Prediction of Myoelectric Biomarkers in Post-Stroke Gait
Abstract
:1. Introduction
- We established an EMG-based neuromuscular disease prediction platform integrating the wireless EMG device, data streaming to a big data server, live signal processing in a big data platform, dashboards for the clients and clinicians for machine learning, and rule-based predictions of neuromuscular diseases;
- We investigated stroke-impaired EMG indices, including power spectrum features using statistical methods and hypothesis tests;
- We utilized the supervised machine learning algorithms to classify myoelectrical features of the stroke patients and the healthy adult group.
2. Materials and Methods
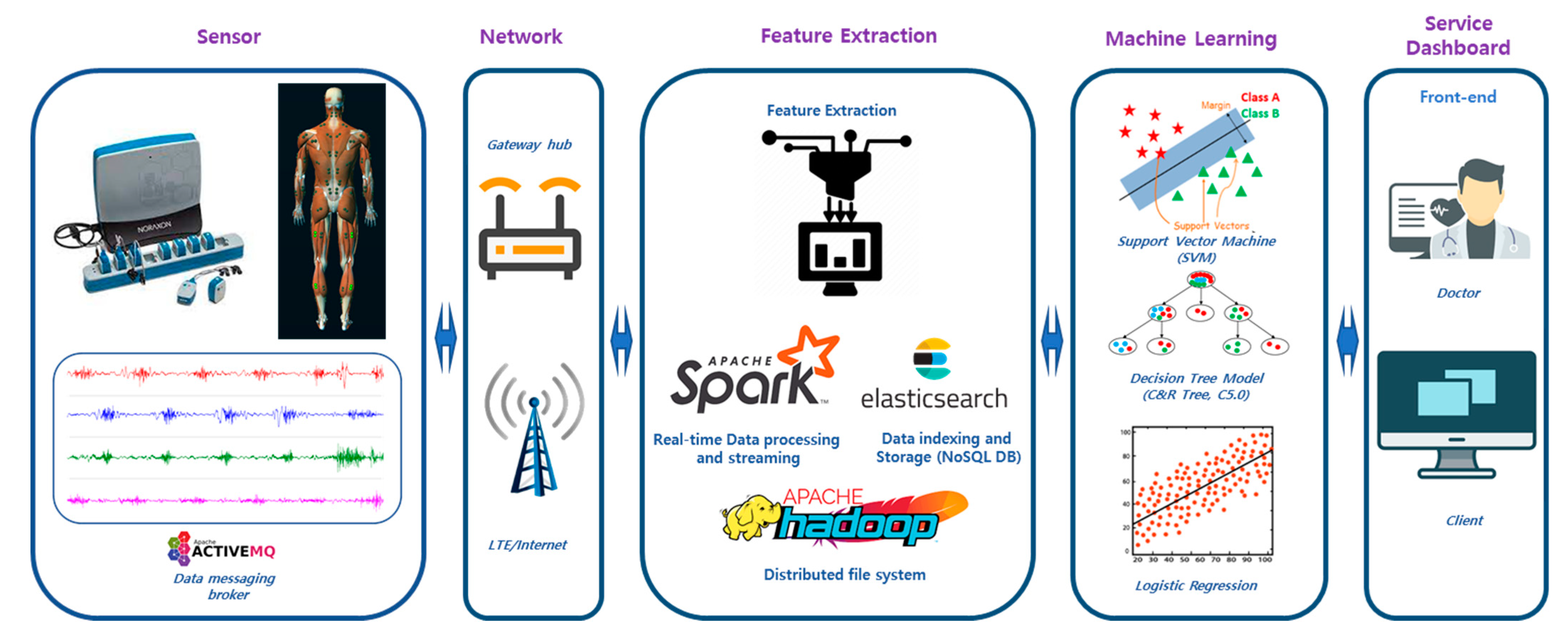
2.1. EMG-Based Disease Prediction System
2.2. Study Protocol
2.3. Demographics of Participants
2.4. Data Acquisition
2.5. Pre-Processing
2.6. Feature Extraction
2.7. Feature Selection
2.8. Machine Learning Algorithms
2.8.1. The Neural Network Model
2.8.2. C5.0 Model
2.8.3. Classification and Regression Tree Model
2.8.4. Support Vector Machine Model
2.8.5. Discriminant Analysis Model
2.8.6. Logistic Regression Model
2.9. Statistical and Machine Learning Analysis
3. Results
3.1. Statistical Investigation
Stroke-Impaired Myoelectric Biomarker
3.2. Machine-Learning-Based Post-Stroke Gait Prediction
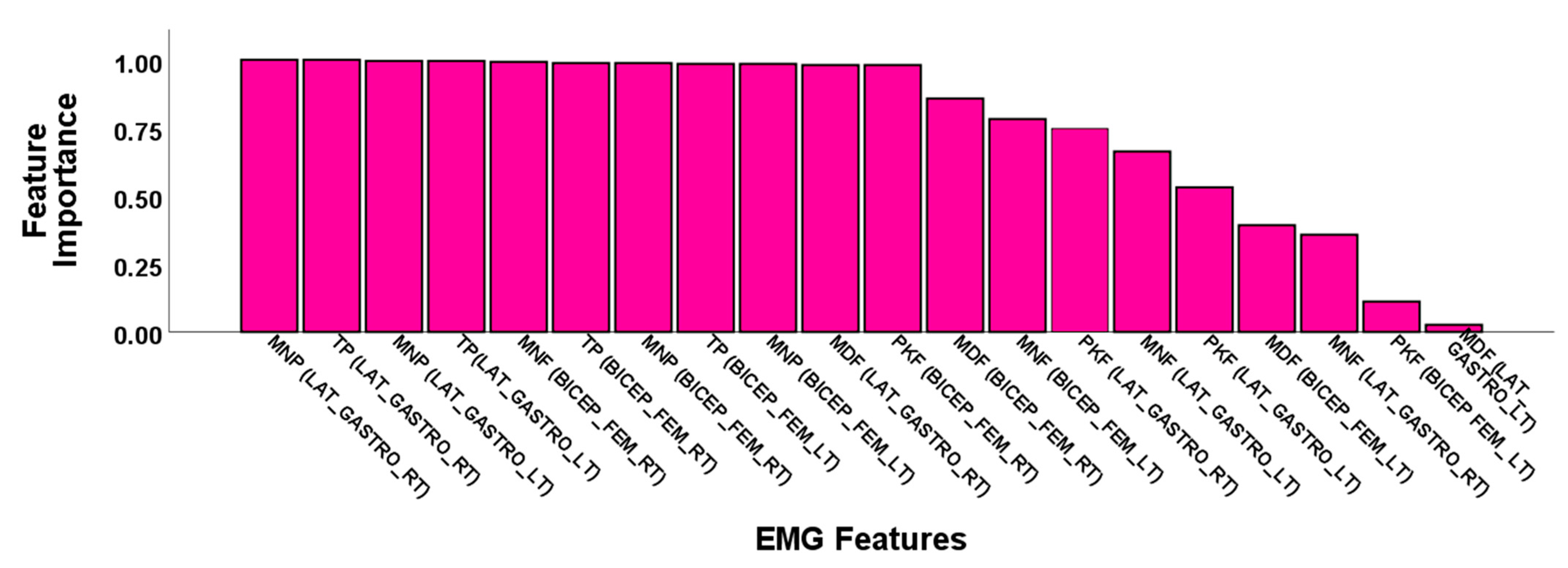
3.2.1. Feature Selection Results
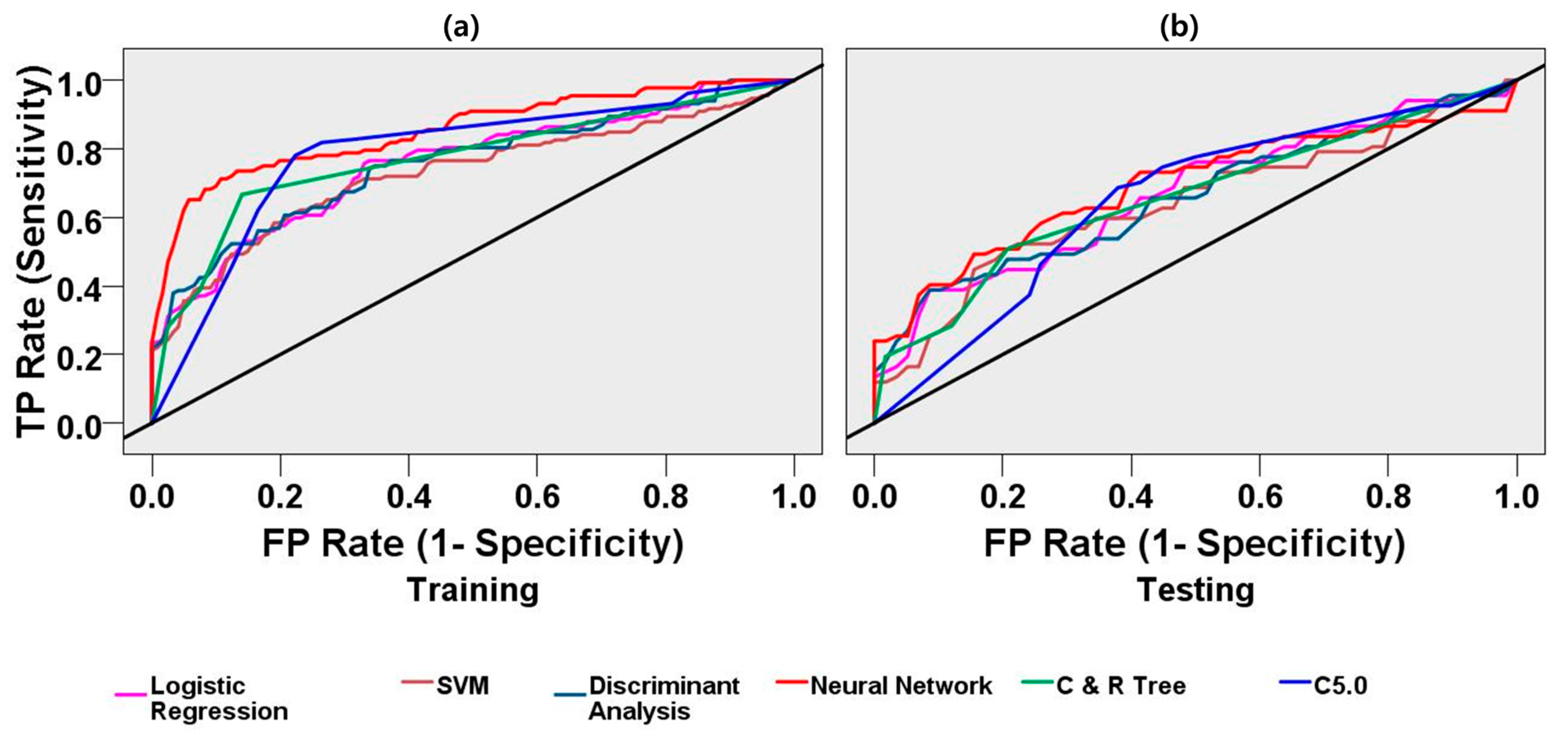
3.2.2. Classification Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2016: Monitoring Health for the Sdgs Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Balami, J.S.; Chen, R.-L.; Grunwald, I.Q.; Buchan, A.M. Neurological complications of acute ischaemic stroke. Lancet Neurol. 2011, 10, 357–371. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; MacLeod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.J. HealthSOS: Real-Time Health Monitoring System for Stroke Prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Park, S.J.; Hussain, I.; Hong, S.; Kim, D.; Park, H.; Benjamin, H.C.M. Real-time Gait Monitoring System for Consumer Stroke Prediction Service. In Proceedings of the 2020 IEEE International Conference on Consumer Electronics (ICCE), Las Vegas, NV, USA, 4–6 January 2020; pp. 1–4. [Google Scholar]
