Annular Fiber Probe for Interstitial Illumination in Photoacoustic Guidance of Radiofrequency Ablation
Abstract
:1. Introduction
2. Methods
2.1. Probe Design and Experimental Setup
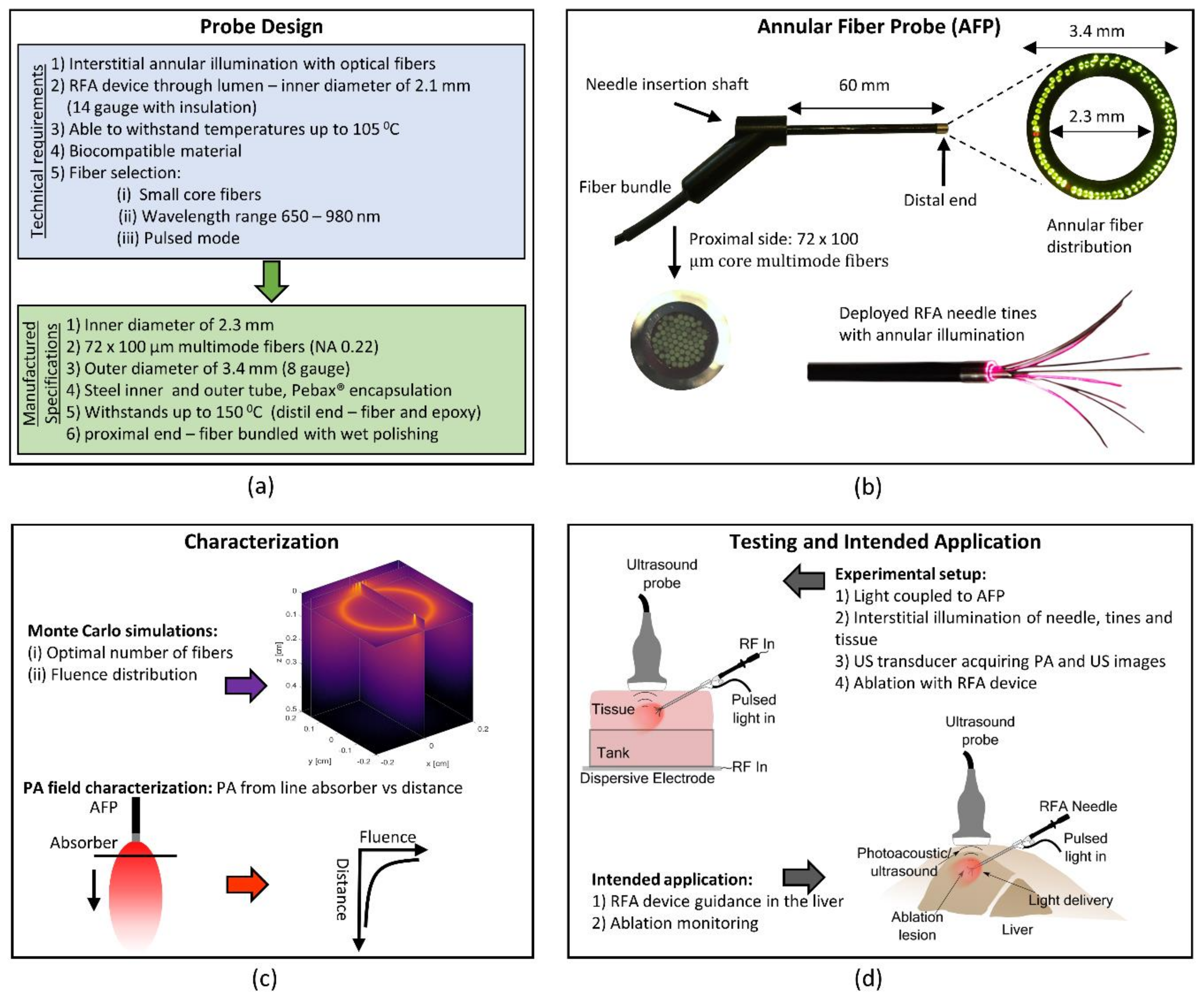
2.1.1. Annular Fiber Probe Design
2.1.2. Experimental Setup
2.2. Device Testing and Characterization
2.2.1. Monte Carlo Simulations
2.2.2. PA Field Characterization with an Absorber
2.3. Validation In Ex Vivo Tissues
2.3.1. Sample Preparation
2.3.2. Surface Versus Interstitial Illumination
2.3.3. Probe and Tine Visualization
2.3.4. Blood Vessel Targeting
2.3.5. Tumor-Mimicking Target Visibility
2.3.6. Ablated Tissue Targeting
3. Results
3.1. Device Testing and Characterization
3.1.1. Monte Carlo Simulation Results
3.1.2. PA Field Characterization with Absorber
3.2. Validation in Ex Vivo Tissues
3.2.1. Surface Versus Interstitial Illumination
3.2.2. Probe and Tine Visualization
3.2.3. Blood Vessel Targeting
3.2.4. Tumor-Mimicking Target Visibility
3.2.5. Ablated Tissue Targeting
4. Discussion
4.1. Device Testing and Characterization
4.1.1. Monte Carlo Simulations
4.1.2. PA Field Characterization with Absorber
4.2. Validation in Ex Vivo Tissues
4.2.1. Surface Versus Interstitial Illumination
4.2.2. Probe and Tine Visualization
4.2.3. Blood Vessel Targeting
4.2.4. Tumor-Mimicking Target Visibility
4.2.5. Ablated Tissue Targeting
4.2.6. Laser Fluence
4.2.7. Reconstruction Artifacts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waller, L.P.; Deshpande, V.; Pyrsopoulos, N. Hepatocellular carcinoma: A comprehensive review. World J. Hepatol. 2015, 7, 2648–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, F.C.-L.; Chok, K.S.-H. Colorectal liver metastases: An update on multidisciplinary approach. World J. Hepatol. 2019, 11, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Dhir, M.; Melin, A.A.D.; Douaiher, J.; Lin, C.; Zhen, W.K.; Hussain, S.M.; Geschwind, J.-F.H.; Doyle, M.B.M.; Abou-Alfa, G.K.; Are, C. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann. Surg. 2016, 263, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Tanis, E.; Nordlinger, B.; Mauer, M.; Sorbye, H.; Coevorden, F.V.; Gruenberger, T.; Schlag, P.; Punt, C.; Ledermann, J.; Ruers, T. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur. J. Cancer 2014, 50, 912–919. [Google Scholar]
- Mulier, S.; Ni, Y.; Jamart, J.; Ruers, T.; Marchal, G.; Michel, L. Local Recurrence After Hepatic Radiofrequency Coagulation: Multivariate Meta-Analysis and Review of Contributing Factors. Ann. Surg. 2005, 242, 158–171. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Rhim, H.; Goldberg, S.N.; Dodd, G.D.; Solbiati, L.; Lim, H.K.; Tonolini, M.; Cho, O.K. Essential Techniques for Successful Radiofrequency Thermal Ablation of Malignant Hepatic Tumors. Radiographics 2001, 21, S17–S39. [Google Scholar] [CrossRef]
