Surface Wettability Tuning of Acrylic Resin Photoresist and Its Aging Performance
Abstract
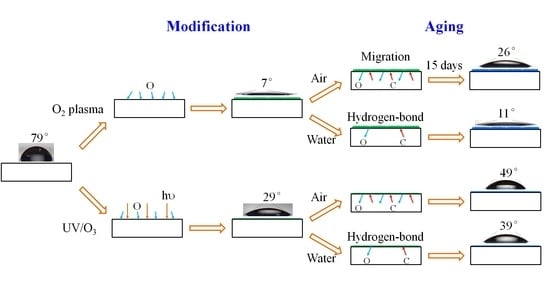
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Photoresist Surface Preparation and Modification
2.3. Contact Angle Measurement
2.4. Physical Roughness and Chemical Characterizations
3. Results and Discussions
3.1. Hydrophilic Treatment by Oxygen Plasma or UV/Ozone to Photoresist Surface
3.1.1. Wettability Tuning
3.1.2. Chemical and Physical Characterizations
3.2. Wettability Aging with Time in Air or in Water Atmosphere
3.2.1. Contact Angle Variation during Aging
3.2.2. Chemical and Physical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Midwoud, P.M.; Janse, A.; Merema, M.T.; Groothuis, G.M.; Verpoorte, E. Comparison of biocompatibility and adsorption properties of different plastics for advanced microfluidic cell and tissue culture models. Anal. Chem. 2012, 84, 3938–3944. [Google Scholar] [CrossRef]
- Matsui, H.; Oaki, Y.; Imai, H. Surface-functionalized hydrophilic monolayer of titanate and its application for dopamine detection. Chem. Commun. 2016, 52, 9466–9469. [Google Scholar] [CrossRef]
- Wang, K.; Hou, D.; Wang, J.; Wang, Z.; Tian, B.; Liang, P. Hydrophilic surface coating on hydrophobic PTFE membrane for robust anti-oil-fouling membrane distillation. Appl. Surf. Sci. 2018, 450, 57–65. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Zinadini, S.; Gholami, F. Preparation and characterization of high flux PES nanofiltration membrane using hydrophilic nanoparticles by phase inversion method for application in advanced wastewater treatment. J. Appl. Res. Water Wastewater 2016, 3, 232–235. [Google Scholar]
- Lu, Z.; Wang, P.; Zhang, D. Super-hydrophobic film fabricated on aluminium surface as a barrier to atmospheric corrosion in a marine environment. Corros. Sci. 2015, 91, 287–296. [Google Scholar] [CrossRef]
- Syed, J.A.; Tang, S.; Meng, X. Super-hydrophobic multilayer coatings with layer number tuned swapping in surface wettability and redox catalytic anti-corrosion application. Sci. Rep. 2017, 7, 4403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Q.; Zhou, J.E.; Wang, Y.; Liang, J.; Zhang, X.; Cerneaux, S.; Wang, X.; Zhu, Z.; Dong, Y. Application of ceramic microfiltration membrane modified by nano-TiO2 coating in separation of a stable oil-in-water emulsion. J. Membr. Sci. 2014, 456, 128–133. [Google Scholar] [CrossRef]
- Zhong, H.; Jiang, Y.; Zeng, G.; Liu, Z.; Liu, L.; Liu, Y.; Yang, X.; Lai, M.; He, Y. Effect of low-concentration rhamnolipid on adsorption of Pseudomonas aeruginosa ATCC 9027 on hydrophilic and hydrophobic surfaces. J. Hazard. Mater. 2015, 285, 383–388. [Google Scholar] [CrossRef]
- Oribayo, O.; Pan, Q.; Feng, X.; Rempel, G.L. Hydrophobic Surface Modification of FMSS and its Application as Effective Sorbents for Oil Spill Clean-ups and Recovery. Aiche J. 2017, 63, 4090–4102. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, J.; Ye, H.; Zhao, F.; Zeng, B. Preparation of hydrophilic surface-imprinted ionic liquid polymer on multi-walled carbon nanotubes for the sensitive electrochemical determination of imidacloprid. RSC Adv. 2017, 7, 4704–4709. [Google Scholar] [CrossRef] [Green Version]
- Roques-Carmes, T.; Palmier, S.; Hayes, R.A.; Schlangen, L.J.M. The effect of the oil/water interfacial tension on electrowetting driven fluid motion. Colloids Surf. A 2005, 267, 56–63. [Google Scholar] [CrossRef]