- Park, S.J.; Hong, S.; Damee, K.; Seo, Y.; Hussain, I.; Hur, J.H.; Jin, W. Development of a Real-Time Stroke Detection System for Elderly Drivers Using Quad-Chamber Air Cushion and Iot Devices. In Proceedings of the WCX™18: SAE World Congress Experience, Detroit, MI, USA, 10–12 April 2018. [Google Scholar]
- Hong, S.; Kim, D.; Park, H.; Seo, Y.; Iqram, H.; Park, S. Gait Feature Vectors for Post-stroke Prediction using Wearable Sensor. Korean Soc. Emot. Sensib. 2019, 22, 55–64. [Google Scholar] [CrossRef]
- Sawacha, Z.; Spolaor, F.; Piątkowska, W.; Cibin, F.; Ciniglio, A.; Guiotto, A.; Ricca, M.; Polli, R.; Murgia, A. Feasibility and Reliability Assessment of Video-Based Motion Analysis and Surface Electromyography in Children with Fragile X during Gait. Sensors 2021, 21, 4746. [Google Scholar] [CrossRef] [PubMed]
- Dimyan, M.; Cohen, L.G. Neuroplasticity in the context of motor rehabilitation after stroke. Nat. Rev. Neurol. 2011, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, M.M.; Settle, K.; Harvey, R.; Rymer, W.Z. Neuromuscular Abnormalities Associated with Spasticity of Upper Extremity Muscles in Hemiparetic Stroke. J. Neurophysiol. 2007, 98, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Hussain, I.; Park, S.-J. Quantitative Evaluation of Task-Induced Neurological Outcome after Stroke. Brain Sci. 2021, 11, 900. [Google Scholar] [CrossRef]
- Park, S.J.; Hong, S.; Kim, D.; Hussain, I.; Seo, Y. Intelligent In-Car Health Monitoring System for Elderly Drivers in Connected Car. In Proceedings of the WCX™18: SAE World Congress Experience, Detroit, MI, USA, 10–12 April 2018. [Google Scholar]
- Park, S.J.; Hong, S.; Kim, D.; Seo, Y.; Hussain, I. Knowledge Based Health Monitoring During Driving. In Communications in Computer and Information Science; Springer: Cham, Switzerland, 2018; pp. 387–392. [Google Scholar]
- Hussain, I.; Young, S.; Kim, C.H.; Benjamin, H.C.M.; Park, S.J. Quantifying Physiological Biomarkers of a Microwave Brain Stimulation Device. Sensors 2021, 21, 1896. [Google Scholar] [CrossRef]
- Park, H.; Hong, S.; Hussain, I.; Kim, D.; Seo, Y.; Park, S.J. Gait monitoring system for stroke prediction of aging adults. In Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2020; pp. 93–97. [Google Scholar]
