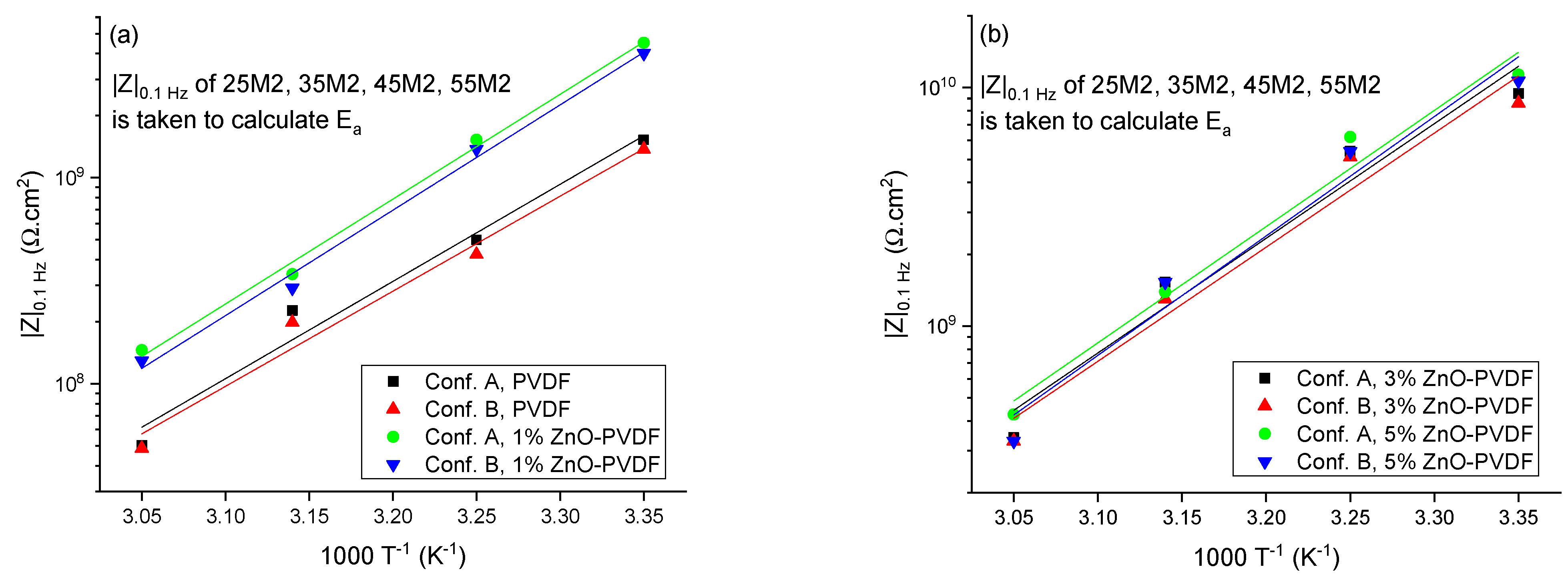
| Sample | Ea from Conf. A (kJ.mol−1) | Ea from Conf. B (kJ.mol−1) | Error (%) |
|---|---|---|---|
| Coating with PVDF | 55.91 ± 0.31 | 55.62 ± 0.31 | 0.52 |
| Coating with 1% ZnO-PVDF | 58.37 ± 0.15 | 58.05 ± 0.19 | 0.55 |
| Coating with 3% ZnO-PVDF | 60.65 ± 0.38 | 60.41 ± 0.37 | 0.41 |
| Coating with 5% ZnO-PVDF | 61.53 ± 0.41 | 61.31 ± 0.48 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, T.; D’Souza, N.; Dahotre, N. Low-Cost Reliable Corrosion Sensors Using ZnO-PVDF Nanocomposite Textiles. Sensors 2021, 21, 4147. https://doi.org/10.3390/s21124147
Chowdhury T, D’Souza N, Dahotre N. Low-Cost Reliable Corrosion Sensors Using ZnO-PVDF Nanocomposite Textiles. Sensors. 2021; 21(12):4147. https://doi.org/10.3390/s21124147
Chicago/Turabian StyleChowdhury, Tonoy, Nandika D’Souza, and Narendra Dahotre. 2021. "Low-Cost Reliable Corrosion Sensors Using ZnO-PVDF Nanocomposite Textiles" Sensors 21, no. 12: 4147. https://doi.org/10.3390/s21124147
APA StyleChowdhury, T., D’Souza, N., & Dahotre, N. (2021). Low-Cost Reliable Corrosion Sensors Using ZnO-PVDF Nanocomposite Textiles. Sensors, 21(12), 4147. https://doi.org/10.3390/s21124147






