Development of Gas Sensor Array for Methane Reforming Process Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Gas Sensors
2.2. Gas Mixtures Preparation
- (1)
- Assumption of the desired concentration of individual chemical substances in the gas mixture (, ppm v/v),
- (2)
- Assumption of the total volume of the gas mixture (, mL),
- (3)
- Determination of the volume of individual substances that must be dosed into the Tedlar bag (, mL):
- (4)
- Determination of the air volume (, mL), which must be dosed into the Tedlar bag:
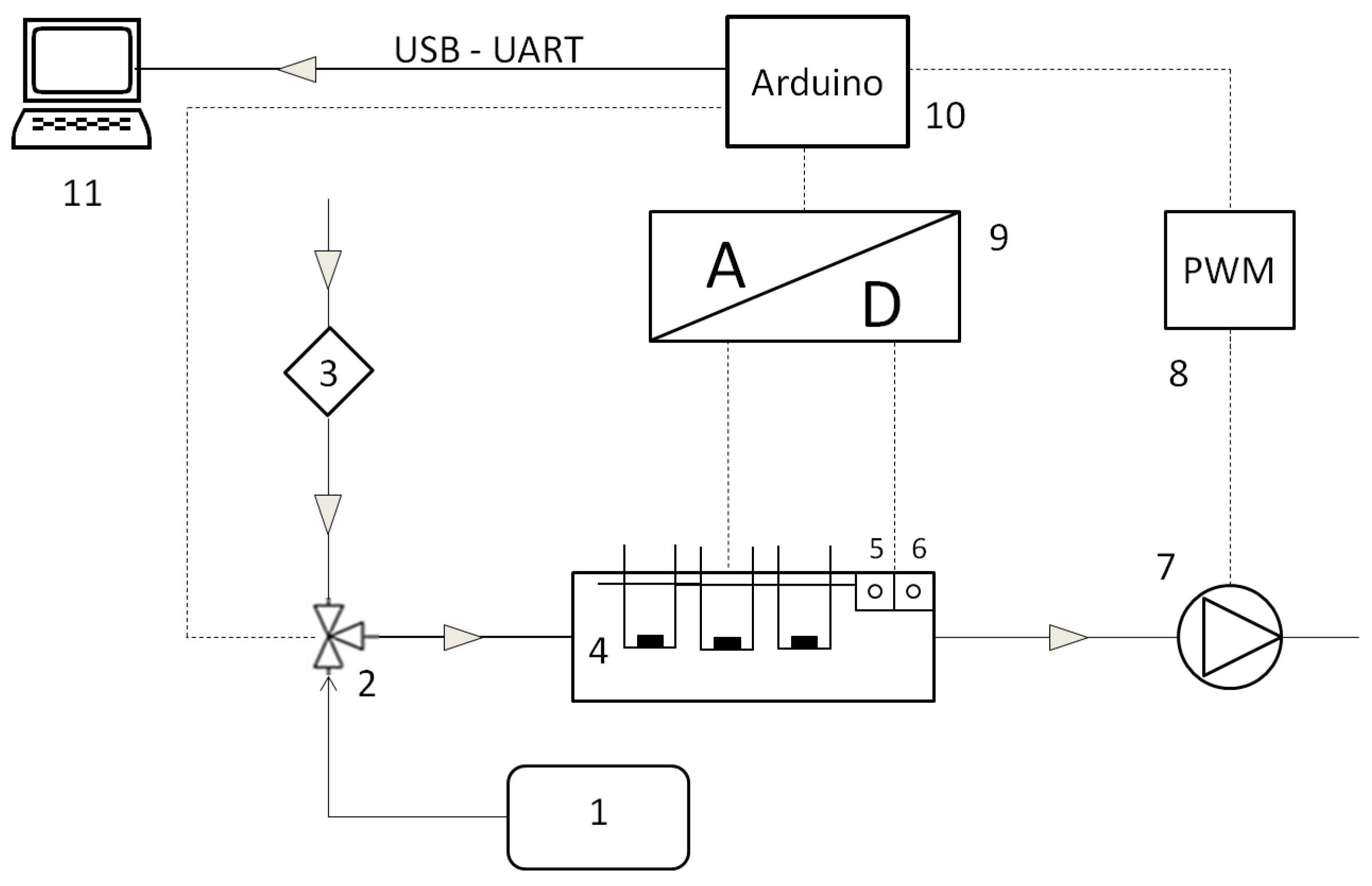
2.3. Gas Sensor Array Measurements
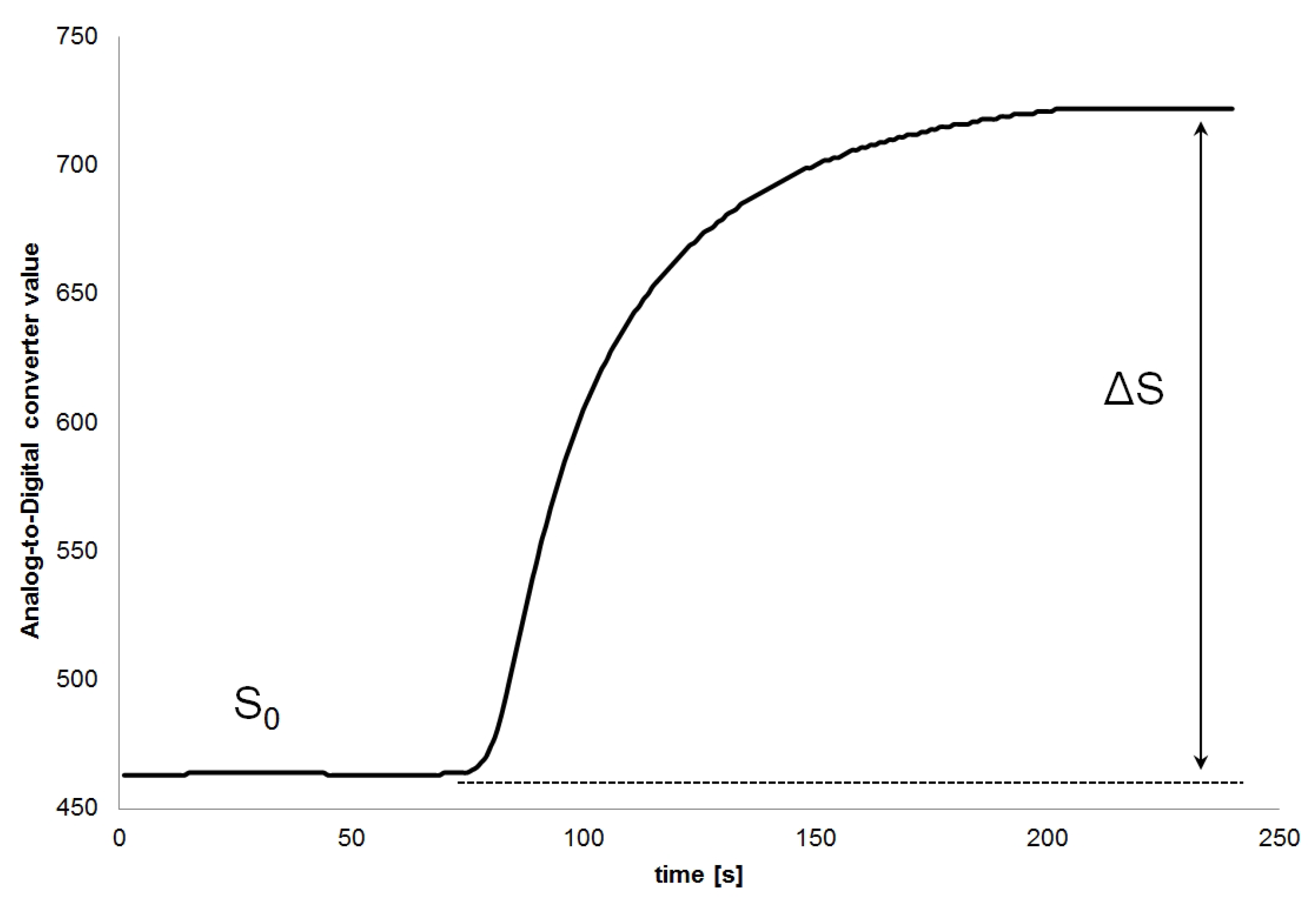
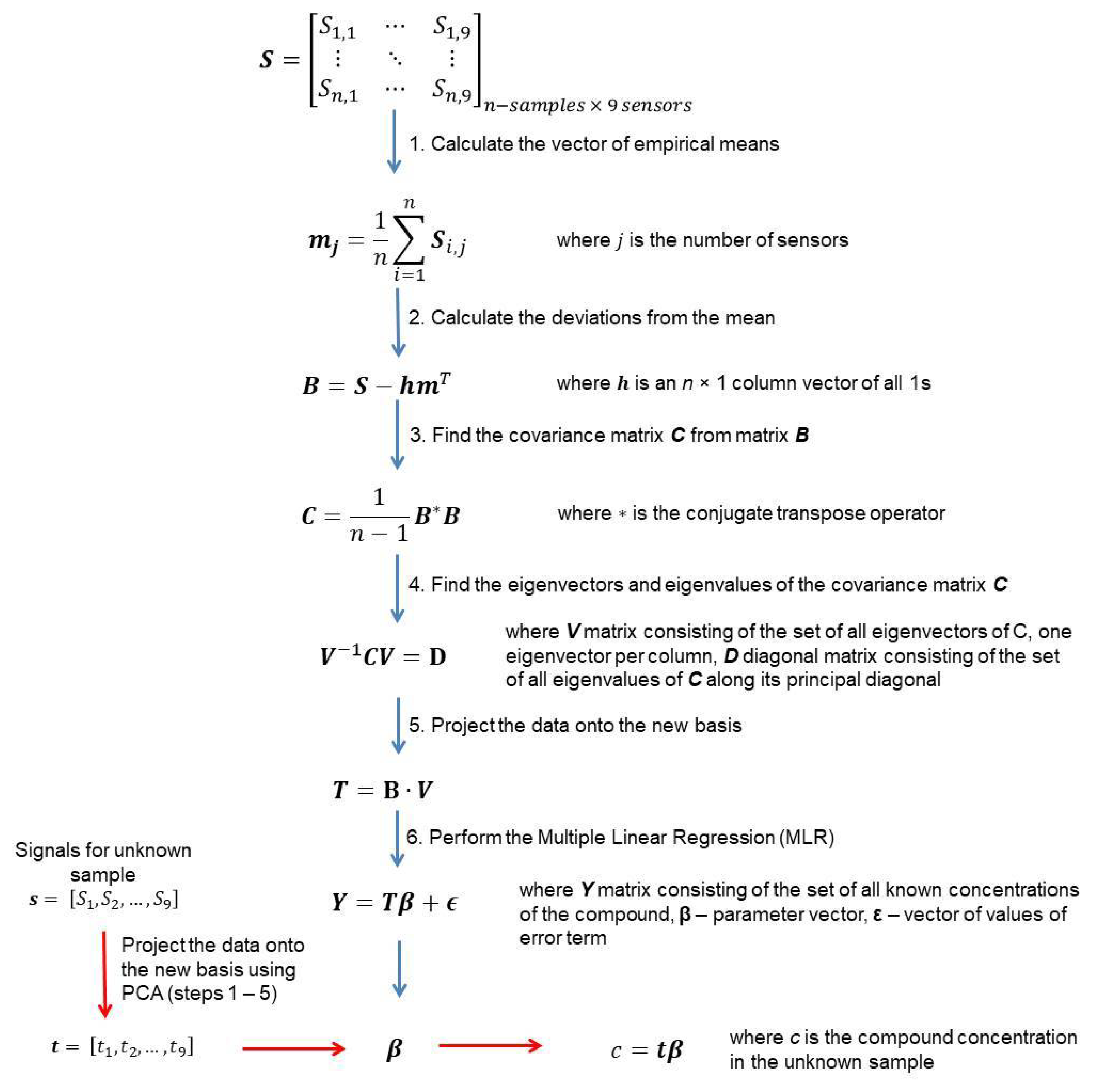
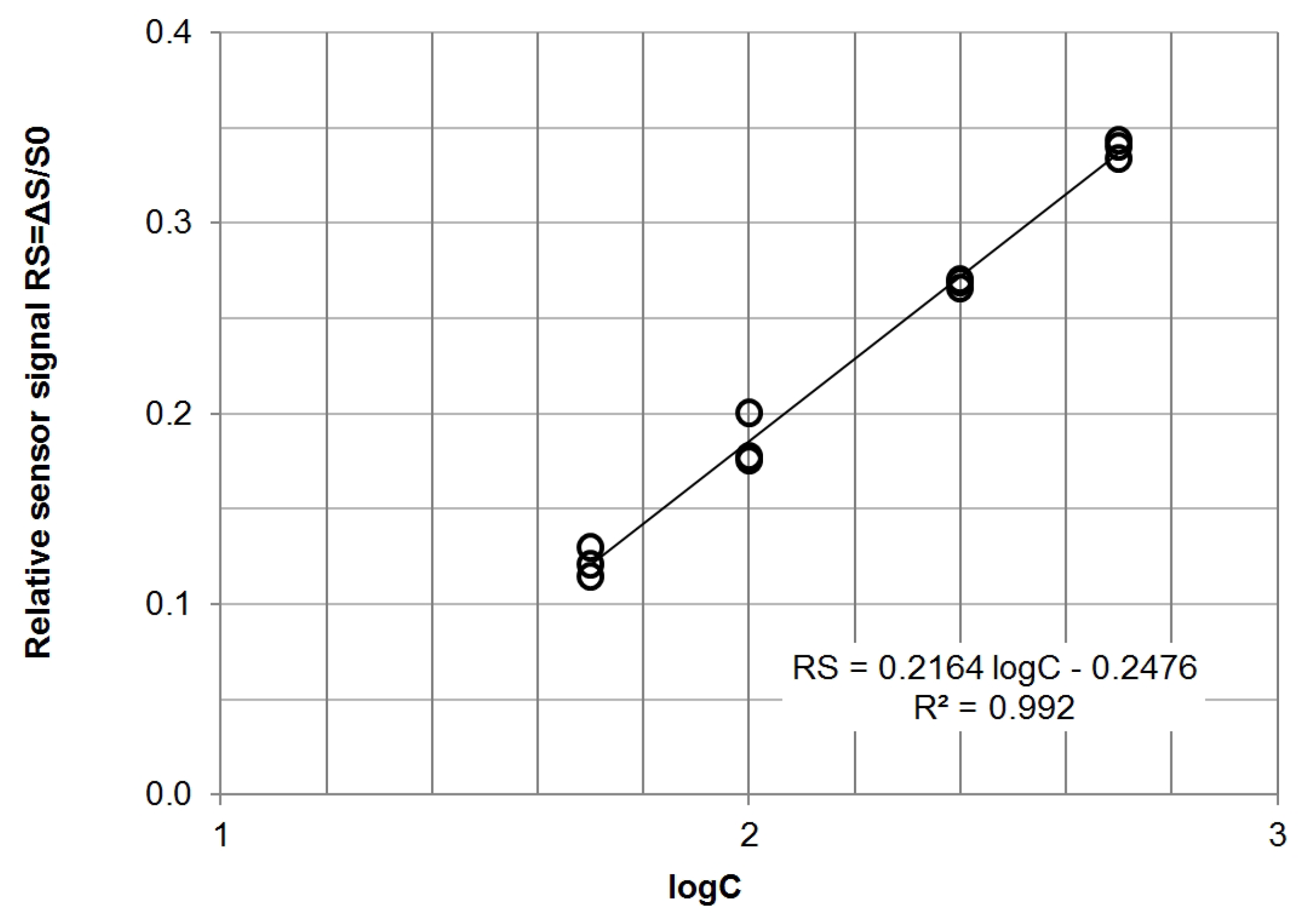
2.4. Data Analysis
- (1)
- Determination of the principal components using the principal component analysis (PCA) method. It allows obtaining an uncorrelated matrix of variables.
- (2)
- Development of the Multiple Linear Regression (MLR) model with the use of principal components as variables.
3. Results
- Inlet molar ratio (IMR):
- Outlet molar ratio (OMR):
- Methane conversion level (MCL):
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Szulczyński, B.; Wasilewski, T.; Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Namieśnik, J.; Gębicki, J. Different Ways to Apply a Measurement Instrument of E-Nose Type to Evaluate Ambient Air Quality with Respect to Odour Nuisance in a Vicinity of Municipal Processing Plants. Sensors 2017, 17, 2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gȩbicki, J.; Byliński, H.; Namieśnik, J. Measurement techniques for assessing the olfactory impact of municipal sewage treatment plants. Environ. Monit. Assess. 