Mask-Type Sensor for Pulse Wave and Respiration Measurements and Eye Blink Detection
Abstract
:1. Introduction
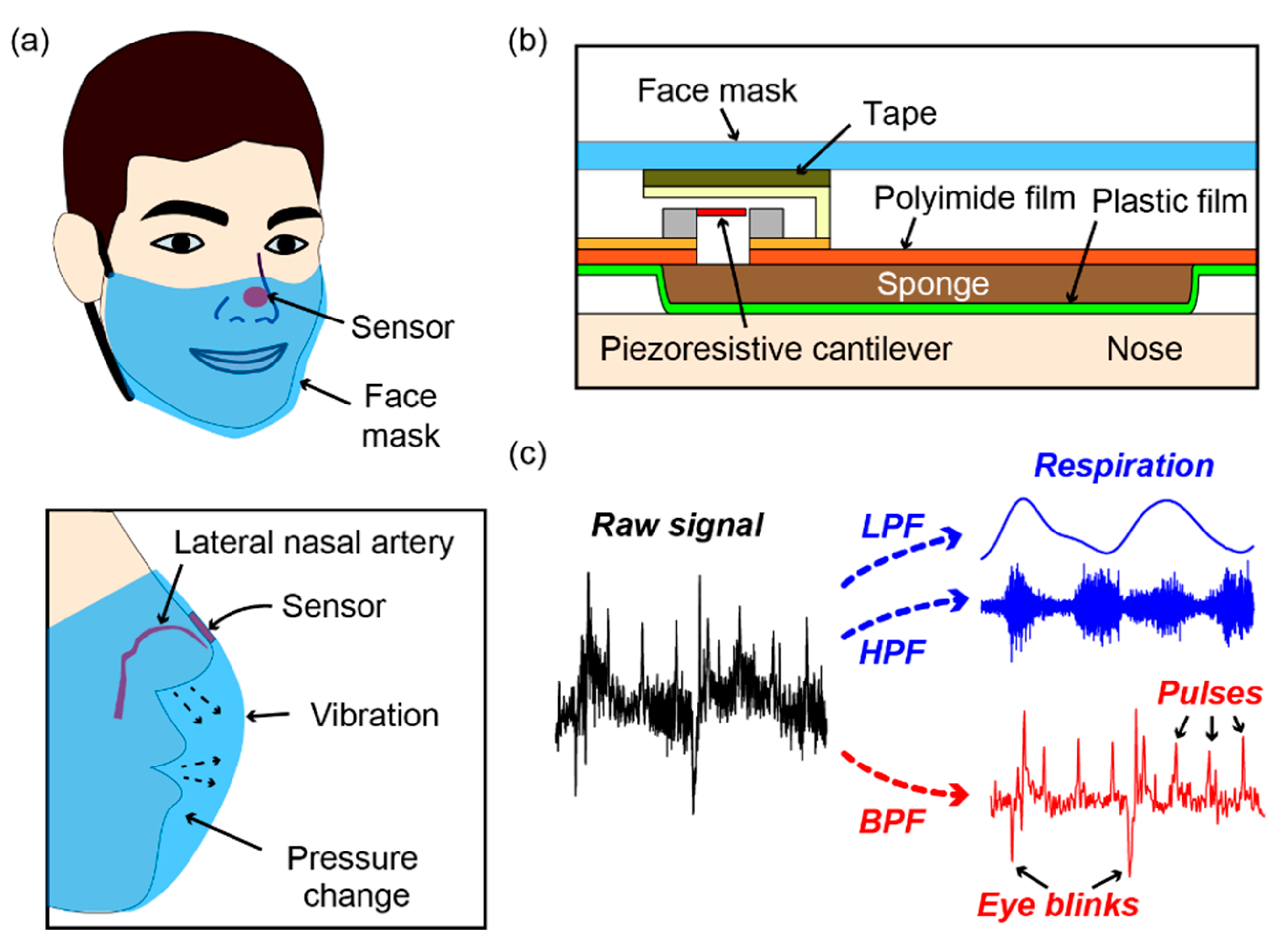
2. Sensor Fabrication
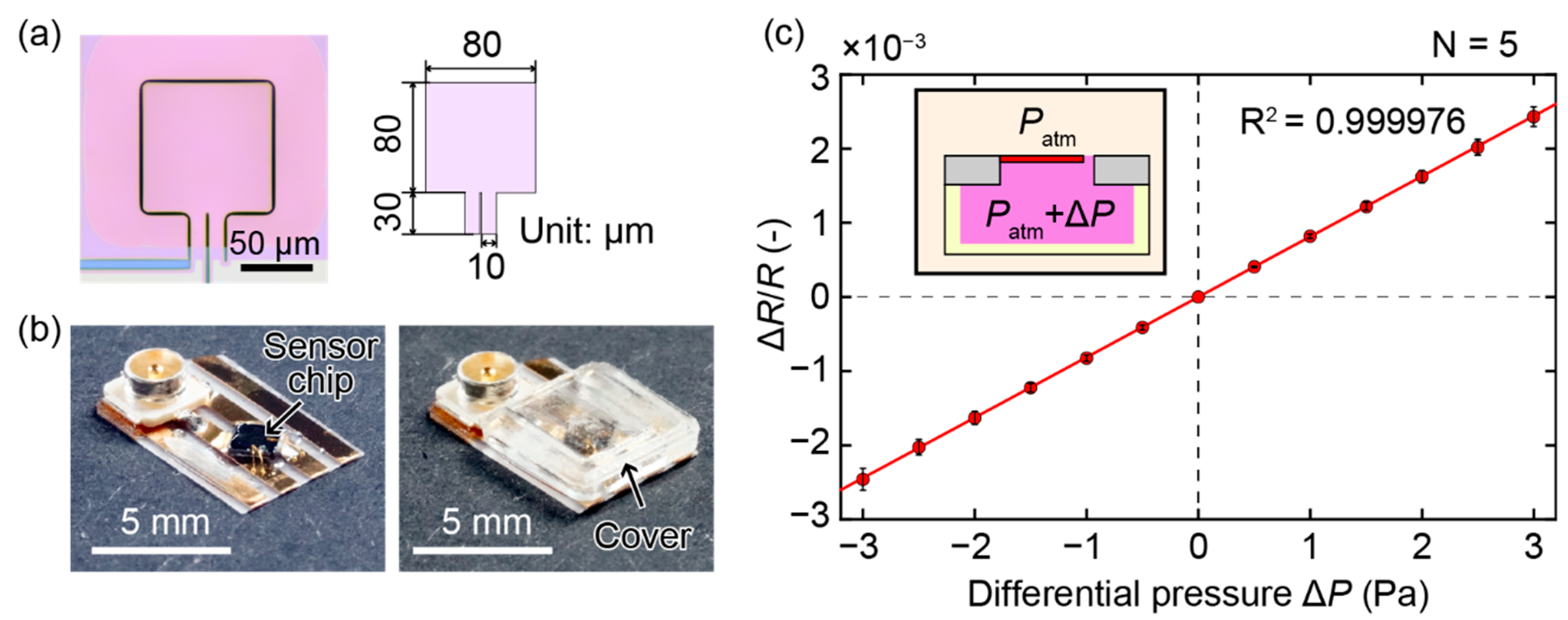
2.1. Piezoresistive Cantilever
2.2. Sensor Device
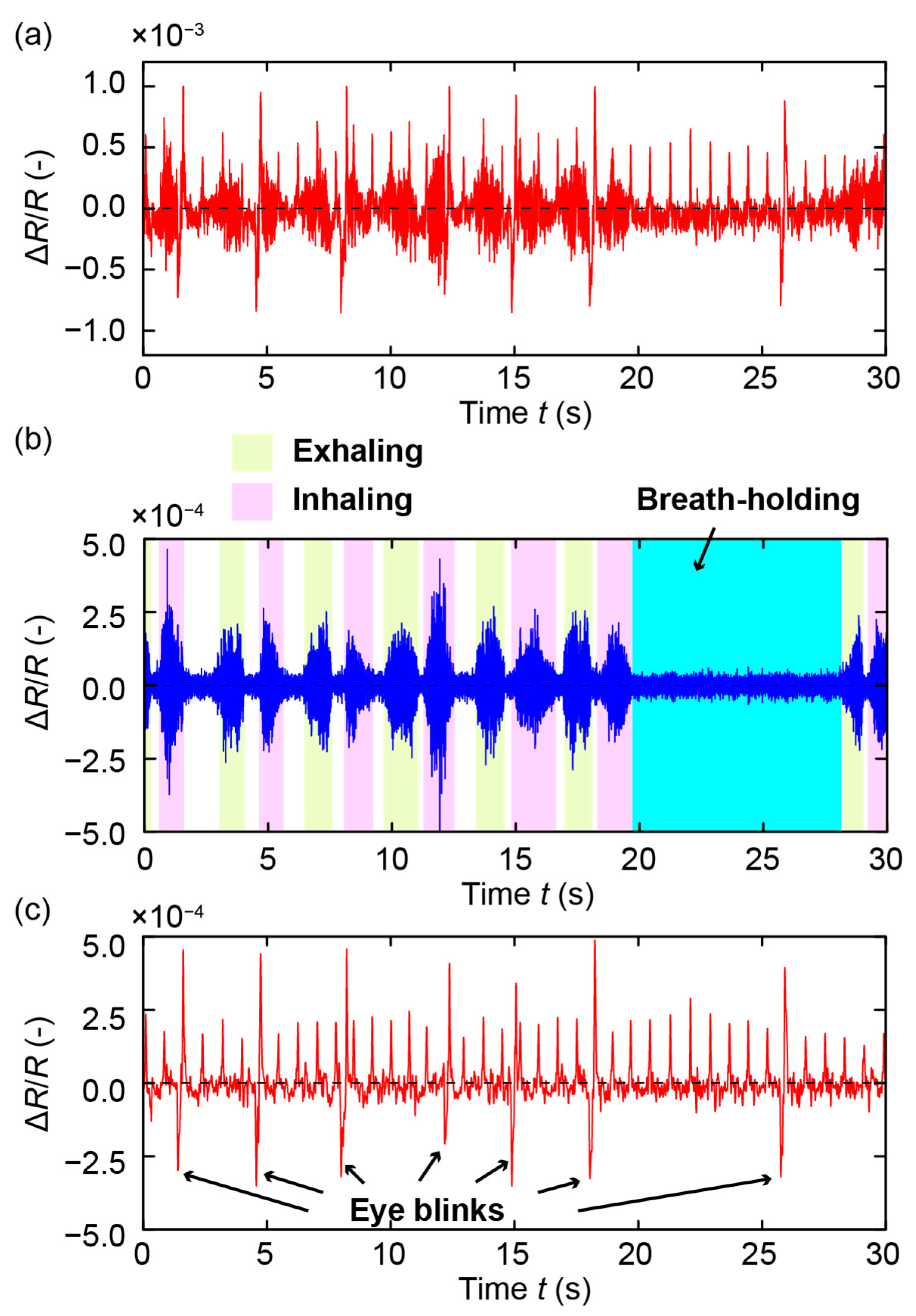
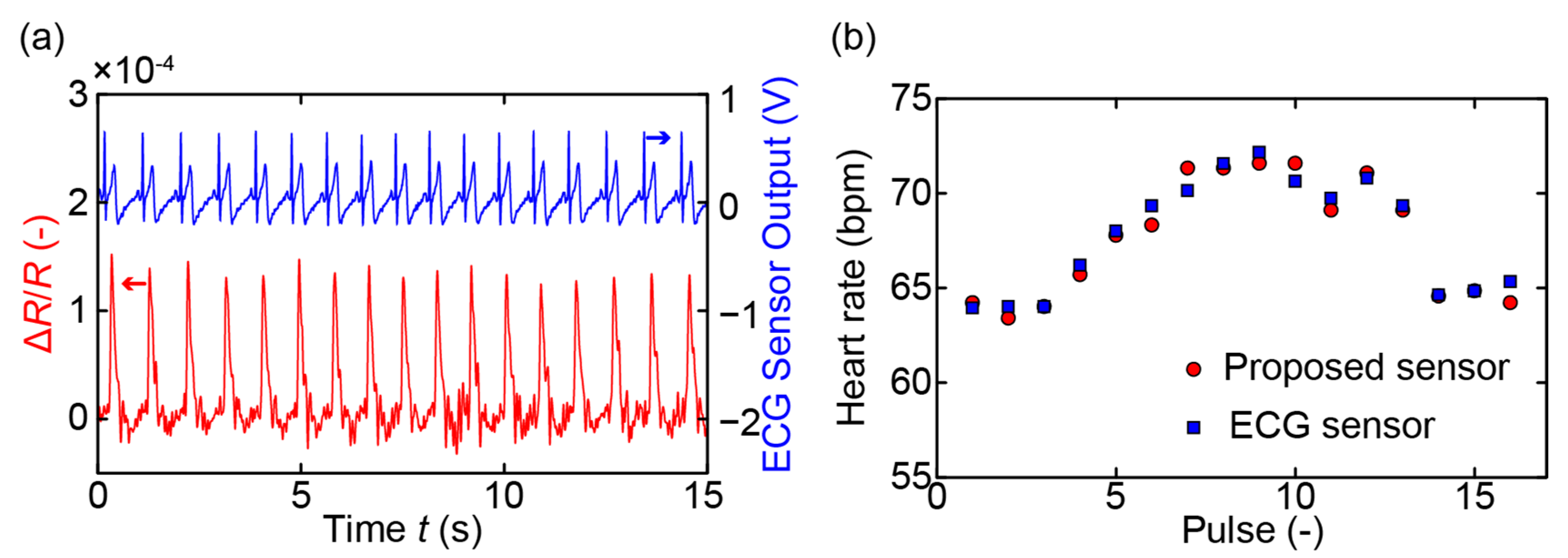
3. Measurements and Results
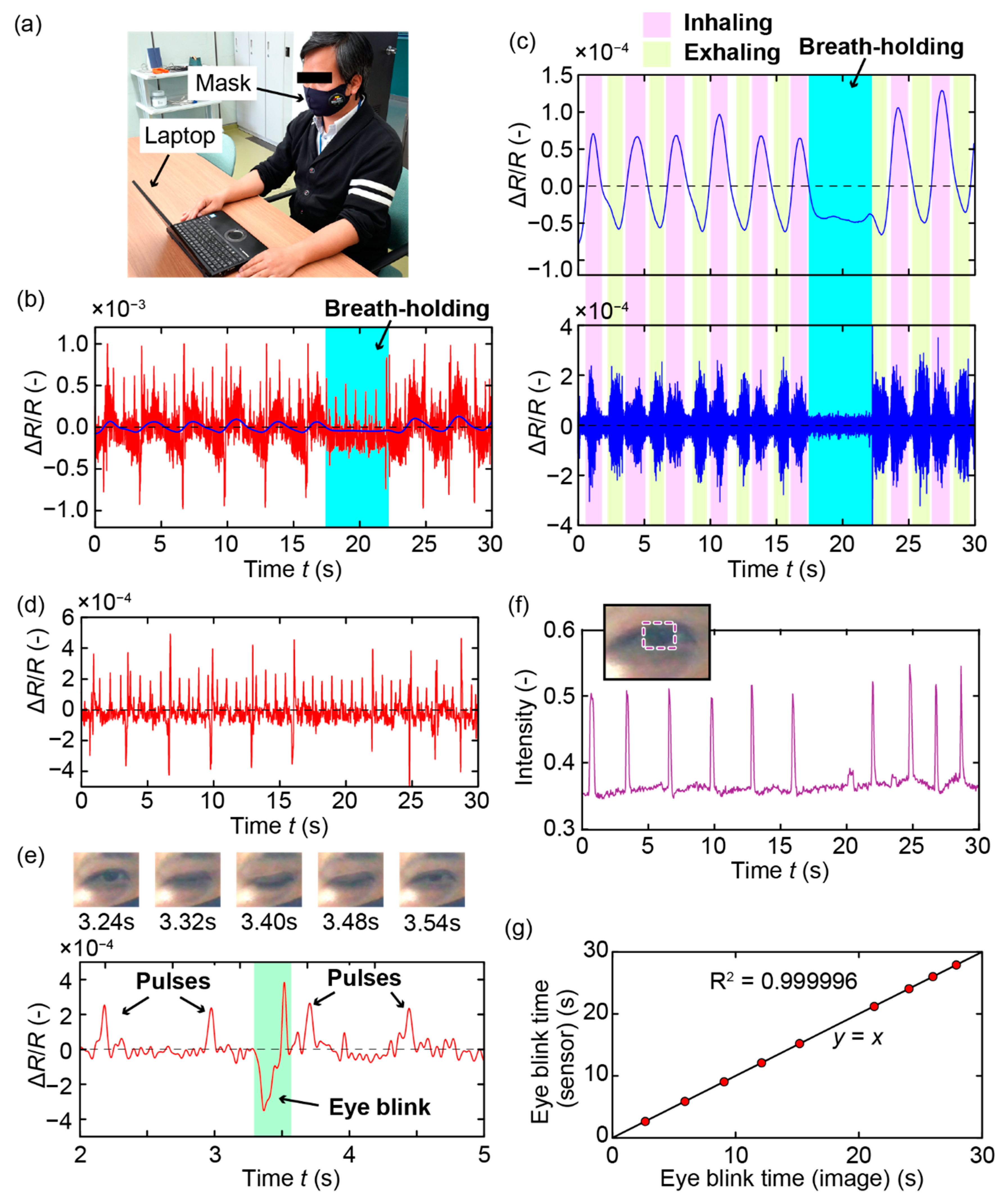
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Koivistoinen, T.; Lyytikäinen, L.-P.; Aatola, H.; Luukkaala, T.; Juonala, M.; Viikari, J.; Lehtimäki, T.; Raitakari, O.T.; Kähönen, M.; Hutri-Kähönen, N. Pulse Wave Velocity Predicts the Progression of Blood Pressure and Development of Hypertension in Young Adults. Hypertension 2018, 71, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-N.; Gao, H.-Q.; Li, B.