Underwater Holographic Sensor for Plankton Studies In Situ including Accompanying Measurements
Abstract
:1. Introduction
- •
- pronounced seasonal thermocline (0.19 deg/m or more);
- •
- pronounced top quasi-homogeneous layer (6 m or more);
- •
- presence of areas in the top quasi-homogeneous layer with the concentration of zooplankton of not less than 0.4 g/m3 and phytoplankton-5.6 g/m3.
- •
- Fishing vessels constantly present in the ocean. Despite the fact that the commercial interests of fishermen do not motivate them to oceanographic activities, mutually beneficial cooperation may be based on the principle of “accurate forecast in exchange for marine data”. An example of this approach to fisheries is RECOPESCA [13,14], where fishing vessels are voluntarily equipped with small sensors that record data on average catches and physical parameters, such as temperature or salinity. Information modules installed on board vessels automatically transmit data to the ground base station and the relevant monitoring center;
- •
- Distributed ocean monitoring systems with measurement instruments for environmental diagnostics located near hazardous economic facilities [15]; These may include burial sites, industrial facilities, large ferry services, nuclear power stations, oil platforms, gas pipelines, etc.;
- •
Sensor Requirements for Accompanying Measurements
- •
- plankton concentration;
- •
- plankton concentration by major taxa;
- •
- average size and size dispersion of individuals;
- •
- particle size distribution;
- •
- average size of individuals and size dispersion within major taxa;
- •
- particle size distribution within major taxa;
- •
- water turbidity (turbidimetric parameter);
- •
- suspension statistics (histogram by non-living particle size);
- •
- parameters characterizing the vital activity of test organisms.
2. Materials and Methods
- A buoy with variable buoyancy is used. In this case, measurements are made without the ability to control data recording and processing parameters in real time. Autonomous on-board power is used. This option of power supply, as well as the methods of communication, information reading, and constructing a route for the considered case are presented in line No. 1, Table 2. The same mode may be applied for AUV with Wi-Fi or radio frequency data transmission when, for example, a glider is surfacing.
- A hydrobiological probe is submerged from a shipboard (including the accompanying one), equipped with a standard winch with a paired SPC cable. Thus, the previous scenario is realized, but with ship power and the possibility of laying specified routes (line No. 2, Table 2);
- A hydrobiological probe is submerged from a shipboard equipped with a standard winch with a FOCL SPC cable. This ensures real-time data processing (line No. 3, Table 2).
- Launch of the DHC software on the on-board computer, probe submersion, implementation of items 1 and 2, Figure 3b (depending on the task, partial implementation of item 3 is possible);
- If it is necessary to back up the hydrophysical data, the hydrophysics software, which works with SCADA support (Supervision Control And Data Acquisition) of ZETVIEW system, is launched before submersion [44];
- To transmit data to the ship computer after lifting the probe, a Wi-Fi network or direct cable connection is used;
- Data processing (items 3–7, Figure 3b) using parallel calculation algorithms and architectures is performed on the ship computer.
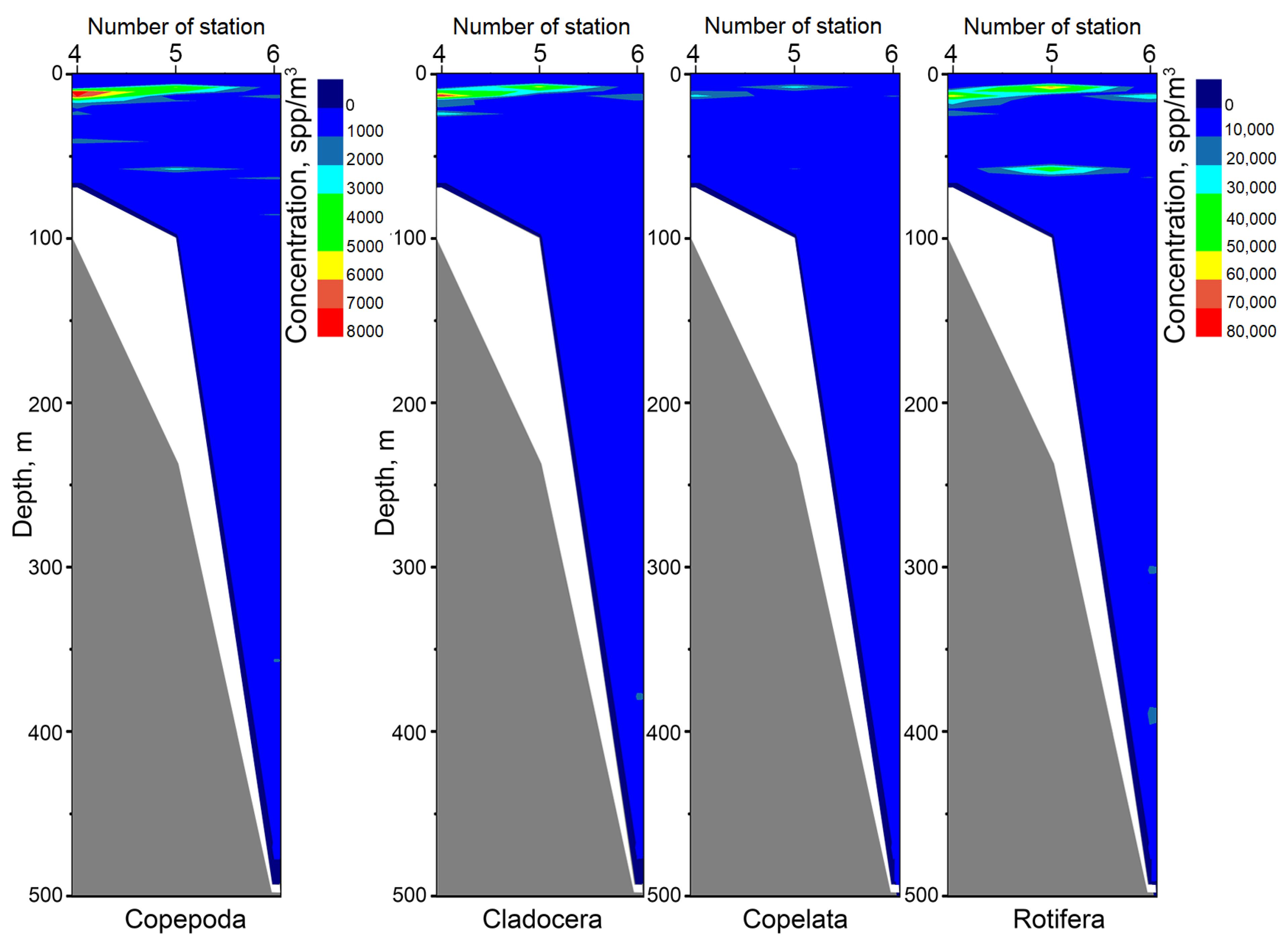
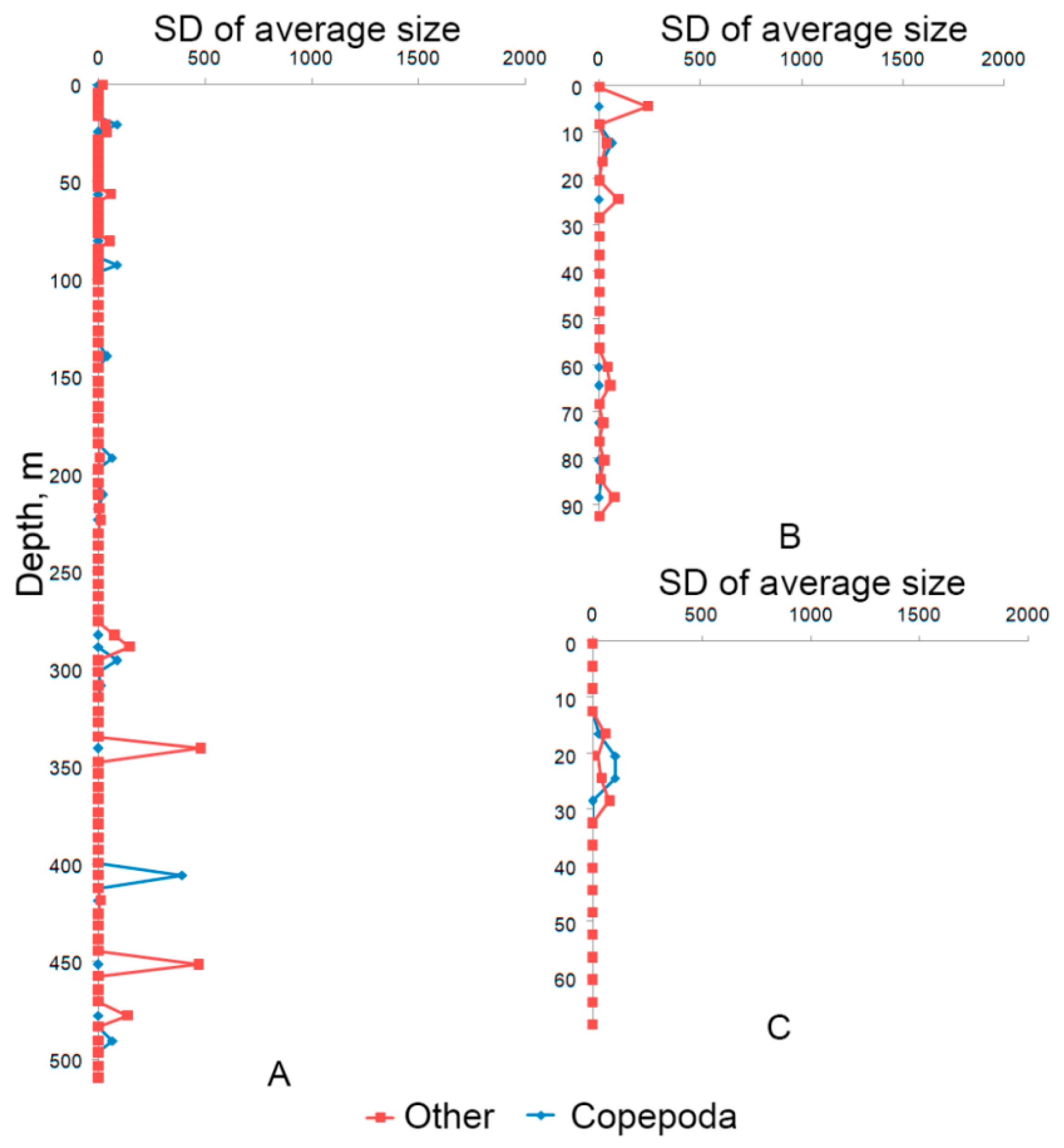
3. Results and Discussion
- 6%—according to the total concentration of zooplankton individuals,
- 23%—Cladocera concentration,
- 11%—Copepoda concentration,
- 11%—Other taxon concentration.
4. Conclusions
- •
- plankton concentration;
- •
- plankton concentration by major taxa;
- •
- average size and size dispersion of individuals;
- •
- particle size distribution;
- •
- average size of individuals and size dispersion within major taxa;
- •
- particle size distribution within major taxa;
- •