- Jong, K.P.D.; Ruiter, S.J.S.; Pennings, J. Stereotactic image guided microwave ablation of HCC: A step forward and still a long way to go. Liver. Int. 2019, 39, 1798–1800. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Tan, S.; Peng, W.; Jiang, Y.; Luo, C. Radiofrequency ablation versus laparoscopic hepatectomy for treatment of hepatocellular carcinoma: A systematic review and meta-analysis. World. J. Surg. Onc. 2002, 18, 199. [Google Scholar]
- Napoleone, M.; Kielar, A.Z.; Hibbert, R.; Saif, S.; Kwan, B.Y. Local tumor progression patterns after radiofrequency ablation of colorectal cancer liver metastases. Diagn. Interv. Radiol. 2016, 22, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.K.C.; Chok, K.S.H.; Chan, A.C.Y.; Cheung, T.T.; Wong, T.C.L.; Fung, J.Y.Y.; Yuen, J.; Poon, R.T.P.; Fan, S.T.; Lo, C.M. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br. J. Surg. 2017, 104, 1775–1784. [Google Scholar] [CrossRef]
- Kang, T.W.; Kim, J.M.; Rhim, H.; Lee, M.W.; Kim, Y.-S.; Lim, H.K.; Choi, D.; Song, K.D.; Kwon, C.H.D.; Joh, J.-W.; et al. Small Hepatocellular Carcinoma: Radiofrequency Ablation versus Nonanatomic Resection—Propensity Score Analyses of Long-term Outcomes. J. Vasc. Interv. 2015, 275, 908–919. [Google Scholar] [CrossRef]
- Thanos, L.; Mylona, S.; Galani, P.; Pomoni, M.; Pomoni, A.; Koskinas, I. Overcoming the heat-sink phenomenon: Successful radiofrequency thermal ablation of liver tumors in contact with blood vessels. Diagn. Interv. Radiol. 2008, 14, 51–56. [Google Scholar]
- Lee, L.-H.; Hwang, J.-I.; Cheng, Y.-C.; Wu, C.-Y.; Lee, S.-W.; Yang, S.-S.; Yeh, H.-Z.; Chang, C.-S.; Lee, T.-Y. Comparable Outcomes of Ultrasound versus Computed Tomography in the Guidance of Radiofrequency Ablation for Hepatocellular Carcinoma. PLoS ONE 2017, 12, e0169655. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-E.; Kim, Y.-S.; Rhim, H.; Lim, H.K.; Lee, M.W.; Choi, D.; Shin, S.W.; Cho, S.K. Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: Analysis focused on the feasibility with the use of ultrasonography guidance. Eur. J. Radiol. 2011, 79, e80–e84. [Google Scholar] [CrossRef]
- Chiou, S.-Y.; Liu, J.-B.; Needleman, L. Current Status of Sonographically Guided Radiofrequency Ablation Techniques. J. Ultrasound Med. 2007, 26, 487–499. [Google Scholar] [CrossRef]
- Künzli, B.M.; Abitabile, P.; Maurer, C.A. Radiofrequency ablation of liver tumors: Actual limitations and potential solutions in the future. World J. Hepatol. 2011, 3, 8–14. [Google Scholar] [CrossRef]
- Reusz, G.; Sarkany, P.; Gal, J.; Csomos, A. Needle-related ultrasound artifacts and their importance in anaesthetic practice. Br. J. Anaesth. 2014, 5, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Sivasubramanian, K.; Periyasamy, V.; Pramanik, M. Non-invasive sentinel lymph node mapping and needle guidance using clinical handheld photoacoustic imaging system in small animal. J. Biophotonics 2018, 11, e201700061. [Google Scholar] [CrossRef]
- Francis, K.J.; Manohar, S. Photoacoustic imaging in percutaneous radiofrequency ablation: Device guidance and ablation visualization. Phys. Med. Biol. 2019, 64, 184001. [Google Scholar] [CrossRef]