-Y.; Cheng, M.; Ma, Y.-B.; Zhang, Z.-M.; Gao, X.-M.; Liu, Y.-P.; Wang, M. Pulse Wave Velocity as a Marker of Arteriosclerosis and Its Comorbidities in Chinese Patients. Hypertens. Res. 2007, 30, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruickshank, K.; Riste, L.; Anderson, S.G.; Wright, J.S.; Dunn, G.; Gosling, R.G. Aortic Pulse-Wave Velocity and Its Relationship to Mortality in Diabetes and Glucose Intolerance. Circulation 2002, 106, 2085–2090. [Google Scholar] [CrossRef] [Green Version]
- Chu, M.; Nguyen, T.; Pandey, V.; Zhou, Y.; Pham, H.N.; Bar-Yoseph, R.; Radom-Aizik, S.; Jain, R.; Cooper, D.M.; Khine, M. Respiration rate and volume measurements using wearable strain sensors. NPJ Digit. Med. 2019, 2, 8. [Google Scholar] [CrossRef]
- Schmidt, J.; Laarousi, R.; Stolzmann, W.; Karrer-Gauß, K. Eye blink detection for different driver states in conditionally automated driving and manual driving using EOG and a driver camera. Behav. Res. Methods 2018, 50, 1088–1101. [Google Scholar] [CrossRef] [Green Version]
- Divjak, M.; Bischof, H. In Eye Blink Based Fatigue Detection for Prevention of Computer Vision Syndrome. In Proceedings of the 11th IAPR Conference on Machine Vision Applications, MVA 2009, Yokohama, Japan, 20–22 May 2009; pp. 350–353. [Google Scholar]
- Quer, G.; Radin, J.M.; Gadaleta, M.; Baca-Motes, K.; Ariniello, L.; Ramos, E.; Kheterpal, V.; Topol, E.J.; Steinhubl, S.R. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat. Med. 2021, 27, 73–77. [Google Scholar] [CrossRef]
- Jeong, H.; Rogers, J.A.; Xu, S. Continuous on-body sensing for the COVID-19 pandemic: Gaps and opportunities. Sci. Adv. 2020, 6, eabd4794. [Google Scholar] [CrossRef]