2016, 188, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Franchi, D.; Gonzatti, F.; Miotto, M.; Kuhn, V.; Farret, F. Use of infrared matrix sensor for temperature measurement and monitoring of PEM/FC stacks. Sens. Actuators Phys. 2019, 293, 119–127. [Google Scholar] [CrossRef]
- Aguilera, T.; Lozano, J.; Paredes, J.A.; Álvarez, F.J.; Suárez, J.I. Electronic Nose Based on Independent Component Analysis Combined with Partial Least Squares and Artificial Neural Networks for Wine Prediction. Sensors 2012, 12, 8055–8072. [Google Scholar] [CrossRef]
- Bachinger, T.; Mandenius, C.F. Searching for process information in the aroma of cell cultures. Trends Biotechnol. 2000, 18, 494–500. [Google Scholar] [CrossRef]
- Bachinger, T.; Mandenius, C.F. Physiologically Motivated Monitoring of Fermentation Processes by Means of an Electronic Nose. Eng. Life Sci. 2001, 1, 33–42. [Google Scholar] [CrossRef]
- Ghosh, S.; Tudu, B.; Bhattacharyya, N.; Bandyopadhyay, R. A recurrent Elman network in conjunction with an electronic nose for fast prediction of optimum fermentation time of black tea. Neural Comput. Appl. 2019, 31, 1165–1171. [Google Scholar] [CrossRef]