- Wiacek, A.; Bell, M.A.L. Photoacoustic-guided surgery from head to toe. Biomed. Opt. Express 2021, 12, 2079–2117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Yao, J. A Practical Guide to Photoacoustic Tomography in the Life Sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef] [PubMed]
- Kempski, K.M.; Wiacek, A.; Graham, M.; González, E.; Goodson, B.; Allman, J.P.D.; Hou, H.; Beck, S.; He, J.; Bell, M.A.L. In vivo photoacoustic imaging of major blood vessels in the pancreas and liver during surgery. J. Biomed. Opt. 2019, 24, 121905. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Xiao, C.; Chen, K.; Zheng, J.; Zhang, J.; Zhao, X.; Xue, X. Different optical properties between human hepatocellular carcinoma tissues and non-tumorous hepatic tissues In Vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 515. [Google Scholar] [CrossRef]
- Germer, C.-T.; Roggan, A.; Ritz, J.P.; Isbert, C.; Albrecht, D.; Müller, G.; Buhr, H.J. Optical Properties of Native and Coagulated Human Liver Tissue and Liver Metastases in the Near Infrared Range. Lasers Surg. Med. 1998, 23, 194–203. [Google Scholar] [CrossRef]
- Xia, W.; Singh, M.K.A.; Maneas, E.; Sato, N.; Shigeta, Y.; Agano, T.; Ourselin, S.; West, S.J.; Desjardins, A.E. Handheld Real-Time LED-Based Photoacoustic and Ultrasound Imaging System for Accurate Visualization of Clinical Metal Needles and Superficial Vasculature to Guide Minimally Invasive Procedures. Sensors 2018, 18, 1394. [Google Scholar] [CrossRef] [Green Version]
- Piras, D.; Grijsen, C.; Schutte, P.; Steenbergen, W.; Manohar, S. Photoacoustic needle: Minimally invasive guidance to biopsy. J. Biomed. Opt. 2013, 18, 070502. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, E.A.; Jain, A.; Bell, M.A.L. Combined ultrasound and photoacoustic image guidance of spinal pedicle cannulation demonstrated with intact ex vivo specimens. IEEE Trans. Biomed. Eng. 2020. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X. Real-time monitoring of high-intensity focused ultrasound ablations with photoacoustic technique: An in vitro study. Med. Phys. 2011, 38, 5345–5350. [Google Scholar] [CrossRef]
- Iskander-Rizk, S.; Kruizinga, P.; Beurskens, R.; Springeling, G.; Groot Mastik, F.; de Groot, N.M.S.; Knops, P.; van der Steen, A.F.W.; Soest, G. Real-time photoacoustic assessment of radiofrequency ablation lesion formation in the left atrium. Photoacoustics 2019, 16, 100150. [Google Scholar] [CrossRef]
- Rebling, J.; Landa, F.J.O.; Deán-Ben, X.L.; Douplik, A.; Razansky, D. Integrated catheter for simultaneous radio frequency ablation and optoacoustic monitoring of lesion progression. Opt. Lett. 2018, 43, 1886–1889. [Google Scholar] [CrossRef]
- Dana, N.; Biase, L.D.; Natale, A.; Emelianov, S.; Bouchard, R. In vitro photoacoustic visualization of myocardial ablation lesions. Heart Rhythm 2014, 11, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Desjardins, A.E.; Ourselin, S.; Vercauteren, T.; Xia, W. Minimally invasive photoacoustic imaging: Current status and future perspectives. Photoacoustics 2019, 16, 100146. [Google Scholar] [CrossRef]