- Hussain, I.; Park, S.J. Big-Ecg: Cardiographic Predictive Cyber-Physical System for Stroke Management. IEEE Access 2021, 9. under review. [Google Scholar]
- Fujita, K.; Kobayashi, Y.; Sato, M.; Hori, H.; Sakai, R.; Ogawa, T.; Sugano, T.; Kawabata, K.; Hitosugi, M. Kinematic and Electrophysiological Characteristics of Pedal Operation by Elderly Drivers during Emergency Braking. Health 2021, 9, 852. [Google Scholar] [CrossRef]
- Lu, Z.; Tong, R.K.-Y.; Zhang, X.; Li, S.; Zhou, P. Myoelectric Pattern Recognition for Controlling a Robotic Hand: A Feasibility Study in Stroke. IEEE Trans. Biomed. Eng. 2018, 66, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Van Kammen, K.; Boonstra, A.M.; Van Der Woude, L.H.V.; Reinders-Messelink, H.A.; Otter, R.D. Differences in muscle activity and temporal step parameters between Lokomat guided walking and treadmill walking in post-stroke hemiparetic patients and healthy walkers. J. Neuroeng. Rehabil. 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Solanki, D.; Kumar, S.; Shubha, B.; Lahiri, U. Implications of Physiology-Sensitive Gait Exercise on the Lower Limb Electromyographic Activity of Hemiplegic Post-Stroke Patients: A Feasibility Study in Low Resource Settings. IEEE J. Transl. Eng. Heal. Med. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Agostini, V.; Ghislieri, M.; Rosati, S.; Balestra, G.; Knaflitz, M. Surface Electromyography Applied to Gait Analysis: How to Improve Its Impact in Clinics? Front. Neurol. 2020, 11, 994. [Google Scholar] [CrossRef]
- Drost, G.; Stegeman, D.F.; van Engelen, B.G.; Zwarts, M.J. Clinical applications of high-density surface EMG: A systematic review. J. Electromyogr. Kinesiol. 2006, 16, 586–602. [Google Scholar] [CrossRef]
- Frigo, C.; Crenna, P. Multichannel SEMG in clinical gait analysis: A review and state-of-the-art. Clin. Biomech. 2009, 24, 236–245. [Google Scholar] [CrossRef]
- Dreher, T.; Brunner, R.; Végvári, D.; Heitzmann, D.; Gantz, S.; Maier, M.W.; Braatz, F.; Wolf, S.I. The Effects of Muscle-Tendon Surgery on Dynamic Electromyographic Patterns and Muscle Tone in Children with Cerebral Palsy. Gait Posture 2013, 38, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Intiso, D.; Santilli, V.; Grasso, M.G.; Rossi, R.; Caruso, I. Rehabilitation of Walking with Electromyographic Biofeedback in Foot-Drop after Stroke. Stroke 1994, 25, 1189–1192. [Google Scholar] [CrossRef] [Green Version]