- Hidayat, S.N.; Nuringtyas, T.R.; Triyana, K. Electronic Nose Coupled with Chemometrics for Monitoring of Tempeh Fermentation Process. In Proceedings of the 2018 4th International Conference on Science and Technology (ICST), Yogyakarta, Indonesia, 7–8 August 2018; pp. 1–6. [Google Scholar]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. A portable electronic nose as an expert system for aroma-based classification of saffron. Chemom. Intell. Lab. Syst. 2016, 156, 148–156. [Google Scholar] [CrossRef]
- Lidén, H.; Mandenius, C.F.; Gorton, L.; Meinander, N.Q.; Lundström, I.; Winquist, F. On-line monitoring of a cultivation using an electronic nose. Anal. Chim. Acta 1998, 361, 223–231. [Google Scholar] [CrossRef]
- Li, G.; Yuan, L.; Wang, X.; Meng, Y.; Li, J.; Zhao, Y.; Peng, Y. Rapid quantification analysis of alcohol during the green jujube wine fermentation by electronic nose. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 330, p. 052046. [Google Scholar]
- Mandenius, C.F.; Eklöv, T.; Lundström, I. Sensor fusion with on-line gas emission multisensor arrays and standard process measuring devices in baker’s yeast manufacturing process. Biotechnol. Bioeng. 1997, 55, 427–438. [Google Scholar] [CrossRef]
- Tan, J.; Balasubramanian, B.; Sukha, D.; Ramkissoon, S.; Umaharan, P. Sensing fermentation degree of cocoa (Theobroma cacao L.) beans by machine learning classification models based electronic nose system. J. Food Process. Eng. 2019, 42, e13175. [Google Scholar] [CrossRef]
- Tan, C.; Xie, D.; Liu, Y.; Peng, W.; Li, X.; Ai, L.; Wu, C.; Wen, C.; Huang, X.; Guo, J. Identification of different bile species and fermentation times of bile arisaema based on an intelligent electronic nose and least squares support vector machine. Anal. Chem. 2018, 90, 3460–3466. [Google Scholar] [CrossRef]