- Ali, A.; Shaker, K.; Nawab, Y.; Jabbar, M.; Hussain, T.; Militky, J.; Baheti, V. Hydrophobic treatment of natural fibers and their composites—A review. J. Ind. Text. 2016, 47, 2153–2183. [Google Scholar] [CrossRef]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.; Lo Porto, C.; Favia, P. Plasma Nano-Texturing of Polymers for Wettability Control: Why, What and How. Coatings 2019, 9, 640. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Lu, X.; Lu, Q. Electrically conductive PEDOT coating with self-healing superhydrophobicity. Langmuir 2014, 30, 4671–4677. [Google Scholar] [CrossRef]
- Jaleh, B.; Etivand, E.S.; Mohazzab, B.F.; Nasrollahzadeh, M.; Varma, R.S. Improving Wettability: Deposition of TiO2 Nanoparticles on the O2 Plasma Activated Polypropylene Membrane. Int. J. Mol. Sci. 2019, 20, 3309. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.M.; Fontoura, C.P.; Garcia, C.S.C.; Martins, S.T.; Henriques, J.A.P.; Figueroa, C.A.; Roesch-Ely, M.; Aguzzoli, C. Investigation of plasma treatment on UHMWPE surfaces: Impact on physicochemical properties, sterilization and fibroblastic adhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 264–275. [Google Scholar] [CrossRef]
- Chen, F.; Liu, J.; Yao, C.; Huang, S.; Song, J.; Sun, J.; Xu, W.; Liu, X. Stability of plasma treated superhydrophobic surfaces under different ambient conditions. J. Colloid Interface Sci. 2016, 470, 221–228. [Google Scholar] [CrossRef]
- Jung, S.-G.; Choi, K.B.; Park, C.H.; Shim, Y.S.; Park, C.H.; Park, Y.W.; Ju, B.-K. Effects of Cl2 plasma treatment on stability, wettability, and electrical properties of ITO for OLEDs. Opt. Mater. 2019, 93, 51–57. [Google Scholar] [CrossRef]
- Deynse, A.V.; Cools, P.; Leys, C.; Morent, R.; Geyter, N.D. Surface modification of polyethylene in an argon atmospheric pressure plasma jet. Surf. Coat. Technol. 2015, 276, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Correia, D.M.; Nunes-Pereira, J.; Alikin, D.; Kholkin, A.L.; Carabineiro, S.A.C.; Rebouta, L.; Rodrigues, M.S.; Vaz, F.; Costa, C.M.; Lanceros-Méndez, S. Surface wettability modification of poly(vinylidene fluoride) and copolymer films and membranes by plasma treatment. Polymer 2019, 169, 138–147. [Google Scholar] [CrossRef]
- Lock, E.H.; Petrovykh, D.Y.; Mack, P.; Carney, T.; White, R.G.; Walton, S.G.; Fernsler, R.F. Surface composition, chemistry, and structure of polystyrene modified by electron-beam-generated plasma. Langmuir 2010, 26, 8857–8868. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Tsai, M.S.; Chen, W.T.; Hong, Y.Z.; Chien, P.Y.; Huang, C.H.; Woon, W.Y.; Lin, C.T. A low-damage plasma surface modification method of stacked graphene bilayers for configurable wettability and electrical properties. Nanotechnology 2019, 30, 245709. [Google Scholar] [CrossRef]
- Peng, S.; Ma, Y. Fabrication of hydrophilic and oil-repellent surface via CF4 plasma treatment. Mater. Des. 2017, 139, 293–297. [Google Scholar] [CrossRef]
- Saloum, S.; Shaker, S.A.; Alkafri, M.N.; Obaid, A.; Hussin, R. Hydrogenated Silicon Carbonitride Thin Film Nanostructuring Using SF6 Plasma: Structural and Optical Analysis. Silicon 2020, 12, 2957–2966. [Google Scholar] [CrossRef]
- Holc, M.; Zaplotnik, R.; Mozetic, M.; Vesel, A. Surface Modification and Aging of Polyacrylonitrile Butadiene Styrene Polymer Induced by Treatment in RF Oxygen Plasma. IEEE Trans. Plasma Sci. 2018, 46, 3669–3676. [Google Scholar] [CrossRef]