- water turbidity (calculated as the total fraction of the cross-sectional area of volume overlapped by the sections of the particles);
- •
- suspension statistics (histogram by non-living particle size).
- •
- small dimensions (length—320.5 mm; diameter—142 mm; weight—9 kg);
- •
- large analyzed volume per one exposure—0.5 l, in the count formation mode in 1 m—5 l;
- •
- possibility to calculate measurement errors of marine particle parameters and perform averaging according to the specified volume;
- •
- capability of combining with standard hydrophysical sensors (temperature sensor, pressure sensor, etc.).
- •
- 6%—total concentration of zooplankton,
- •
- 23%—Cladocera concentration,
- •
- 11%—Copepoda concentration,
- •
- 11%—Other taxon concentration.
- •
- adding the particle compactness parameter to the number of features for classification
- •
- introduction of elements of adaptive control of the sample size of experimental data in the formation of counts for plankton
- •
- comparison of the results of suspension turbidimetry with alternative measurement methods.
5. Patents
- Dyomin V.V., Polovtsev I.G., Olshukov A.S. The method of registration of plankton. The patent of the Russian Federation No. 623984 dated 31.08.2016.
- Dyomin V.V., Davydova A.Yu., Kirillov N.S., Olshukov A.S., Polovtsev I.G. The method of recording the integral size-quantitative characteristics of plankton. The patent of the Russian Federation No. 2690976 dated 09.11.2018.
- Dyomin V.V., Olshukov A.S., Davydova A.Yu., Kirillov N.S. DHC-Plankton V1.2. Certificate of state registration of computer programs No. 2019667359 dated 23.12.2019.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunagawa, S.; Acinas, S.G.; Bork, P.; Bowler, C.; Acinas, S.G.; Babin, M.; Bork, P.; Boss, E.; Bowler, C.; Cochrane, G.; et al. Tara Oceans: Towards Global Ocean Ecosystems Biology. Nat. Rev. Microbiol. 2020, 18, 428–445. [Google Scholar] [CrossRef]
- Graham, G.W.; Nimmo Smith, W.A.M. The Application of Holography to the Analysis of Size and Settling Velocity of Suspended Cohesive Sediments. Limnol. Oceanogr. Methods 2010, 8, 1–15. [Google Scholar] [CrossRef]
- Schmid, M.S.; Aubry, C.; Grigor, J.; Fortier, L. The LOKI Underwater Imaging System and an Automatic Identification Model for the Detection of Zooplankton Taxa in the Arctic Ocean. Methods Oceanogr. 2016, 15, 129–160. [Google Scholar] [CrossRef]
- Balykin, P.A.; Bonk, A.A.; Startsev, A.V. Stock Assessment and Management of Marine Fish Fisheries. In Textbook for Students in the Direction 111400.62, 111.68, 35.03.08, 35.04.07 “Aquatic Biological Resources and Aquaculture” Full-Time and Part-Time Education; World Wildlife Fund (WWF): Morges, Switzerland, 2014; p. 68. [Google Scholar]
- Lombard, F.; Boss, E.; Waite, A.M.; Vogt, M.; Uitz, J.; Stemmann, L.; Sosik, H.M.; Schulz, J.; Romagnan, J.-B.; Picheral, M.; et al. Globally Consistent Quantitative Observations of Planktonic Ecosystems. Front. Mar. Sci. 2019, 6, 196. [Google Scholar] [CrossRef] [Green Version]
- Chiba, S.; Batten, S.; Martin, C.S.; Ivory, S.; Miloslavich, P.; Weatherdon, L.V. Zooplankton Monitoring to Contribute towards Addressing Global Biodiversity Conservation Challenges. J. Plankton Res. 2018, 40, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller-Karger, F.E.; Miloslavich, P.; Bax, N.J.; Simmons, S.; Costello, M.J.; Sousa Pinto, I.; Canonico, G.; Turner, W.; Gill, M.; Montes, E.; et al. Advancing Marine Biological Observations and Data Requirements of the Complementary Essential Ocean Variables (EOVs) and Essential Biodiversity Variables (EBVs) Frameworks. Front. Mar. Sci. 2018, 5, 211. [Google Scholar] [CrossRef]
- Batten, S.D.; Abu-Alhaija, R.; Chiba, S.; Edwards, M.; Graham, G.; Jyothibabu, R.; Kitchener, J.A.; Koubbi, P.; McQuatters-Gollop, A.; Muxagata, E.; et al. A Global Plankton Diversity Monitoring Program. Front. Mar. Sci. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Argo Data and How to Get It. Available online: http://www.argo.ucsd.edu/Argo_data_and.html (accessed on 21 February 2020).