- Belikova, V.; Panchuk, V.; Legin, E.; Melenteva, A.; Kirsanov, D.; Legin, A. Continuous monitoring of water quality at aeration plant with potentiometric sensor array. Sens. Actuators Chem. 2019, 282, 854–860. [Google Scholar] [CrossRef]
- Oikonomou, P.; Botsialas, A.; Olziersky, A.; Stratakos, I.; Katsikas, S.; Dimas, D.; Sotiropoulos, G.; Goustouridis, D.; Raptis, I.; Sanopoulou, M. Wireless sensor network based on a chemocapacitive sensor array for the real-time monitoring of industrial pollutants. Procedia Eng. 2014, 87, 564–567. [Google Scholar] [CrossRef] [Green Version]
- Giungato, P.; Di Gilio, A.; Palmisani, J.; Marzocca, A.; Mazzone, A.; Brattoli, M.; Giua, R.; De Gennaro, G. Synergistic approaches for odor active compounds monitoring and identification: State of the art, integration, limits and potentialities of analytical and sensorial techniques. TRAC Trends Anal. Chem. 2018, 107, 116–129. [Google Scholar] [CrossRef]
- Gostelow, P.; Parsons, S. Sewage treatment works odour measurement. Water Sci. Technol. 2000, 41, 33–40. [Google Scholar] [CrossRef]
- Gębicki, J.; Dymerski, T.; Rutkowski, S. Identification of odor of volatile organic compounds using classical sensory analysis and electronic nose technique. Environ. Prot. Eng. 2014, 40, 103–116. [Google Scholar] [CrossRef]