- Juárez-Moreno, J.A.; Ávila-Ortega, A.; Oliva, A.I.; Avilés, F.; Cauich-Rodríguez, J.V. Effect of wettability and surface roughness on the adhesion properties of collagen on PDMS films treated by capacitively coupled oxygen plasma. Appl. Surf. Sci. 2015, 349, 763–773. [Google Scholar] [CrossRef]
- Alves, P.; Cardoso, R.; Correia, T.R.; Antunes, B.P.; Correia, I.J.; Ferreira, P. Surface modification of polyurethane films by plasma and ultraviolet light to improve haemocompatibility for artificial heart valves. Colloids Surf. B 2014, 113, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Walther, F.; Davydovskaya, P.; Zürcher, S.; Kaiser, M.; Herberg, H.; Gigler, A.M.; Stark, R.W. Stability of the hydrophilic behavior of oxygen plasma activated SU-8. J. Micromech. Microeng. 2007, 17, 524. [Google Scholar] [CrossRef] [Green Version]
- Jokinen, V.; Suvanto, P.; Franssila, S. Oxygen and nitrogen plasma hydrophilization and hydrophobic recovery of polymers. Biomicrofluidics 2012, 6, 16501–1650110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shui, L.; Hayes, R.A.; Jin, M.; Zhang, X.; Bai, P.; van den Berg, A.; Zhou, G. Microfluidics for electronic paper-like displays. Lab Chip 2014, 14, 2374–2384. [Google Scholar] [CrossRef] [PubMed]
- Mulyana, Y.; Uenuma, M.; Ishikawa, Y.; Uraoka, Y. Reversible Oxidation of Graphene Through Ultraviolet/Ozone Treatment and Its Nonthermal Reduction through Ultraviolet Irradiation. J. Phys. Chem. C 2014, 118, 27372–27381. [Google Scholar] [CrossRef]
- Nguyen, P.Q.M.; Yeo, L.-P.; Lok, B.-K.; Lam, Y.-C. Patterned surface with controllable wettability for inkjet printing of flexible printed electronics. Acs Appl. Mater. Interfaces 2014, 6, 4011–4016. [Google Scholar] [CrossRef] [PubMed]
- Šojić, D.; Despotović, V.; Orčić, D.; Szabó, E.; Arany, E.; Armaković, S.; Illés, E.; Gajda-Schrantz, K.; Dombi, A.; Alapi, T. Degradation of thiamethoxam and metoprolol by UV, O3 and UV/O3 hybrid processes: Kinetics, degradation intermediates and toxicity. J. Hydrol. 2012, 472, 314–327. [Google Scholar] [CrossRef]
- Maeda, H.; Yamagishi, R.; Ishida, E.H.; Kasuga, T. Wettability and dynamics of water droplet on a snail shell. J. Colloid Interface Sci. 2019, 547, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Park, C.-Y.; Moon, S.-Y.; Choi, J.-H.; Chang, B.-J.; Kim, J.-H. Surface-attached brush-type CO2-philic poly(PEGMA)/PSf composite membranes by UV/ozone-induced graft polymerization: Fabrication, characterization, and gas separation properties. J. Membr. Sci. 2019, 589, 117214. [Google Scholar] [CrossRef]
- Sham, M.L.; Li, J.; Ma, P.C.; Kim, J.-K. Cleaning and Functionalization of Polymer Surfaces and Nanoscale Carbon Fillers by UV/Ozone Treatment: A Review. J. Compos. Mater. 2009, 43, 1537–1564. [Google Scholar] [CrossRef]
- Pascual, M.; Kerdraon, M.; Rezard, Q.; Jullien, M.C.; Champougny, L. Wettability patterning in microfluidic devices using thermally-enhanced hydrophobic recovery of PDMS. Soft Matter 2019, 15, 9253–9260. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.C.; Razali, N.A.M. Temporary Wettability Tuning of PCL/PDMS Micro Pattern Using the Plasma Treatments. Materials 2019, 12, 644. [Google Scholar] [CrossRef] [Green Version]