- COPEPOD-the Global Plankton Database Project. Available online: https://www.st.nmfs.noaa.gov/plankton/ (accessed on 14 October 2020).
- JCOMM. Available online: https://www.jcomm.info/index.php?option=com_oe&task=viewDoclistRecord&doclistID=112 (accessed on 27 December 2020).
- Vargas, C.D.; Pollina, T.; Romac, S.; Bescot, N.L.; Henry, N.; Mahé, F.; Malpot, E.; Beaumont, C.; Hardy, M. Plankton Planet: ‘Seatizen’ Oceanography to Assess Open Ocean Life at the Planetary Scale. bioRxiv 2020, 1–36. [Google Scholar] [CrossRef]
- Leblond, E.; Lazure, P.; Laurans, M.; Rioual, C.; Woerther, P.; Quemener, L.; Berthou, P. RECOPESCA: A New Example of Participative Approach to Collect In-Situ Environmental and Fisheries Data. Mercat. Ocean 2010, 37, 40–48. [Google Scholar]
- NeXOS Project. Available online: http://www.nexosproject.eu/ (accessed on 21 February 2020).
- Marchuk, G.I.; Paton, B.E.; Korotaev, G.K.; Zalesny, V.B. Data-Computing Technologies: A New Stage in the Development of Operational Oceanography. Izv. Atmos. Ocean Phys. 2013, 49, 579–591. [Google Scholar] [CrossRef]
- Vayena, E.; Tasioulas, J. “We the Scientists”: A Human Right to Citizen Science. Philos. Technol. 2015, 28, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Vann-Sander, S.; Clifton, J.; Harvey, E. Can Citizen Science Work? Perceptions of the Role and Utility of Citizen Science in a Marine Policy and Management Context. Mar. Policy 2016, 72, 82–93. [Google Scholar] [CrossRef]
- Chai, F.; Johnson, K.S.; Claustre, H.; Xing, X.; Wang, Y.; Boss, E.; Riser, S.; Fennel, K.; Schofield, O.; Sutton, A. Monitoring Ocean Biogeochemistry with Autonomous Platforms. Nat. Rev. Earth Environ. 2020, 1, 315–326. [Google Scholar] [CrossRef]
- Miloslavich, P.; Pearlman, F.; Pearlman, J.; Muller-karger, F.; Appeltans, W.; Batten, S.; Kudela, R.; López, A.L.; Thompson, P. Implementation of Global, Sustained and Multidisciplinary Observations of Plankton Communities. GOOS Report 2018, 230, 1–53. [Google Scholar]
- Dyomin, V.; Davydova, A.; Morgalev, Y.; Olshukov, A.; Polovtsev, I.; Morgaleva, T.; Morgalev, S. Planktonic Response to Light as a Pollution Indicator. J. Great Lakes Res. 2020, 46, 41–47. [Google Scholar] [CrossRef]
- Dyomin, V.V.; Davydova, A.Y.; Morgalev, S.N.; Kirillov, N.S.; Olshukov, A.; Polovtsev, I.; Davydov, S. Monitoring of Plankton Spatial and Temporal Characteristics With the Use of a Submersible Digital Holographic Camera. Front. Mar. Sci. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- SBE 19plus V2 SeaCAT Profiler CTD Sea-Bird Scientific-Overview Sea-Bird. Available online: https://www.seabird.com/profiling/sbe-19plus-v2-seacat-profiler-ctd/family?productCategoryId=54627473770 (accessed on 28 June 2021).
- Cowen, R.K.; Guigand, C.M. In Situ Ichthyoplankton Imaging System (ISIIS): System Design and Preliminary Results. Limnol. Oceanogr. Methods 2008, 6, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Lertvilai, P. The in Situ Plankton Assemblage eXplorer (IPAX): An Inexpensive Underwater Imaging System for Zooplankton Study. Methods Ecol. Evol. 2020, 11, 1042–1048. [Google Scholar] [CrossRef]
- Davis, C.S.; Hu, Q.; Gallager, S.M.; Tang, X.; Ashjian, C.J. Real-Time Observation of Taxa-Specific Plankton Distributions: An Optical Sampling Method. Mar. Ecol. Prog. Ser. 2004, 284, 77–96. [Google Scholar] [CrossRef]
- Ohman, M.D.; Powell, J.R.; Picheral, M.; Jensen, D.W. Mesozooplankton and Particulate Matter Responses to a Deep-Water Frontal System in the Southern California Current System. J. Plankton Res. 2012, 34, 815–827. [Google Scholar] [CrossRef] [Green Version]
- LISST-Holo2-Sequoia ScientificSequoia Scientific. Available online: http://www.sequoiasci.com/product/lisst-holo/ (accessed on 10 June 2021).
- Ouillon, S. Why and How Do We Study Sediment Transport? Focus on Coastal Zones and on Going Methods. Water 2018, 10, 390. [Google Scholar] [CrossRef]
- HoloSea: Submersible Holographic Microscope-4Deep. Available online: http://4-deep.com/products/submersible-microscope/ (accessed on 7 January 2020).
- Rotermund, L.M.; Samson, J. A Submersible Holographic Microscope for 4-D In-Situ Studies of Micro-Organisms in the Ocean with Intensity and Quantitative Phase Imaging. J. Mar. Sci. Res. Dev. 2015, 6, 1–5. [Google Scholar] [CrossRef]
- Sun, H.; Benzie, P.; Burns, N.; Hendry, D.; Player, M.; Watson, J. Underwater Digital Holography for Studies of Marine Plankton. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2008, 366, 1789–1806. [Google Scholar] [CrossRef]
- Nayak, A.R.; McFarland, M.N.; Sullivan, J.M.; Twardowski, M.S. Evidence for Ubiquitous Preferential Particle Orientation in Representative Oceanic Shear Flows. Limnol. Oceanogr. 2018, 63, 122–143. [Google Scholar] [CrossRef] [Green Version]
- Owen, R.B.; Zozulya, A.A. In-Line Digital Holographic Sensor for Monitoring and Characterizing Marine Particulates. Opt. Eng. 2000, 39, 2187–2197. [Google Scholar] [CrossRef]
- Bochdansky, A.B.; Jericho, M.H.; Herndl, G.J. Development and Deployment of a Point-Source Digital Inline Holographic Microscope for the Study of Plankton and Particles to a Depth of 6000 m. Limnol. Oceanogr. Methods 2013, 11, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Dyomin, V.V.; Polovtsev, I.G.; Davydova, A.Y.; Olshukov, A.S. Digital Holographic Camera for Plankton Monitoring. In Proceedings of the Practical Holography XXXIII: Displays, Materials, and Applications, San Francisco, CA, USA, 2–7 February 2019; Bjelkhagen, H.I., Bove, V.M., Eds.; SPIE: Bellingham, WA, USA, 2019. [Google Scholar]
- Nayak, A.R.; Malkiel, E.; McFarland, M.N.; Twardowski, M.S.; Sullivan, J.M. A Review of Holography in the Aquatic Sciences: In Situ Characterization of Particles, Plankton, and Small Scale Biophysical Interactions. Front. Mar. Sci. 2021, 7, 1–16. [Google Scholar] [CrossRef]
- Giering, S.L.C.; Cavan, E.L.; Basedow, S.L.; Briggs, N.; Burd, A.B.; Darroch, L.J.; Guidi, L.; Irisson, J.-O.; Iversen, M.H.; Kiko, R.; et al. Sinking Organic Particles in the Ocean—Flux Estimates from in Situ Optical Devices. Front. Mar. Sci. 2020, 6, 834. [Google Scholar] [CrossRef] [Green Version]
- Benfield, M.C.; Grosjean, P.; Culverhouse, P.F.; Irigoien, X.; Sieracki, M.E.; Lopez-Urrutia, A.; Dam, H.G.; Hu, Q.; Davis, C.S.; Hanson, A.; et al. RAPID: Research on Automated Plankton Identification. Oceanography 2007, 20, 172–218. [Google Scholar] [CrossRef]
- Dyomin, V.; Davydova, A.; Olshukov, A.; Polovtsev, I. Hardware Means for Monitoring Research of Plankton in the Habitat: Problems, State of the Art, and Prospects. In Proceedings of the OCEANS 2019-Marseille, Marseille, France, 17–20 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–5. [Google Scholar]
- Dyomin, V.; Davydova, A.; Davydov, S.; Kirillov, N.; Morgalev, Y.; Olshukov, A.; Polovtsev, I. Hydrobiological Probe for the in Situ Study and Monitoring of Zooplankton. In Proceedings of the 2019 IEEE Underwater Technology (UT), Kaohsiung, Taiwan, 16–19 April 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–6. [Google Scholar]
- Dyomin, V.; Olshukov, A.; Davydova, A. Data Acquisition from Digital Holograms of Particles. In Proceedings of the Unconventional Optical Imaging, Strasbourg, France, 22–26 April 2018; Fournier, C., Georges, M.P., Popescu, G., Eds.; SPIE: Bellingham, WA, USA, 2018; Volume 10677, p. 106773B. [Google Scholar]
- Colier, R.; Burckhardt, C.; Lin, L. Optical Holography; Academic Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Schnars, U.; Jueptner, W. Digital Holography; Springer: Berlin/Heidelberg, Germany, 2005; ISBN 3-540-21934-X. [Google Scholar]
- SCADA System ZETVIEW-Description, Application Features. Available online: https://zetlab.com/en/shop/virtual-devices/scada-zetview/zetview-scada-system/ (accessed on 27 April 2021).