- Di Francesco, F.; Lazzerini, B.; Marcelloni, F.; Pioggia, G. An electronic nose for odour annoyance assessment. Atmos. Environ. 2001, 35, 1225–1234. [Google Scholar] [CrossRef]
- IV, E.A.B.; Koziel, J.A.; Cai, L.; Wright, D. Characterization of Livestock Odors Using Steel Plates, Solid-Phase Microextraction, and Multidimensional Gas Chromatography–Mass Spectrometry–Olfactometry. J. Air Waste Manag. Assoc. 2006, 56, 1391–1403. [Google Scholar] [CrossRef] [Green Version]
- Zarra, T.; Naddeo, V.; Belgiorno, V.; Reiser, M.; Kranert, M. Instrumental characterization of odour: A combination of olfactory and analytical methods. Water Sci. Technol. 2009, 59, 1603–1609. [Google Scholar] [CrossRef]
- Dincer, F.; Odabasi, M.; Muezzinoglu, A. Chemical characterization of odorous gases at a landfill site by gas chromatography–mass spectrometry. J. Chromatogr. 2006, 1122, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of key odorant compounds in freshly distilled cognac using GC-O, GC-MS, and sensory evaluation. J. Agric. Food Chem. 2004, 52, 5670–5676. [Google Scholar] [CrossRef] [PubMed]
- Munoz, R.; Sivret, E.C.; Parcsi, G.; Lebrero, R.; Wang, X.; Suffet, I.M.; Stuetz, R.M. Monitoring techniques for odour abatement assessment. Water Res. 2010, 44, 5129–5149. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.K. The Electronic Nose: Artificial Olfaction Technology; Springer: New Delhi, India, 2014. [Google Scholar]
- Principal Components Regression. Available online: http://www.science.smith.edu/~jcrouser/SDS293/labs/lab11-r.html (accessed on 30 June 2021).