- Van Der Houwen, L.; Scholtes, V.; Becher, J.; Harlaar, J. Botulinum toxin A injections do not improve surface EMG patterns during gait in children with cerebral palsy—A randomized controlled study. Gait Posture 2011, 33, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Rahnama, N.; Lees, A.; Reilly, T. Electromyography of selected lower-limb muscles fatigued by exercise at the intensity of soccer match-play. J. Electromyogr. Kinesiol. 2006, 16, 257–263. [Google Scholar] [CrossRef]
- Van Mastrigt, N.M.; Celie, K.; Mieremet, A.L.; Ruifrok, A.C.C.; Geradts, Z. Critical review of the use and scientific basis of forensic gait analysis. Forensic Sci. Res. 2018, 3, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infarinato, F.; Romano, P.; Goffredo, M.; Ottaviani, M.; Galafate, D.; Gison, A.; Petruccelli, S.; Pournajaf, S.; Franceschini, M. Functional Gait Recovery after a Combination of Conventional Therapy and Overground Robot-Assisted Gait Training Is Not Associated with Significant Changes in Muscle Activation Pattern: An EMG Preliminary Study on Subjects Subacute Post Stroke. Brain Sci. 2021, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Van Asseldonk, E.H.F.; Veneman, J.F.; Ekkelenkamp, R.; Buurke, J.H.; van der Helm, F.C.T.; van der Kooij, H. The Effects on Kinematics and Muscle Activity of Walking in a Robotic Gait Trainer During Zero-Force Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 360–370. [Google Scholar] [CrossRef]
- Otter, A.R.D.; Geurts, A.C.H.; Mulder, T.; Duysens, J. Gait recovery is not associated with changes in the temporal patterning of muscle activity during treadmill walking in patients with post-stroke hemiparesis. Clin. Neurophysiol. 2006, 117, 4–15. [Google Scholar]
- Cui, C.; Gui-Bin, B.; Zeng-Guang, H.; Jun, Z.; Su, G.; Zhou, H.; Peng, L.; Wang, W. Simultaneous Recognition and Assessment of Post-Stroke Hemiparetic Gait by Fusing Kinematic, Kinetic, and Electrophysiological Data. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 856–864. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Garcia-Cossio, E.; Birbaumer, N.; Burdet, E.; Ramos-Murguialday, A. Is EMG a Viable Alternative to BCI for Detecting Movement Intention in Severe Stroke? IEEE Trans. Biomed. Eng. 2018, 65, 2790–2797. [Google Scholar] [CrossRef]