- Hillborg, H.; Tomczak, N.; Olàh, A.; Schönherr, H.; Vancso, G.J. Nanoscale hydrophobic recovery: A chemical force microscopy study of UV/ozone-treated cross-linked poly(dimethylsiloxane). Langmuir 2004, 20, 785. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of oxygen plasma nanotexturing of organic polymer surfaces: From stable super hydrophilic to super hydrophobic surfaces. Langmuir 2009, 25, 11748. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Gillain, C.C.; Adriaensen, Y.; Derclaye, S.; Rouxhet, P.G. Plasma-Oxidized Polystyrene: Wetting Properties and Surface Reconstruction. Langmuir 2000, 16, 8194–8200. [Google Scholar] [CrossRef]
- Mortazavi, M.; Nosonovsky, M. A model for diffusion-driven hydrophobic recovery in plasma treated polymers. Appl. Surf. Sci. 2012, 258, 6876–6883. [Google Scholar] [CrossRef]
- Bilgin, S.; Isik, M.; Yilgor, E.; Yilgor, I. Hydrophilization of silicone–urea copolymer surfaces by UV/ozone: Influence of PDMS molecular weight on surface oxidation and hydrophobic recovery. Polymer 2013, 54, 6665–6675. [Google Scholar] [CrossRef]
- Owen, M.J.; Smith, P.J. Plasma treatment of polydimethylsiloxane. J. Adhes. Sci. Technol. 1997, 8, 1063–1075. [Google Scholar] [CrossRef]
- Oláh, A.; Hillborg, H.; Vancso, G.J. Hydrophobic recovery of UV/ozone treated poly(dimethylsiloxane): Adhesion studies by contact mechanics and mechanism of surface modification. Appl. Surf. Sci. 2005, 239, 410–423. [Google Scholar] [CrossRef]
- O’Connell, C.; Sherlock, R.; Ball, M.D.; Aszalós-Kiss, B.; Prendergast, U.; Glynn, T.J. Investigation of the hydrophobic recovery of various polymeric biomaterials after 172 nm UV treatment using contact angle, surface free energy and XPS measurements. Appl. Surf. Sci. 2009, 255, 4405–4413. [Google Scholar] [CrossRef]
- Anbumani, S.; Silva, A.M.; Roggero, U.F.S.; Silva, A.M.P.A.; Hernández-Figueroa, H.E.; Cotta, M.A. Oxygen plasma-enhanced covalent biomolecule immobilization on SU-8 thin films: A stable and homogenous surface biofunctionalization strategy. Appl. Surf. Sci. 2021, 553, 149502. [Google Scholar] [CrossRef]
- Kearney, P.C.; Ruth, J.M.; Zeng, Q.; Mazzocchi, P. UV-ozonation of paraquat. J. Agric. Food Chem. 1985, 33, 953–957. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Vittal, J.J. Helix inside a Helix: Encapsulation of Hydrogen-Bonded Water Molecules in a Staircase Coordination Polymer. Angew. Chem. Int. Ed. 2004, 116, 5893–5896. [Google Scholar] [CrossRef]
- Dormidontova, E.E. Role of Competitive PEO−Water and Water−Water Hydrogen Bonding in Aqueous Solution PEO Behavior. Macromolecules 2002, 35, 987–1001. [Google Scholar] [CrossRef]
- Tamai, Y.; Tanaka, H.; Nakanishi, K. Molecular Dynamics Study of Polymer−Water Interaction in Hydrogels. 1. Hydrogen-Bond Structure. Macromolecules 1996, 29, 6750–6760. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Li, F.; Tang, B.; Zhou, G. Surface Wettability Tuning of Acrylic Resin Photoresist and Its Aging Performance. Sensors 2021, 21, 4866. https://doi.org/10.3390/s21144866
Dou Y, Li F, Tang B, Zhou G. Surface Wettability Tuning of Acrylic Resin Photoresist and Its Aging Performance. Sensors. 2021; 21(14):4866. https://doi.org/10.3390/s21144866
Chicago/Turabian StyleDou, Yingying, Fahong Li, Biao Tang, and Guofu Zhou. 2021. "Surface Wettability Tuning of Acrylic Resin Photoresist and Its Aging Performance" Sensors 21, no. 14: 4866. https://doi.org/10.3390/s21144866
APA StyleDou, Y., Li, F., Tang, B., & Zhou, G. (2021). Surface Wettability Tuning of Acrylic Resin Photoresist and Its Aging Performance. Sensors, 21(14), 4866. https://doi.org/10.3390/s21144866