- Szulczyński, B.; Rybarczyk, P.; Gębicki, J. Monitoring of n-butanol vapors biofiltration process using an electronic nose combined with calibration models. Monatshefte Chem. Chem. Mon. 2018, 149, 1693–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Abdulrazzaq, N.N.; Al-Sabbagh, B.H.; Rees, J.M.; Zimmerman, W.B. Measuring Vapor and Liquid Concentrations for Binary and Ternary Systems in a Microbubble Distillation Unit via Gas Sensors. Chemosensors 2018, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Portable Infrared Syngas Analyzer Gasboard-3100P. Available online: https://en.gassensor.com.cn/GasAnalyzer/info_itemid_252.html (accessed on 30 June 2021).
- Syngas Analysis System Gasboard-9021. Available online: https://en.gassensor.com.cn/GasAnalyzer/info_itemid_283.html (accessed on 30 June 2021).
- Portable Natural Gas Analyzer Gasboard-3110P. Available online: http://www.gas-analyzers.com/products/syngas-analyzer/Gasboard-3110P.html (accessed on 30 June 2021).
- 970P Portable Multi-Gas Industrial Analyzers. Available online: https://catalog.nova-gas.com/viewitems/ication-specific-product-lines-syngas-gasification/strial-syngas-analyzers-and-gasification-analyzers (accessed on 30 June 2021).
- Online Syngas Analyzer by Vasthi. Available online: https://www.vasthi.com/online-syngas-analyzer (accessed on 30 June 2021).
- Syngas Analyzer Portable SYN-600. Available online: https://www.syngas-analyzer.com/product/Syngas-Analyzer-Portable-SYN-600.html (accessed on 30 June 2021).
- SWG 100 Syngas. Available online: https://www.mru.eu/en/products/detail/swg-100-syngas-1/ (accessed on 30 June 2021).
- MCA 100 SYN P—Portable Syngas Analyzer. Available online: https://www.energy-xprt.com/products/etg-model-mca-100-syn-p-portable-syngas-analyzer-525843 (accessed on 30 June 2021).