- Saponas, T.S.; Tan, D.S.; Morris, D.; Balakrishnan, R. Demonstrating the feasibility of using forearm electromyography for muscle-computer interfaces. In Proceedings of the 26th Annual CHI Conference on Human Factors in Computing Systems—CHI ’08, Firenze, Italy, 5–10 April 2008; pp. 515–524. [Google Scholar]
- Williams, M.R.; Kirsch, R.F. Evaluation of Head Orientation and Neck Muscle EMG Signals as Command Inputs to a Human–Computer Interface for Individuals with High Tetraplegia. IEEE Trans. Neural Syst. Rehabilitation Eng. 2008, 16, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Buurke, J.H.; Nene, A.V.; Kwakkel, G.; Erren-Wolters, V.; IJzerman, M.J.; Hermens, H.J. Recovery of gait after stroke: What changes? Neurorehabilit. Neural Repair 2008, 22, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Wonsetler, E.; Bowden, M.G. A systematic review of mechanisms of gait speed change post-stroke. Part 2: Exercise capacity, muscle activation, kinetics, and kinematics. Top. Stroke Rehabil. 2017, 24, 394–403. [Google Scholar] [CrossRef]
- Androwis, G.J.; Pilkar, R.; Ramanujam, A.; Nolan, K.J. Electromyography Assessment During Gait in a Robotic Exoskeleton for Acute Stroke. Front. Neurol. 2018, 9, 630. [Google Scholar] [CrossRef] [Green Version]
- Sylos-Labini, F.; La Scaleia, V.; D’Avella, A.; Pisotta, I.; Tamburella, F.; Scivoletto, G.; Molinari, M.; Wang, S.; Wang, L.; van Asseldonk, E.; et al. EMG patterns during assisted walking in the exoskeleton. Front. Hum. Neurosci. 2014, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Phinyomark, A.; Thongpanja, S.; Hu, H.; Phukpattaranont, P.; Limsakul, P.P.A.C. The usefulness of mean and median frequencies in electromyography analysis. In Computational Intelligence in Electromyography Analysis—A Perspective on Current Applications and Future Challenges; IntechOpen: London, UK, 2012; pp. 195–220. [Google Scholar]
- Oskoei, M.A.; Hu, H. Support Vector Machine-Based Classification Scheme for Myoelectric Control Applied to Upper Limb. IEEE Trans. Biomed. Eng. 2008, 55, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Al-Mulla, R.; Colley, M.; Sepulveda, F. sEMG techniques to detect and predict localised muscle fatigue. In EMG Methods for Evaluating Muscle and Nerve Function; IntechOpen: London, UK, 2012; pp. 157–186. [Google Scholar]