- WoRMS-World Register of Marine Species. Available online: http://www.marinespecies.org/ (accessed on 27 April 2021).
- Marine Species Identification Portal. Available online: http://species-identification.org/ (accessed on 27 April 2021).
- Dyomin, V.V.; Polovtsev, I.G.; Davydova, A.Y. Fast Recognition of Marine Particles in Underwater Digital Holography. In Proceedings of the 23rd International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics, Irkutsk, Russian Federation, 3–7 July 2017; Romanovskii, O.A., Matvienko, G.G., Eds.; SPIE: Bellingham, WA, USA, 2017; Volume 10466, p. 1046627. [Google Scholar]
- Li, W.; Goodchild, M.F.; Church, R. An Efficient Measure of Compactness for Two-Dimensional Shapes and Its Application in Regionalization Problems. Int. J. Geogr. Inf. Sci. 2013, 27, 1227–1250. [Google Scholar] [CrossRef] [Green Version]
- Dyomin, V.V.; Polovtsev, I.; Davydova, A.; Olshukov, A.S.; Kirillov, N.; Davydov, S. Underwater Holographic Sensors for Plankton Studies in Situ. In Proceedings of the Ocean Sensing and Monitoring XII, Online Only, CA, USA, 27 April–9 May 2020; Hou, W., Arnone, R., Eds.; SPIE: Bellingham, WA, USA, 2020. [Google Scholar]
- Goswami, S.C. Zooplankton Methodology, Collection&Identification–A field Manual; National Institute of Oceanography: Dona Paula, Goa, 2004; p. 16. [Google Scholar]
- Kovalev, A.V.; Mazzocchi, M.G.; Siokou, I.; Kideys, A.E. Zooplankton of the Black Sea and the Eastern Mediterranean: Similarities and Dissimilarities. Mediterr. Mar. Sci. 2001, 2, 69. [Google Scholar] [CrossRef] [Green Version]
- Hernández-León, S.; Montero, I. Zooplankton Biomass Estimated from Digitalized Images in Antarctic Waters: A Calibration Exercise. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Lehette, P.; Hernández-León, S. Zooplankton Biomass Estimation from Digitized Images: A Comparison between Subtropical and Antarctic Organisms. Limnol. Oceanogr. Methods 2009, 7, 304–308. [Google Scholar] [CrossRef]
- Wiebe, P.H. Functional Regression Equations for Zooplankton Displacement Volume Wet Weight, Dry Weight, and Carbon: A Correction. Fish. Bull. 1988, 86, 833–835. [Google Scholar]
- Mack, H.R.; Conroy, J.D.; Blocksom, K.A.; Stein, R.A.; Ludsin, S.A. A Comparative Analysis of Zooplankton Field Collection and Sample Enumeration Methods. Limnol. Oceanogr. Methods 2012, 10, 41–53. [Google Scholar] [CrossRef]
- Turner, J.T. Zooplankton Fecal Pellets, Marine Snow, Phytodetritus and the Ocean’s Biological Pump. Prog. Oceanogr. 2015, 130, 205–248. [Google Scholar] [CrossRef]
- Sigman, D.M.; Hain, M.P. The Biological Productivity of the Ocean | Learn Science at Scitable. Nat. Educ. Knowl. 2012, 3, 1–16. [Google Scholar]
- Deppeler, S.L.; Davidson, A.T. Southern Ocean Phytoplankton in a Changing Climate. Front. Mar. Sci. 2017, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Haklidir, M.; Tut, F.S.; Kapkin, Ş. Possibilities of Production and Storage of Hydrogen in the Black Sea. In Proceeding of the 16th World Hydrog. Energy Conference, Lyon, France, 13–16 June 2006; Volume 4, pp. 3072–3079. [Google Scholar]
- Origin Help-Creating Contour Graphs. Available online: https://www.originlab.com/doc/Origin-Help/Create-Contour-Graph#Algorithm_for_Creating_a_Contour_from_a_Matrix (accessed on 11 May 2021).