- Gruber, P.; Marques, M.P.C.; Szita, N.; Mayr, T. Integration and application of optical chemical sensors in microbioreactors. Lab Chip 2017, 17, 2693–2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demuth, C.; Varonier, J.; Jossen, V.; Eibl, R.; Eibl, D. Novel probes for pH and dissolved oxygen measurements in cultivations from millilitre to benchtop scale. Appl. Microbiol. Biotechnol. 2016, 100, 3853–3863. [Google Scholar] [CrossRef]
- Wang, X.d.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [Green Version]
- Spichiger, S.; Spichiger-Keller, U.E. Process Monitoring with Disposable Chemical Sensors Fit in the Framework of Process Analysis Technology (PAT) for Innovative Pharmaceutical Development and Quality Assurance. Chim. Int. J. Chem. 2010, 64, 803–807. [Google Scholar] [CrossRef]
- Pearce, T.C.; Gardner, J.W.; Friel, S.; Bartlett, P.N.; Blair, N. Electronic nose for monitoring the flavour of beers. Analyst 1993, 118, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Gardner, J.W.; Shin, H.W.; Hines, E.L.; Dow, C.S. An electronic nose system for monitoring the quality of potable water. Sensors Actuators Chem. 2000, 69, 336–341. [Google Scholar] [CrossRef]
- Romero-Flores, A.; McConnell, L.L.; Hapeman, C.J.; Ramirez, M.; Torrents, A. Evaluation of an electronic nose for odorant and process monitoring of alkaline-stabilized biosolids production. Chemosphere 2017, 186, 151–159. [Google Scholar] [CrossRef] [PubMed]


| Sensor Array | Number of Sensors | Process | Reference |
|---|---|---|---|
| An infrared matrix sensor | 1 | Monitor the FC stack temperature distribution | [3] |
| An array of thin film tin oxide sensors prepared by RF sputtering onto alumina and doped with chromium and indium | 16 | Wine classification and prediction based on an electronic nose (e-nose) | [4] |
| Metal oxide semiconductors (MOSs) and Metal Oxide semiconductor field-effect transistors (MOSFETs) | 50 | Monitors bioreactors and highlights their potential for controlling quality and safety, and for the optimization and automatic control of bioprocesses | [5] |
| MOSFET-sensors with catalytic metal gates of palladium, iridium or platinum | 10 | Non-invasive monitoring of the physiological changes in fermentation processes | [6] |
| Five TGS sensors from Figaro, Japan (TGS-832, TGS-823, TGS-2600, TGS-2610 and TGS-2611) | 5 | Predicting the optimum fermentation time at an earlier stage of the process | [7] |
| Sensor array of different types of metal oxide gas sensor (MOSs) | 8 | Study the tempeh fermentation process and the stages of this process | [8] |
| Sensor array was comprised of five sensors supplied by Figaro (Japan) and five sensors obtained from HANWIE Electronics (China) | 10 | Identification of different types of saffron, stigma of Crocus sativus, based on their volatile organic compounds (VOCs) | [9] |
| Semiconducting tin dioxide based sensors and an optical carbon detector | 4 | Monitoring an ethanol batch cultivation with the yeast Saccharomyces cerevisiae | [10] |
| Metal oxide sensor arrays | 10 | Prediction of the alcohol content of the green jujube wine fermentation | [11] |
| Sensor array containing different gas-sensitive semiconductor devices and an infrared gas sensor | 14 | Measuring the emission from a production-scale baker’s yeast manufacturing process and monitor the gas emission from a yeast culture bioreactor during fed-batch operation | [12] |
| Metal oxide sensor arrays | 9 | Determine the fermentation degree of cocoa beans | [13] |
| Metal oxide semiconductors (MOS) chemical sensors | 18 | Identification of different fermentation times and bile species of Bile Arisaema | [14] |
| Potentiometric sensor array: polymeric cation-sesnitive (8), polymeric anion-sensitive (8) and metallic and chalcogenide glass sensor with RedOx sensitivity | 23 | Real-time monitoring of ammonium and nitrate nitrogen in processed water at aeration plant | [15] |
| Hybrid sensor array composed by InterDigitated Chemocapacitora (IDVc) with the appropriate read-out electronic | 8 | The monitoring and evaluation and control of the specific Volatile Organic Compounds (VOCs) | [16] |
| Analysis Method | Advantages | Disadvantages |
|---|---|---|
| Gas chromatography |
|
|