- Barbero, M.; Merletti, R.; Rainoldi, A. Atlas of Muscle Innervation Zones: Understanding Surface Electromyography and Its Applications; Springer: Milan, Italy, 2012. [Google Scholar]
- Park, S.; Hong, S.; Kim, D.; Yu, J.; Hussain, I.; Park, H.; Benjamin, H. Development of intelligent stroke monitoring system for the elderly during sleeping. Sleep Med. 2019, 64, S294. [Google Scholar] [CrossRef]
- Dolin, R.H.; Alschuler, L.; Boyer, B.S.; Beebe, C.; Behlen, F.M.; Biron, P.V.; Shabo, A.S. HL7 Clinical Document Architecture, Release 2. J. Am. Med Informatics Assoc. 2006, 13, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Azza, A.; Bajda-Pawlikowski, K.; Abadi, D.; Silberschatz, A.; Rasin, A. Hadoopdb: An Architectural Hybrid of Mapreduce and Dbms Technologies for Analytical Workloads. Proc. VLDB Endow. 2009, 2, 922–933. [Google Scholar]
- Strzecha, K.; Krakós, M.; Więcek, B.; Chudzik, P.; Tatar, K.; Lisowski, G.; Mosorov, V.; Sankowski, D. Processing of Emg Signals with High Impact of Power Line and Cardiac Interferences. Appl. Sci. 2021, 11, 4625. [Google Scholar] [CrossRef]
- Hyvarinen, A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 1999, 10, 626–634. [Google Scholar] [CrossRef] [Green Version]
- McCool, P.; Fraser, G.D.; Chan, A.D.C.; Petropoulakis, L.; Soraghan, J.J. Identification of Contaminant Type in Surface Electromyography (EMG) Signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 774–783. [Google Scholar] [CrossRef]
- Fraser, G.D.; Chan, A.D.C.; Green, J.R.; MacIsaac, D.T. Automated Biosignal Quality Analysis for Electromyography Using a One-Class Support Vector Machine. IEEE Trans. Instrum. Meas. 2014, 63, 2919–2930. [Google Scholar] [CrossRef]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Thongpanja, S.; Phinyomark, A.; Phukpattaranont, P.; Limsakul, C. Mean and Median Frequency of EMG Signal to Determine Muscle Force based on Time-Dependent Power Spectrum. Elektron. ir Elektrotechnika 2013, 19, 51–56. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Bishop, C.M. Neural Networks for Pattern Recognition; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Quinlan, J.R. Data Mining Tools See5 and C5. 0. 2004. Available online: http://www.rulequest.com/see5-info.html (accessed on 5 May 2021).