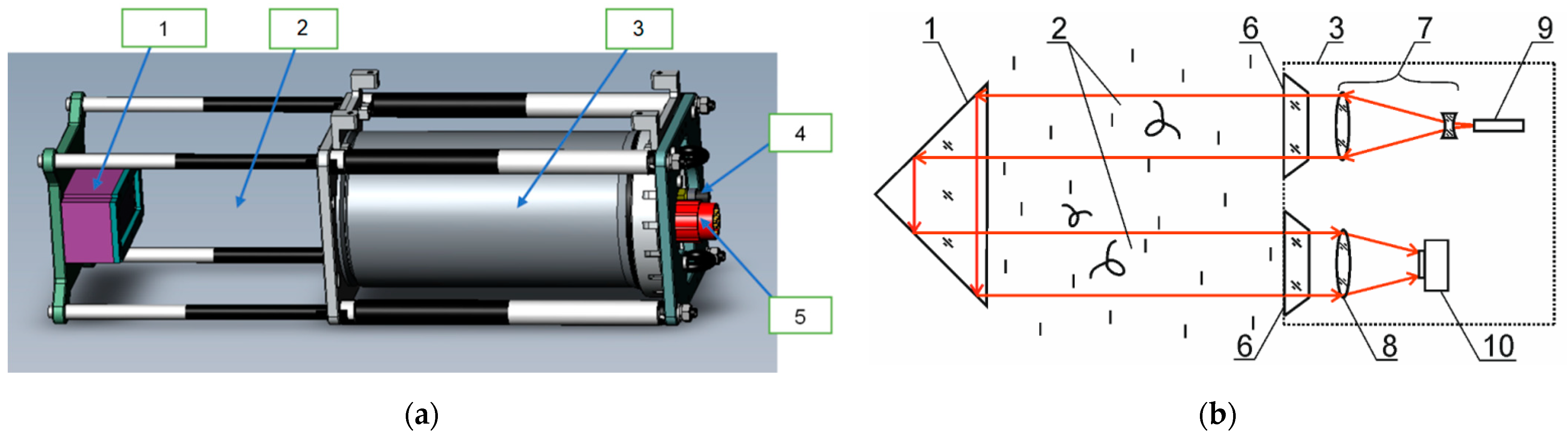
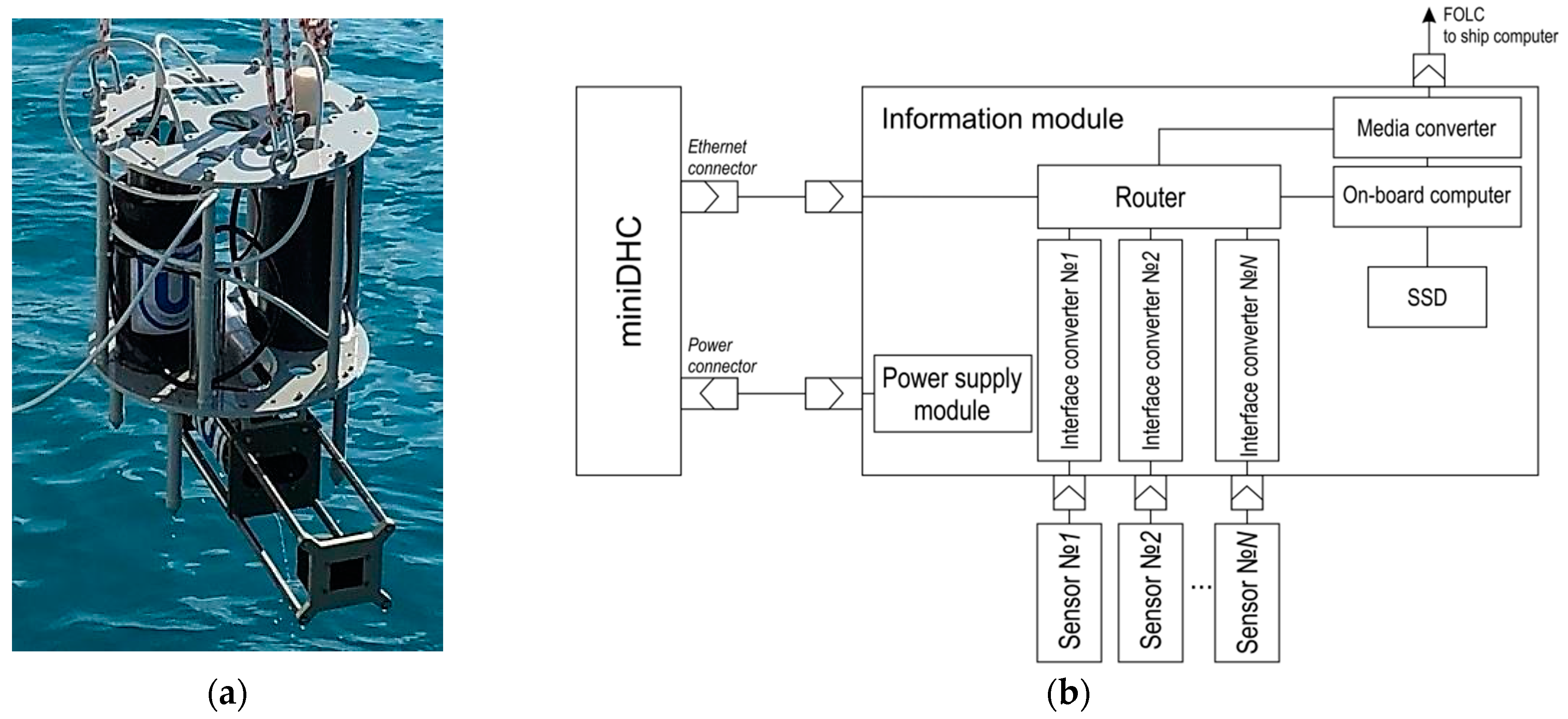
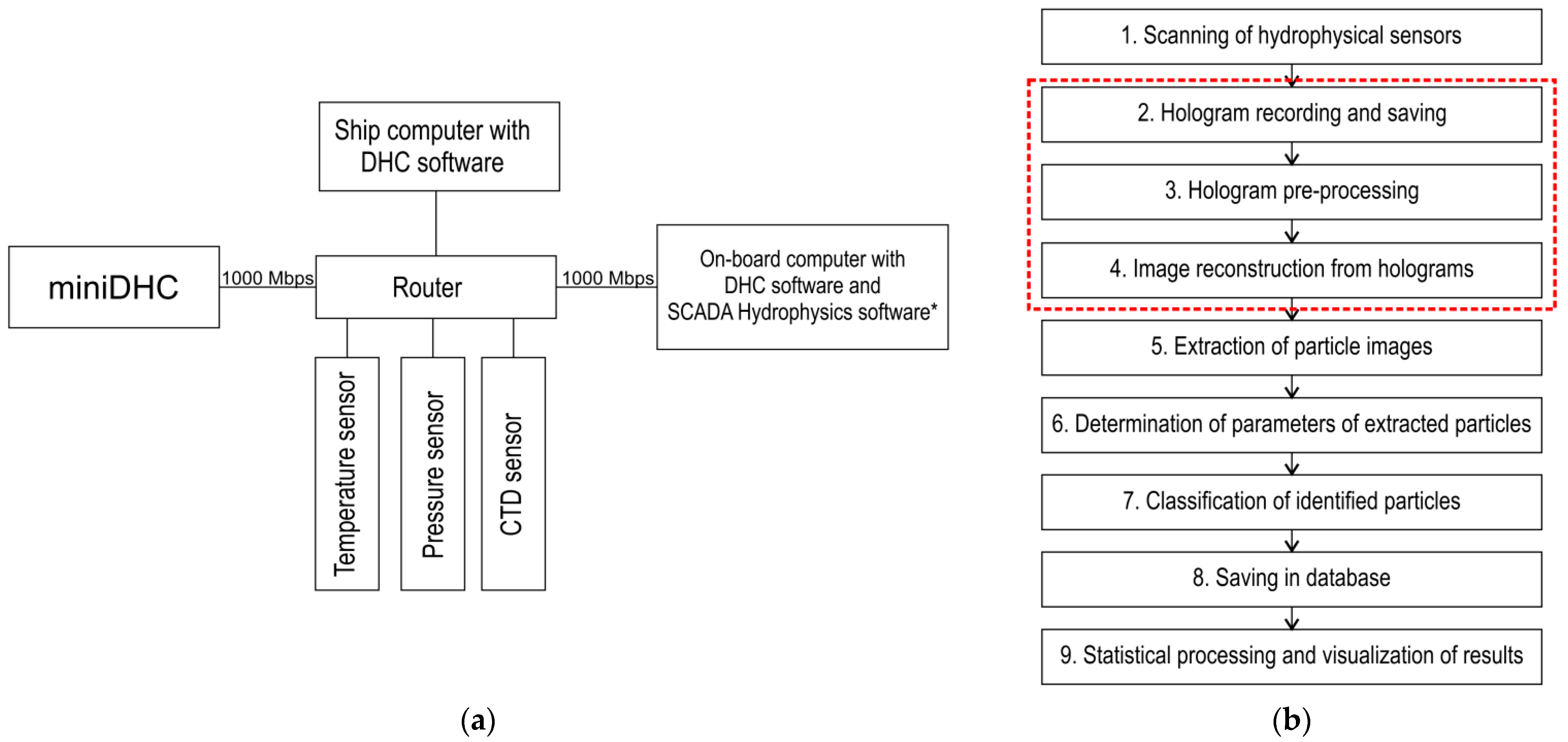
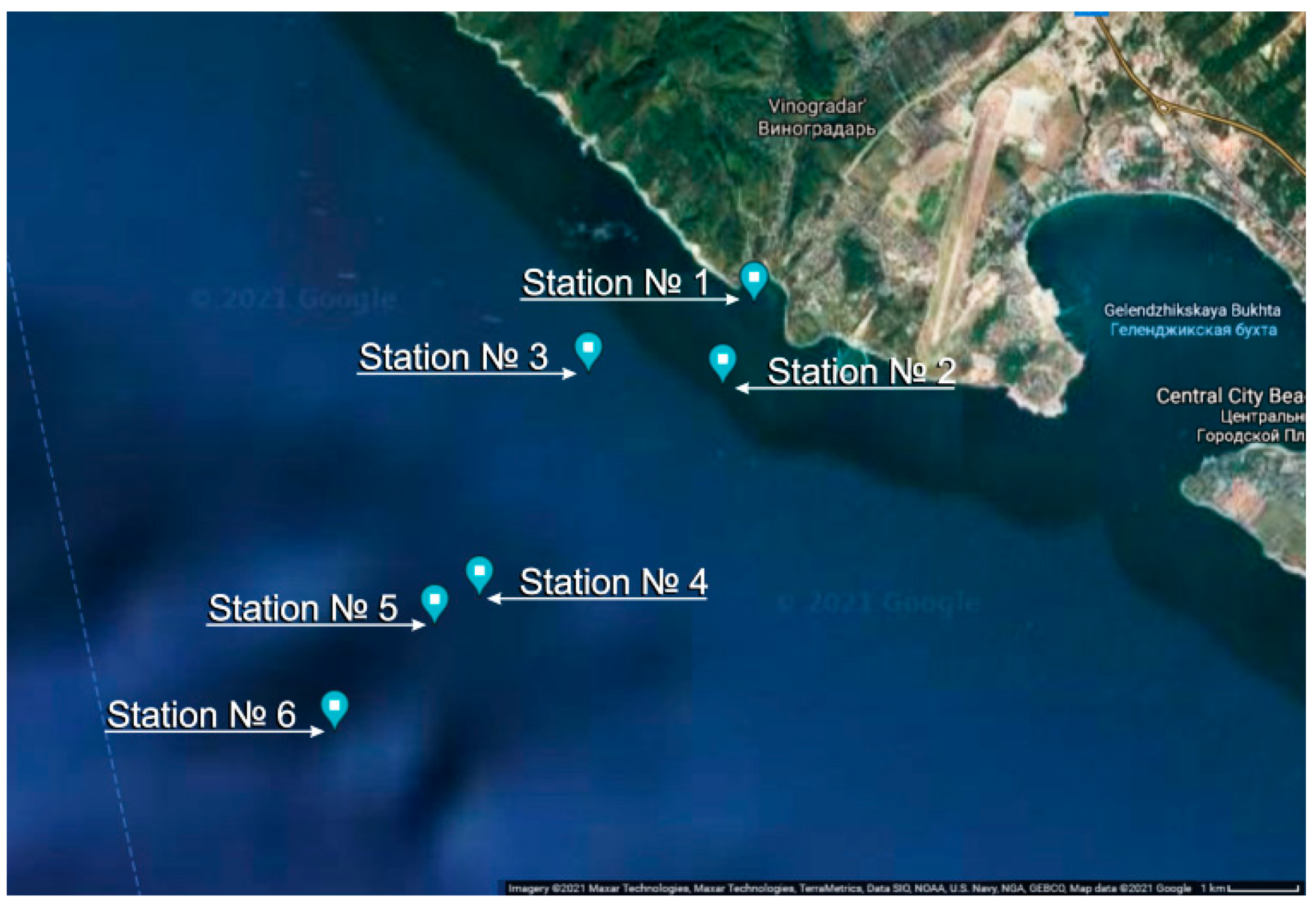
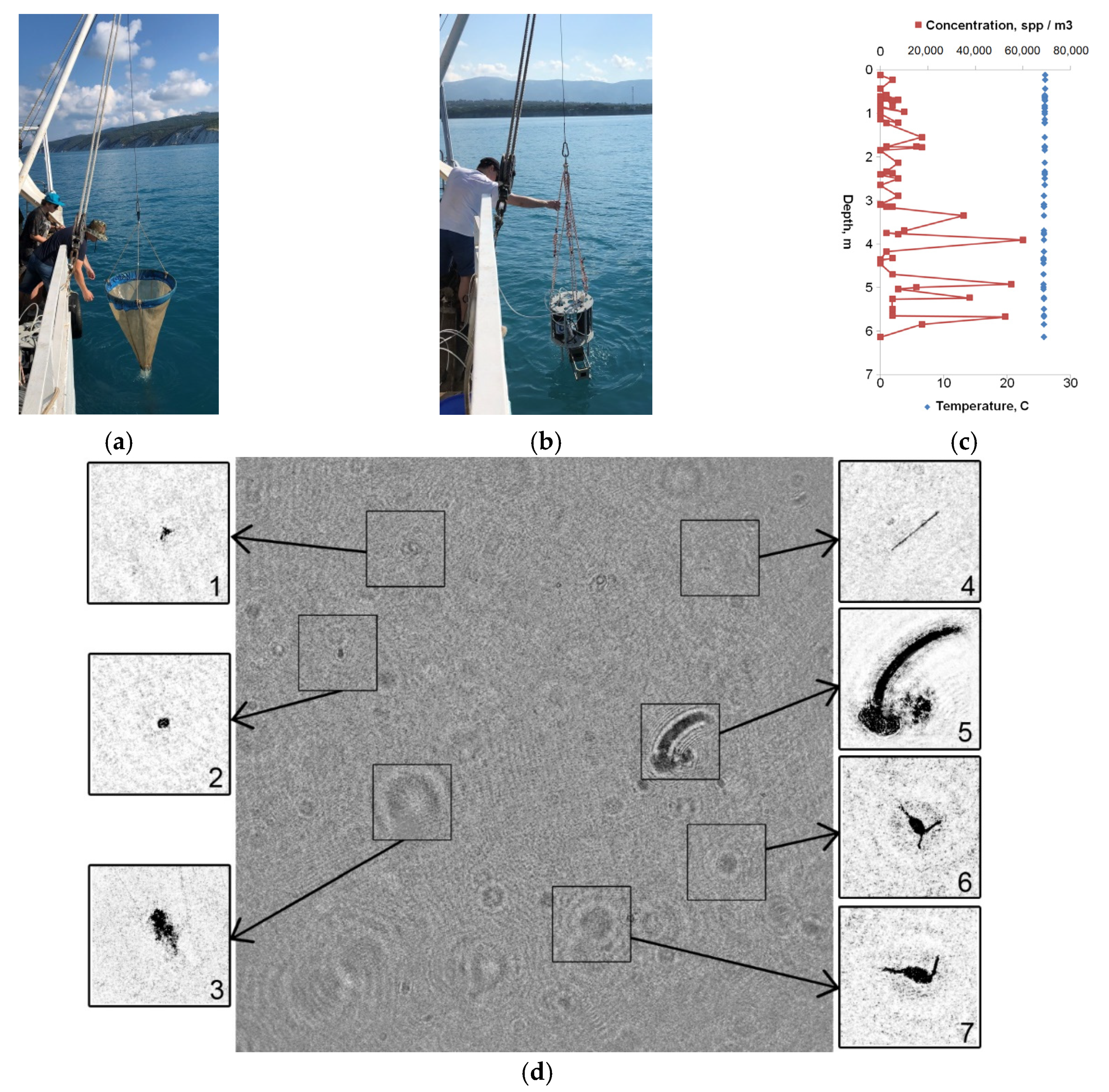
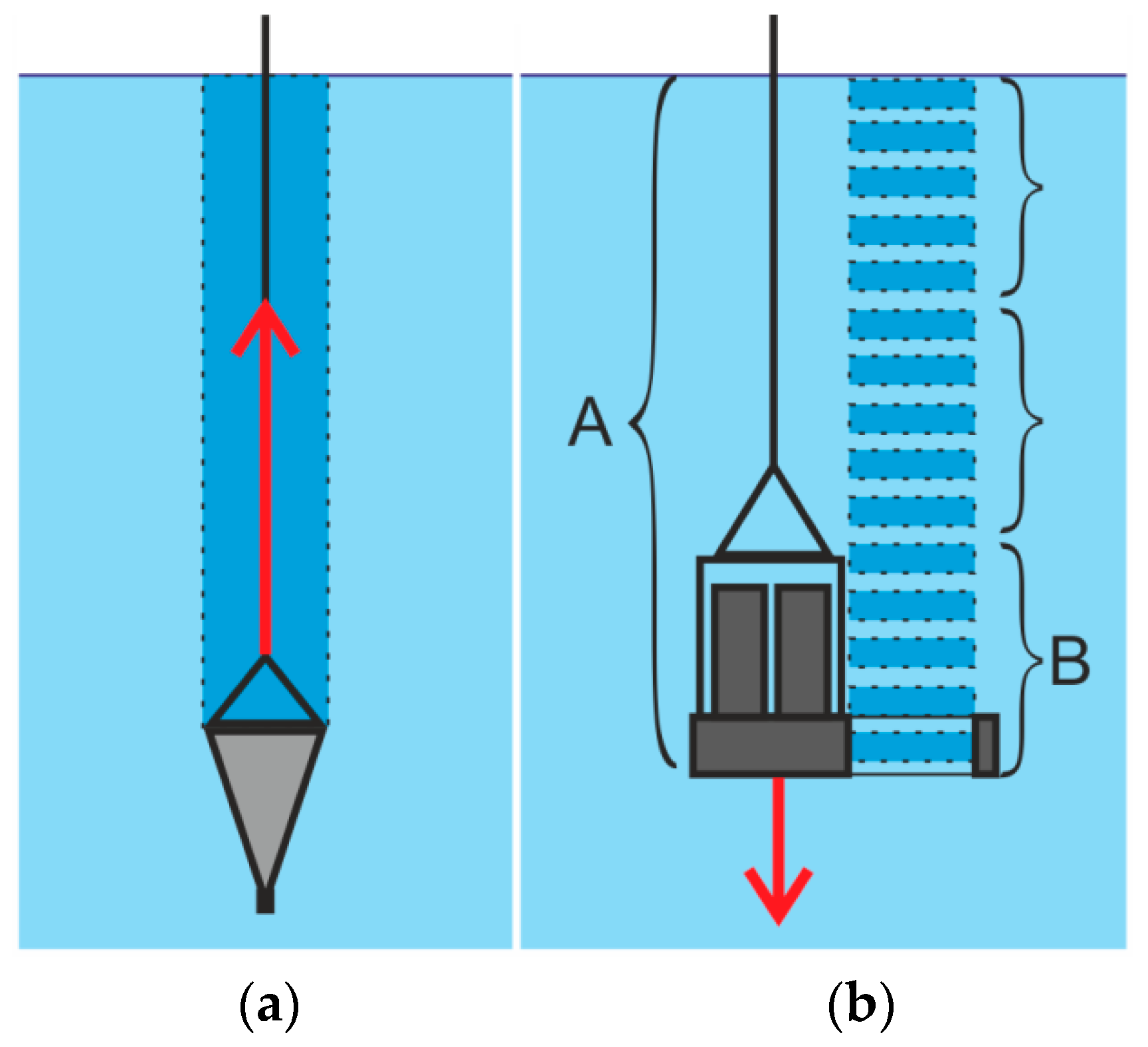
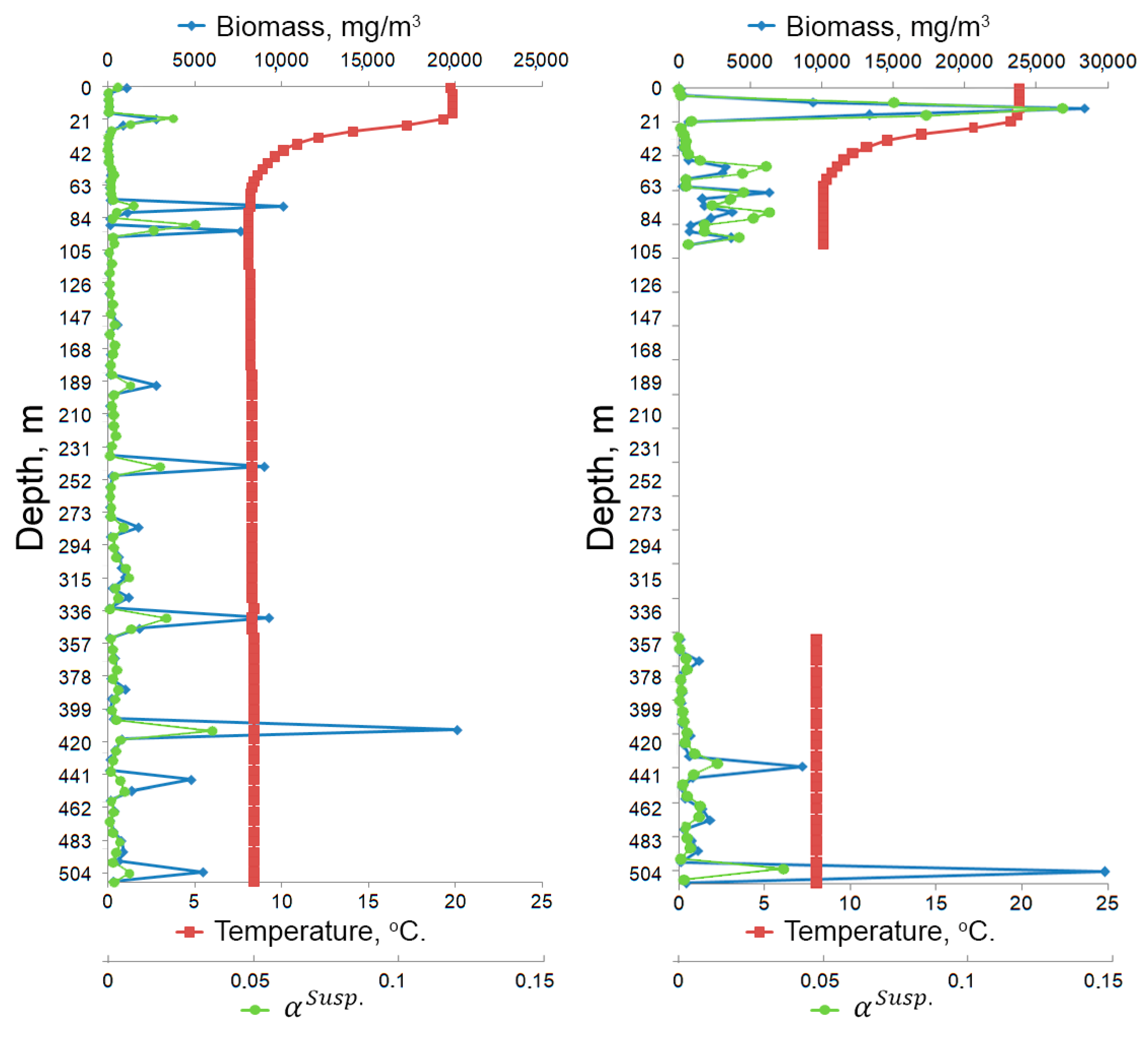

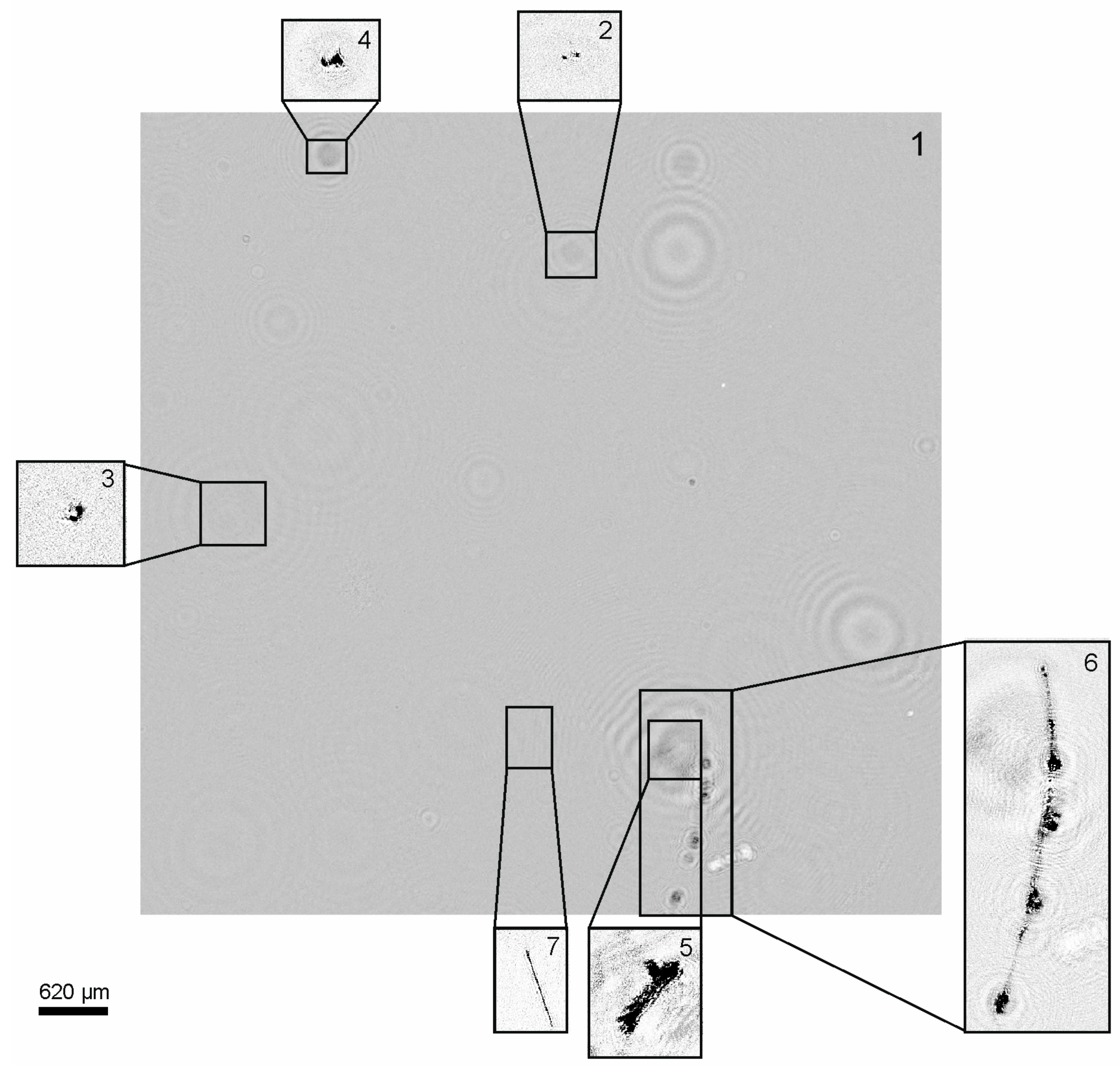

| Parameter | Value |
|---|---|
| Power characteristics: | |
| – power voltage, V | 12 |
| – power consumption, W | 20 |
| Volume studied per one exposure, l | 0.5 |
| Averaging volume, l | 5 |
| Working volume length, mm | 338.4 |
| Submersion depth, m, not more than | 500 |
| Size of measured particles, mm | 0.1–28 |
| Submersion speed, m/s | 0.1–1.0 |
| Discreteness of counts formed in real time at the submersion speed of 0.3 m/s, m | 6 |
| Ethernet transmission rate, GB/s | 1 |
| Hydrostatic pressure tolerance, atm | 60 |
| Overall dimensions (length × diameter), mm | 320.5 × 142 |
| Weight, kg, not more than | 9 |