| Gas sensor arrays |
|
|
| Sensor Type | Model | Detected Gases |
|---|---|---|
| Catalytic | TGS6810 | methane, propane, iso-butane |
| Catalytic | TGS6812 | methane, propane, hydrogen |
| Electrochemical | TGS4161 | carbon dioxide |
| Electrochemical | TGS5042 | carbon monoxide |
| Metal Oxide Semiconductor | TGS2600 | methane, carbon monoxide, hydrogen |
| Metal Oxide Semiconductor | TGS2602 | hydrogen, toluene, ethanol |
| Metal Oxide Semiconductor | TGS2603 | hydrogen, ethanol |
| Metal Oxide Semiconductor | TGS2611 | ethanol, hydrogen, methane |
| Metal Oxide Semiconductor | TGS3870 | carbon monoxide, methane |
| Metal Oxide Semiconductor | TGS823 | carbon monoxide, methane, iso-butane |
| Metal Oxide Semiconductor | TGS8100 | methane, hydrogen, ethanol |
| TGS2600 | 0.114 | 0.034 | 0.216 | 0.332 |
| TGS2602 | 0.089 | 0.022 | 0.091 | 0.087 |
| TGS2603 | 0.082 | 0.025 | 0.085 | 0.221 |
| TGS2611 | 0.196 | 0.015 | 0.102 | 0.146 |
| TGS3870 | 0.165 | 0.012 | 0.033 | 0.074 |
| TGS4161 | 0.012 | 0.307 | 0.013 | 0.022 |
| TGS5042 | 0.019 | 0.009 | 0.247 | 0.042 |
| TGS6810 | 0.005 | 0.002 | 0.005 | 0.005 |
| TGS6812 | 0.004 | 0.001 | 0.006 | 0.005 |
| TGS823 | 0.211 | 0.019 | 0.233 | 0.185 |
| TGS8100 | 0.052 | 0.011 | 0.138 | 0.201 |
| DRM Inlet Stream | DRM Outlet Stream | |
|---|---|---|
| total number of mixtures | 36 | 81 |
| 50, 100, 200, 300, 400, 500 ppm v/v | 10, 50, 100 ppm v/v | |
| 50, 100, 200, 300, 400, 500 ppm v/v | 10, 50, 100 ppm v/v | |
| - | 100, 250, 500 ppm v/v | |
| - | 100, 250, 500 ppm v/v |
| Producer | Model | Technology | Range | Accuracy | Response Time | Reference |
|---|---|---|---|---|---|---|
| Gdańsk University of Technology | Sensor matrix prototype | MOS and EC gas sensors | , , , : 0–100% (using dilution system) | 5% Full Scale (FS) | <90 s to 90% step range (MOS) | - |
| Cubic Sensor and Instrument Co. | Portable Infrared Syngas Analyzer Gasboard-3100P | , , (NDIR) (TCD) | : 0–30% : 0–25% : 0–10% : 0–30% | 2% Full Scale (FS) | <15 s to 90% step range (NDIR) | [31] |
| Cubic Sensor and Instrument Co. | Syngas Analysis System Gasboard-9021 | , , (NDIR) (TCD) | : 0–30% : 0–25% : 0–10% : 0–30% | , , < 1% FS <2% FS | <15 s to 90% step range (NDIR) | [32] |
| Hubei Cubic-Ruiyi Instrument CO. | Portable Natural Gas Analyzer Gasboard-3110P | , (NDIR) | : 0–5% : 0–100% | <2% FS | <15 s to 90% step range (NDIR) | [33] |
| Nova Analytical Systems (a Unit of Tenova Goodfellow Inc.) | 970P Portable Multi-Gas Industrial Analyzers | , , (NDIR) (TCD) | : 0–2% or 0–50% : 0–2% or 0–50% : 0–2% or 0–50% : 0–2% or 0–50% | 0.1% for all gases <1% FS in 8 h | 20–30 s to 90% step range | [34] |
| VASTHI Instruments Pvt Ltd | Online Syngas Analyzer by Vasthi | , , (NDIR) (TCD) | : 0–100% : 0–100% : 0–50% : 0–50% | , , : 0.5% from range or ± 3% : ±5 ppm or 5% | 45 s to 90% step range | [35] |
| Wuhan Tianyu Intelligent Control Technology Co., Ltd. (TIANYU) | Syngas Analyzer Portable SYN-600 | , , (NDIR) (TCD-MEMS) | : 0–100% : 0–100% : 0–100% : 0–100% | , , : ±2% FS : ±3% FS | 45 s to 90% step range | [36] |
| MRU GmbH | SWG 100 Syngas | , , (NDIR) (TCD) | : 0–10/100% : 0–10/100% : 0–10/100% : 0–10/100% | no data | no data | [37] |
| ETG Risorse e Technologia S.r.l. | MCA 100 SYN P – Portable Syngas Analyzer | , , (NDIR) (TCD) | Modified according to the needs of customer | , , , : ±2% FS | no data | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyniewski, D.; Szulczyński, B.; Dymerski, T.; Gębicki, J. Development of Gas Sensor Array for Methane Reforming Process Monitoring. Sensors 2021, 21, 4983. https://doi.org/10.3390/s21154983
Dobrzyniewski D, Szulczyński B, Dymerski T, Gębicki J. Development of Gas Sensor Array for Methane Reforming Process Monitoring. Sensors. 2021; 21(15):4983. https://doi.org/10.3390/s21154983
Chicago/Turabian StyleDobrzyniewski, Dominik, Bartosz Szulczyński, Tomasz Dymerski, and Jacek Gębicki. 2021. "Development of Gas Sensor Array for Methane Reforming Process Monitoring" Sensors 21, no. 15: 4983. https://doi.org/10.3390/s21154983
APA StyleDobrzyniewski, D., Szulczyński, B., Dymerski, T., & Gębicki, J. (2021). Development of Gas Sensor Array for Methane Reforming Process Monitoring. Sensors, 21(15), 4983. https://doi.org/10.3390/s21154983