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Chapman and Hall/CRC: Belmont, CA, USA, 1984; pp. 151–166. [Google Scholar]
- Suykens, J.A.K.; Vandewalle, J. Least Squares Support Vector Machine Classifiers. Neural Process. Lett. 1999, 9, 293–300. [Google Scholar] [CrossRef]
- Huang, J.; Ling, C. Using AUC and accuracy in evaluating learning algorithms. IEEE Trans. Knowl. Data Eng. 2005, 17, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Knaflitz, M.; Merletti, R.; De Luca, C.J. Inference of motor unit recruitment order in voluntary and electrically elicited contractions. J. Appl. Physiol. 1990, 68, 1657–1667. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Bian, G.-B.; Hou, Z.-G.; Zhao, J.; Zhou, H. A Multimodal Framework Based on Integration of Cortical and Muscular Activities for Decoding Human Intentions About Lower Limb Motions. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 889–899. [Google Scholar] [CrossRef]
- Castiblanco, J.C.; Ortmann, S.; Mondragon, I.F.; Alvarado-Rojas, C.; Jöbges, M.; Colorado, J.D. Myoelectric pattern recognition of hand motions for stroke rehabilitation. Biomed. Signal Process. Control. 2020, 57, 101737. [Google Scholar] [CrossRef]
- Angelova, S.; Ribagin, S.; Raikova, R.; Veneva, I. Power frequency spectrum analysis of surface EMG signals of upper limb muscles during elbow flexion—A comparison between healthy subjects and stroke survivors. J. Electromyogr. Kinesiol. 2018, 38, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Rasool, G.; Afsharipour, B.; Suresh, N.L.; Rymer, W.Z. Spatial Analysis of Multichannel Surface EMG in Hemiplegic Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1802–1811. [Google Scholar] [CrossRef]
- Lieber, R.L.; Steinman, S.; Barash, I.A.; Chambers, H. Structural and functional changes in spastic skeletal muscle. Muscle Nerve 2004, 29, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Lukács, M. Electrophysiological signs of changes in motor units after ischaemic stroke. Clin. Neurophysiol. 2005, 116, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Lukács, M.; Vécsei, L.; Beniczky, S. Changes in Muscle Fiber Density Following a Stroke. Clin. Neurophysiol. 2009, 120, 1539–1542. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Wilson, K.M.; Lock, B.A.; Kamper, D.G. Subject-Specific Myoelectric Pattern Classification of Functional Hand Movements for Stroke Survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhou, P. High-Density Myoelectric Pattern Recognition Toward Improved Stroke Rehabilitation. IEEE Trans. Biomed. Eng. 2012, 59, 1649–1657. [Google Scholar] [CrossRef] [PubMed]


| EMG Features | Muscle | Control | Stroke | t-Test | ||
|---|---|---|---|---|---|---|
| Mean | Standard Dev. | Mean | Standard Dev. | p-Value | ||
| Median Power Frequency (MDF), Hz | BICEP FEM. LT (BFLT) | 39.87 | 63.75 | 32.90 | 29.36 | 0.17 |
| BICEP FEM. RT (BFRT) | 39.67 | 61.43 | 29.83 | 26.78 | 0.04 * | |
| LAT. GASTRO. LT (LGLT) | 53.94 | 63.66 | 47.03 | 37.78 | 0.19 | |
| LAT. GASTRO. RT (LGRT) | 53.12 | 64.01 | 38.76 | 34.50 | 0.01 * | |
| Mean Power Frequency (MNF), Hz | BICEP FEM. LT (BFLT) | 175.93 | 65.45 | 160.00 | 64.21 | 0.02 * |
| BICEP FEM. RT (BFRT) | 166.21 | 59.40 | 149.00 | 63.33 | 0.01 * | |
| LAT. GASTRO. LT (LGLT) | 166.87 | 57.92 | 162.29 | 68.03 | 0.48 | |
| LAT. GASTRO. RT (LGRT) | 157.27 | 60.174 | 152.51 | 40.29 | 0.36 | |
| Peak Power Frequency (PKF), Hz | BICEP FEM. LT (BFLT) | 20.69 | 61.06 | 18.20 | 22.98 | 0.59 |
| BICEP FEM. RT (BFRT) | 21.80 | 58.40 | 12.94 | 14.92 | 0.04 * | |
| LAT. GASTRO. LT (LGLT) | 23.57 | 60.89 | 22.09 | 27.29 | 0.76 | |
| LAT. GASTRO. RT (LGRT) | 29.86 | 63.38 | 20.25 | 29.44 | 0.06 | |
| Mean Power (MNP), V2/Hz | BICEP FEM. LT (BFLT) | 0.0045 | 0.0040 | 0.0273 | 0.1178 | 0.01 * |
| BICEP FEM. RT (BFRT) | 0.0072 | 0.0116 | 0.0470 | 0.1921 | 0.01 * | |