| Approach | Measurement Management Link | Data Transmission to a Ship | Power Supply | On-Board Computer | Visual Information on Ship Computer | Route |
|---|---|---|---|---|---|---|
| Measurements with autonomous on-board power supply | - | WI-FI+ twisted pair | Battery or carrier power (buoy, glider, etc.) | Available, carrier computer (buoy, glider, etc.) may be used | - | Random, determined by the carrier motion algorithm (buoy, glider, etc.) |
| Ship-fed measurements | - | WI-FI+ twisted pair | Battery, ship’s SPC cable | Available | - | Route of an accompanying ship with a winch |
| Real-time measurement and processing | FOCL SPC cable 500 m or more | FOCL | Ship’s SPC cable | Available | Possible in real time | Route of an accompanying ship with a winch with FOCL SPC cable |
| Taxa | Presence of Antennas | H, µm | M |
|---|---|---|---|
| 1. Chaetognatha | YES | >200 | 0–0.2 |
| 2. Copepoda | YES | >200 | 0.2–0.5 |
| 3. Copelata | YES | >200 | 0.5–0.66 |
| 4. Cladocera | YES | >200 | 0.66–0.9 |
| 5. Other | YES | >200 | 0.9–1 |
| 6. Rotifera | YES | ≤200 | 0–0.9 |
| 7. Phytoplankton chain | NO | ANY | 0–0.25 |
| 8. Marine snow | NO | ANY | 0.25–0.9 |
| 9. Suspension | NO | ≤200 | 0.9–1 |
| Traditional Classification of Plankton Sample Using a Net | Plankton Classification Using the miniDHC | |||
|---|---|---|---|---|
| n/n | Organism | Concentration, spp/m3 | Taxon | Concentration, spp/m3 |
| 1 | Sagitta setosa < 10 mm | 7.05 | Chaetognatha | 0 |
| 2 | Copepoda < 1 mm | 430.53 | Copepoda | 720.33 |
| 3 | Nauplii Copepoda | 38.95 | ||
| 4 | Acartia clausi | 0.84 | ||
| 5 | Centropages kroyeri | 307.37 | ||
| 6 | Oithona davisae | 34.74 | ||
| 7 | Harpacticoida | 0.63 | ||
| 8 | Oikopleura dioica | 0.32 | Appendicularia | 0 |
| 9 | Plepois polyphemoides | 54.74 | Cladocera | 42.37 |
| 10 | Noctiluca miliaris | 0.11 | Other | 400.84 |
| 11 | Larvae Gastropoda | 326.32 | ||
| 12 | Larvae Bivalvia | 0.32 | ||
| 13 | Larvae Polychaeta | 1.05 | ||
| 14 | Larvae Decapoda | 1.37 | ||
| 15 | Nauplii Cirripedia | 42.11 | ||
| 16 | Cypris st., Ostracoda | 0.11 | ||
| 17 | Ova Fish | 1.79 | ||
| 18 | Actinotrocha | 0.21 | ||
| Total | 1248.53 | 1163.54 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyomin, V.; Davydova, A.; Polovtsev, I.; Olshukov, A.; Kirillov, N.; Davydov, S. Underwater Holographic Sensor for Plankton Studies In Situ including Accompanying Measurements. Sensors 2021, 21, 4863. https://doi.org/10.3390/s21144863
Dyomin V, Davydova A, Polovtsev I, Olshukov A, Kirillov N, Davydov S. Underwater Holographic Sensor for Plankton Studies In Situ including Accompanying Measurements. Sensors. 2021; 21(14):4863. https://doi.org/10.3390/s21144863
Chicago/Turabian StyleDyomin, Victor, Alexandra Davydova, Igor Polovtsev, Alexey Olshukov, Nikolay Kirillov, and Sergey Davydov. 2021. "Underwater Holographic Sensor for Plankton Studies In Situ including Accompanying Measurements" Sensors 21, no. 14: 4863. https://doi.org/10.3390/s21144863