- Ritz, J.-P.; Roggan, A.; Isbert, C.; Müller, G.; Buhr, H.J.; Germer, C.-T. Optical Properties of Native and Coagulated Porcine Liver Tissue Between 400 and 2400 nm. Lasers Surg. Med. 2001, 29, 205–212. [Google Scholar] [CrossRef]
- Bell, M.A.L.; Kuo, N.; Song, D.Y.; Boctor, E.M. Short-lag spatial coherence beamforming of photoacoustic images for enhanced visualization of prostate brachytherapy seeds. Biomed. Opt. Express 2013, 4, 1964–1977. [Google Scholar] [CrossRef] [Green Version]
- Rascevska, E.; Francis, K.J.; Manohar, S. Annular illumination photoacoustic probe for needle guidance in medical interventions. In Proceedings of the European Conference on Biomedical Optics, Munich Germany, 23–25 June 2019; Optical Society of America: Washington DC, USA, 2019; p. 11077_20. [Google Scholar]
- Eddins, B.; Bell, M.A.L. Design of a multifiber light delivery system for photoacoustic-guided surgery. J. Biomed. Opt. 2017, 22, 041011. [Google Scholar] [CrossRef]
- Marti, D.; Aasbjerg, R.N.N.; Andersen, P.E.E.; Hansen, A.K.K. MCmatlab: An open-source, user-friendly, MATLAB-integrated three-dimensional Monte Carlo light transport solver with heat diffusion and tissue damage. J. Biomed. Opt. 2018, 23, 121622. [Google Scholar] [CrossRef]
- Lanka, P.; Francis, K.J.; Kruit, H.; Farina, A.; Cubeddu, R.; Sekar, S.K.V.; Manohar, S.; Pifferi, A. Optical signatures of radiofrequency ablation in biological tissues. Sci. Rep. 2021, 11, 6579. [Google Scholar] [CrossRef]
- Giannios, P.; Toutouzas, K.G.; Matiatou, M.; Stasinos, K.; Konstadoulakis, M.M.; Zografos, G.C.; Moutzouris, K. Visible to near-infrared refractive properties of freshly-excised human-liver tissues: Marking hepatic malignancies. Sci. Rep. 2016, 6, 27910. [Google Scholar] [CrossRef] [Green Version]
- Boudoux, C. Light transport in tissue. In Fundamentals of Biomedical Optics; Blurb: Montréal, QC, Canada, 2016; p. 241. [Google Scholar]
- Marquez, G.; Wang, L.V.; Lin, S.-P.; Schwartz, J.A.; Thomsen, S.L. Anisotropy in the absorption and scattering spectra of chicken breast tissue. Appl. Opt. 1998, 37, 798–804. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ruarus, A.H.; Vroomen, L.G.P.H.; van Tilborg, A.A.J.M.; Scheffer, H.J.; Nielsen, K.; de Jong, M.C.; de Vries, J.J.J.; Zonderhuis, B.M.; Eker, H.H.; et al. Colorectal liver metastases: Surgery versus thermal ablation (COLLISION)—A phase III single-blind prospective randomized controlled trial. BMC Cancer 2018, 18, 821. [Google Scholar] [CrossRef]
- Joseph, F.K.; Kruit, H.; Rascevska, E.; Manohar, S. Minimally invasive photoacoustic imaging for device guidance and monitoring of radiofrequency ablation. Proc. SPIE 2020, 11240, 362–367. [Google Scholar]
- Gould, T.; Wang, Q.; Pfefer, T.J. Optical-thermal light-tissue interactions during photoacoustic breast imaging. Biomed. Opt. Express 2014, 5, 832–847. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Tokumine, J.; Lefor, A.K.; Nakazawa, H.; Yamamoto, K.; Karasawa, H.; Nagase, M.; Yorozu, T. Photoacoustic needle improves needle tip visibility during deep peripheral nerve block. Sci. Rep. 2021, 11, 8432. [Google Scholar] [CrossRef]