| LAT. GASTRO. LT (LGLT) | 0.0073 | 0.0197 | 0.0250 | 0.0794 | 0.01 * | |
| LAT. GASTRO. RT (LGRT) | 0.0127 | 0.0237 | 0.0142 | 0.0696 | 0.78 | |
| Total Power (TP), V2/Hz | BICEP FEM. LT (BFLT) | 4.66 | 4.15 | 27.96 | 120.72 | 0.01 * |
| BICEP FEM. RT (BFRT) | 7.35 | 11.94 | 48.13 | 196.93 | 0.01 * | |
| LAT. GASTRO. LT (LGLT) | 7.48 | 20.21 | 25.67 | 81.46 | 0.01 * | |
| LAT. GASTRO. RT (LGRT) | 12.98 | 24.32 | 14.52 | 71.36 | 0.78 | |
| Model | Accuracy (ACC) | Sensitivity | Specificity | Precision | Negative Predictive Value | AUC | Gini |
|---|---|---|---|---|---|---|---|
| Neural Network | 0.80 | 0.71 | 0.89 | 0.88 | 0.74 | 0.85 | 0.70 |
| C5.0 | 0.78 | 0.78 | 0.78 | 0.79 | 0.76 | 0.79 | 0.57 |
| C&R Tree | 0.76 | 0.67 | 0.86 | 0.84 | 0.70 | 0.77 | 0.54 |
| Logistic Regression | 0.71 | 0.74 | 0.67 | 0.71 | 0.70 | 0.75 | 0.51 |
| SVM | 0.68 | 0.64 | 0.73 | 0.72 | 0.65 | 0.72 | 0.45 |
| Discriminant Analysis | 0.68 | 0.67 | 0.69 | 0.71 | 0.66 | 0.75 | 0.51 |
| Model | Accuracy (ACC) | Sensitivity | Specificity | Precision | Negative Predictive Value | AUC | Gini |
|---|---|---|---|---|---|---|---|
| Neural Network | 0.65 | 0.57 | 0.74 | 0.72 | 0.60 | 0.69 | 0.38 |
| C5.0 | 0.66 | 0.69 | 0.62 | 0.68 | 0.63 | 0.65 | 0.30 |
| C&R Tree | 0.64 | 0.51 | 0.79 | 0.74 | 0.58 | 0.66 | 0.32 |
| Logistic Regression | 0.61 | 0.63 | 0.59 | 0.64 | 0.58 | 0.66 | 0.32 |
| SVM | 0.62 | 0.60 | 0.64 | 0.66 | 0.58 | 0.64 | 0.27 |
| Discriminant Analysis | 0.59 | 0.60 | 0.59 | 0.63 | 0.56 | 0.65 | 0.31 |
| Study | Study Sample | EMG Features | Findings | Application |
|---|---|---|---|---|
| Lu et al. [18] | Eight post-stroke subjects | Root mean square (RMS), 4th order auto regressive (AR) Coefficients, and waveform length (WL) | Mean classification accuracy, GNB): 84.8%; SVM: 83.3%; paired t-Test, p: 0.125 | Classification of six hand motion patterns for controlling a robotic hand |
| Lee et al. [67] | Twenty stroke patients | Mean absolute value (MAV), the number of zero crossing (ZC), the slope sign change (SSC), and WL | Mean classification accuracy, LDA: = 71.3% for moderately impaired subjects. | Classification of task-specific hand movements |
| Castiblanco et al. [61] | Eighteen stroke patients and twenty-eight healthy control | MAV, RMS, SSC, MNF, mean power (MNP), MDF, and spectral moments (SM) | Accuracy classification of stroke and control group, KNN: 0.87; SVM: 0.82, and LDA: 0.74. | Identification of the fingers and hand motions for robotics-based rehabilitation. |
| Angelova et al. [62] | Ten stroke patients and fifteen healthy adults | Power spectrum features: MNF, MDF, Maximal power | MNF and MDF are lower for stroke patients compared with healthy control group. | Identification of changes in features during elbow flexion. |
| Proposed study | Forty-eight stroke patients and seventy-five healthy adults | MNF, MDF, PKF, TP, MP | Classification performance, neural network model: precision: 88%, specificity: 89%, accuracy: 80%. | Prediction of stroke-impaired myoelectrical changes through statistics and machine learning for understanding post-stroke impairment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, I.; Park, S.-J. Prediction of Myoelectric Biomarkers in Post-Stroke Gait. Sensors 2021, 21, 5334. https://doi.org/10.3390/s21165334
Hussain I, Park S-J. Prediction of Myoelectric Biomarkers in Post-Stroke Gait. Sensors. 2021; 21(16):5334. https://doi.org/10.3390/s21165334
Chicago/Turabian StyleHussain, Iqram, and Se-Jin Park. 2021. "Prediction of Myoelectric Biomarkers in Post-Stroke Gait" Sensors 21, no. 16: 5334. https://doi.org/10.3390/s21165334
APA StyleHussain, I., & Park, S.-J. (2021). Prediction of Myoelectric Biomarkers in Post-Stroke Gait. Sensors, 21(16), 5334. https://doi.org/10.3390/s21165334