- Natarajan, A.; Su, H.-W.; Heneghan, C. Assessment of physiological signs associated with COVID-19 measured using wearable devices. NPJ Digit. Med. 2020, 3, 156. [Google Scholar] [CrossRef]
- Miller, D.J.; Capodilupo, J.V.; Lastella, M.; Sargent, C.; Roach, G.D.; Lee, V.H.; Capodilupo, E.R. Analyzing changes in respiratory rate to predict the risk of COVID-19 infection. PLoS ONE 2020, 15, e0243693. [Google Scholar] [CrossRef]
- Mishra, T.; Wang, M.; Metwally, A.A.; Bogu, G.K.; Brooks, A.W.; Bahmani, A.; Alavi, A.; Celli, A.; Higgs, E.; Dagan-Rosenfeld, O.; et al. Pre-symptomatic detection of COVID-19 from smartwatch data. Nat. Biomed. Eng. 2020, 4, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Ates, H.C.; Yetisen, A.K.; Güder, F.; Dincer, C. Wearable devices for the detection of COVID-19. Nat. Electron. 2021, 4, 13–14. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Ichiki, M. MEMS-Based Sensor for Simultaneous Measurement of Pulse Wave and Respiration Rate. Sensors 2019, 19, 4942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.-V.; Mizuki, Y.; Tsukagoshi, T.; Takahata, T.; Ichiki, M.; Shimoyama, I. MEMS-Based Pulse Wave Sensor Utilizing a Piezoresistive Cantilever. Sensors 2020, 20, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuki, Y.; Nguyen, T.; Takahata, T.; Shimoyama, I. Highly Sensitive Pulse Wave Sensor with a Piezoresistive Cantilever Inside an Air Chamber. In Proceedings of the 2019 IEEE 32nd International Conference on Micro Electro Mechanical Systems (MEMS), Seoul, Korea, 27–31 January 2019; pp. 611–614. [Google Scholar]
- Takahashi, H.; Dung, N.M.; Matsumoto, K.; Shimoyama, I. Differential pressure sensor using a piezoresistive cantilever. J. Micromech. Microeng. 2012, 22, 5. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-V.; Ichiki, M. Mask-Type Sensor for Pulse Wave and Respiration Measurements and Eye Blink Detection. Sensors 2021, 21, 4895. https://doi.org/10.3390/s21144895
Nguyen T-V, Ichiki M. Mask-Type Sensor for Pulse Wave and Respiration Measurements and Eye Blink Detection. Sensors. 2021; 21(14):4895. https://doi.org/10.3390/s21144895
Chicago/Turabian StyleNguyen, Thanh-Vinh, and Masaaki Ichiki. 2021. "Mask-Type Sensor for Pulse Wave and Respiration Measurements and Eye Blink Detection" Sensors 21, no. 14: 4895. https://doi.org/10.3390/s21144895
APA StyleNguyen, T.-V., & Ichiki, M. (2021). Mask-Type Sensor for Pulse Wave and Respiration Measurements and Eye Blink Detection. Sensors, 21(14), 4895. https://doi.org/10.3390/s21144895