- Hotta, N.; Tagaya, T.; Maeno, T.; Ayada, M.; Sato, K.; Ishikawa, T.; Okumura, A.; Fukuzawa, Y.; Kakumu, S. Advanced dynamic flow imaging with contrast-enhanced ultrasonography for the evaluation of tumor vascularity in liver tumors. Clin. Imaging 2005, 29, 34–41. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Bach, A.M.; Winston, C.B.; Hann, L.E.; Heffernan, N.; Loumeau, T.; DeMatteo, R.P.; Fong, Y.; Blumgart, L.H. What is the yield of intraoperative ultrasonography during partial hepatectomy for malignant disease? J. Am. Coll. Surg. 2001, 192, 577–583. [Google Scholar] [CrossRef]
- Kolkman, R.G.; Bosschaart, N.; Kok, B. Photoacoustic Imaging of Valves in Superficial Veins. Lasers Surg. Med. 2006, 38, 740–744. [Google Scholar] [CrossRef]
- Fetzer, D.T.; Rodgers, S.K.; Harris, A.C.; Kono, Y.; Wasnik, A.P.; Kamaya, A.; Sirlin, C. Screening and Surveillance of Hepatocellular Carcinoma: An Introduction to Ultrasound Liver Imaging Reporting and Data System. Radiol. Clin. N. Am. 2017, 55, 1197–1209. [Google Scholar] [CrossRef]
- Jacques, S.L. Role of tissue optics and pulse duration on tissue effects during high-power laser irradiation. Appl. Opt. 1993, 32, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wiacek, A.; Kempski, K.M.; Palmer, T.; Izzi, J.; Beck, S.; Bell, M.A.L. Empirical assessment of laser safety for photoacoustic-guided liver surgeries. Biomed. Opt. Express 2021, 12, 1205–1216. [Google Scholar] [CrossRef] [PubMed]







| Optical Parameter | Native Liver Tissue | Ablated Liver Tissue at 70 °C | ||||
|---|---|---|---|---|---|---|
| Wavelength | 650 | 900 | 1050 | 650 | 900 | 1050 |
| 1.23 | 0.73 | 0.60 | 0.70 | 0.23 | 0.24 | |
| 8.26 | 5.04 | 3.85 | 31.48 | 24.90 | 18.341 | |
| 118.01 | 72.03 | 55.02 | 314.79 | 248.98 | 183.41 | |
| 0.93 | 0.93 | 0.93 | 0.90 | 0.90 | 0.90 | |
| 1.33 | 1.33 | 1.33 | 1.33 | 1.33 | 1.33 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruit, H.; Joseph Francis, K.; Rascevska, E.; Manohar, S. Annular Fiber Probe for Interstitial Illumination in Photoacoustic Guidance of Radiofrequency Ablation. Sensors 2021, 21, 4458. https://doi.org/10.3390/s21134458
Kruit H, Joseph Francis K, Rascevska E, Manohar S. Annular Fiber Probe for Interstitial Illumination in Photoacoustic Guidance of Radiofrequency Ablation. Sensors. 2021; 21(13):4458. https://doi.org/10.3390/s21134458
Chicago/Turabian StyleKruit, Hindrik, Kalloor Joseph Francis, Elina Rascevska, and Srirang Manohar. 2021. "Annular Fiber Probe for Interstitial Illumination in Photoacoustic Guidance of Radiofrequency Ablation" Sensors 21, no. 13: 4458. https://doi.org/10.3390/s21134458
APA StyleKruit, H., Joseph Francis, K., Rascevska, E., & Manohar, S. (2021). Annular Fiber Probe for Interstitial Illumination in Photoacoustic Guidance of Radiofrequency Ablation. Sensors, 21(13), 4458. https://doi.org/10.3390/s21134458






