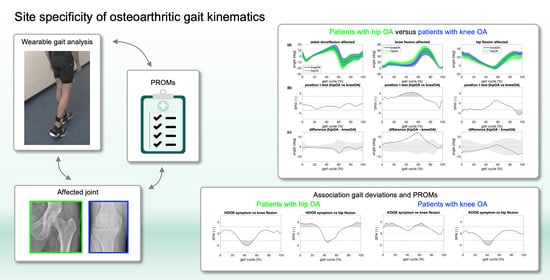
Assessing Site Specificity of Osteoarthritic Gait Kinematics with Wearable Sensors and Their Association with Patient Reported Outcome Measures (PROMs): Knee versus Hip Osteoarthritis
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Demographic and Clinical Evaluation
2.3. Gait Analysis
2.4. Data Processing
2.5. Statistical Analyses
3. Results
3.1. Overall Group Effect in Joint Kinematics
3.2. Patients with OA Versus Controls
3.3. Patients with Hip OA Versus Patients with Knee OA
3.4. Association between Gait Deviations from Controls and PROMs
3.4.1. Knee OA
3.4.2. Hip OA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, L.B.; Helmick, C.G.; Schwartz, T.A.; Renner, J.B.; Tudor, G.; Koch, G.G.; Dragomir, A.D.; Kalsbeek, W.D.; Luta, G.; Jordan, J.M. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthr. Cartil. 2010, 18, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Eckhard, L.; Munir, S.; Wood, D.; Talbot, S.; Brighton, R.; Walter, B.; Bare, J. The ceiling effects of patient reported outcome measures for total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2021, 107, 102758. [Google Scholar] [CrossRef]
- Terwee, C.B.; van der Slikke, R.M.; van Lummel, R.C.; Benink, R.J.; Meijers, W.G.; de Vet, H.C. Self-reported physical functioning was more influenced by pain than performance-based physical functioning in knee-osteoarthritis patients. J. Clin. Epidemiol. 2006, 59, 724–731. [Google Scholar] [CrossRef]
- Laslett, L.L.; Pelletier, J.-P.; Cicuttini, F.M.; Jones, G.; Martel-Pelletier, J. Measuring Disease Progression in Osteoarthritis. Curr. Treatm. Opt. Rheumatol. 2016, 2, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Ornetti, P.; Maillefert, J.F.; Laroche, D.; Morisset, C.; Dougados, M.; Gossec, L. Gait analysis as a quantifiable outcome measure in hip or knee osteoarthritis: A systematic review. Joint Bone Spine 2010, 77, 421–425. [Google Scholar] [CrossRef]
- Ornetti, P.; Laroche, D.; Morisset, C.; Beis, J.N.; Tavernier, C.; Maillefert, J.F. Three-dimensional kinematics of the lower limbs in hip osteoarthritis during walking. J. Back Musculoskelet. Rehabil. 2011, 24, 201–208. [Google Scholar] [CrossRef]
- Sharma, L.; Hurwitz, D.E.; Thonar, E.J.; Sum, J.A.; Lenz, M.E.; Dunlop, D.D.; Schnitzer, T.J.; Kirwan-Mellis, G.; Andriacchi, T.P. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheumatol. 1998, 41, 1233–1240. [Google Scholar] [CrossRef]
- Miyazaki, T.; Wada, M.; Kawahara, H.; Sato, M.; Baba, H.; Shimada, S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann. Rheum. Dis. 2002, 61, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Mündermann, A.; Dyrby, C.O.; Andriacchi, T.P. Secondary gait changes in patients with medial compartment knee osteoarthritis: Increased load at the ankle, knee, and hip during walking. Arthritis Rheumatol. 2005, 52, 2835–2844. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Mündermann, A.; Smith, R.L.; Alexander, E.J.; Dyrby, C.O.; Koo, S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann. Biomed. Eng. 2004, 32, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Shimoura, K.; Ono, T.; Aoyama, T.; Takahashi, M. Proximal gait adaptations in individuals with knee osteoarthritis: A systematic review and meta-analysis. J. Biomech. 2019, 87, 127–141. [Google Scholar] [CrossRef]
- Paoloni, M.; Di Sante, L.; Dimaggio, M.; Bernetti, A.; Mangone, M.; Di Renzo, S.; Santilli, V. Kinematic and kinetic modifications in walking pattern of hip osteoarthritis patients induced by intra-articular injections of hyaluronic acid. Clin. Biomech. (Bristol Avon) 2012, 27, 661–665. [Google Scholar] [CrossRef]
- Skwara, A.; Ponelis, R.; Tibesku, C.O.; Rosenbaum, D.; Fuchs-Winkelmann, S. Gait patterns after intraarticular treatment of patients with osteoarthritis of the knee--hyaluronan versus triamcinolone: A prospective, randomized, doubleblind, monocentric study. Eur. J. Med. Res. 2009, 14, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Bahl, J.S.; Nelson, M.J.; Taylor, M.; Solomon, L.B.; Arnold, J.B.; Thewlis, D. Biomechanical changes and recovery of gait function after total hip arthroplasty for osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2018, 26, 847–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosdian, L.; Dobson, F.; Wrigley, T.V.; Paterson, K.; Bennell, K.; Dowsey, M.; Choong, P.; Allison, K.; Hinman, R.S. Longitudinal changes in knee kinematics and moments following knee arthroplasty: A systematic review. Knee 2014, 21, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.; Hunt, M.A.; Ferber, R. Biomechanical deviations during level walking associated with knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res. (Hoboken) 2013, 65, 1643–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Straaten, R.; De Baets, L.; Jonkers, I.; Timmermans, A. Mobile assessment of the lower limb kinematics in healthy persons and in persons with degenerative knee disorders: A systematic review. Gait Posture 2018, 59, 229–241. [Google Scholar] [CrossRef]
- McCarthy, I.; Hodgins, D.; Mor, A.; Elbaz, A.; Segal, G. Analysis of knee flexion characteristics and how they alter with the onset of knee osteoarthritis: A case control study. BMC Musculoskelet. Disord. 2013, 14, 169. [Google Scholar] [CrossRef] [Green Version]
- Tadano, S.; Takeda, R.; Sasaki, K.; Fujisawa, T.; Tohyama, H. Gait characterization for osteoarthritis patients using wearable gait sensors (H-Gait systems). J. Biomech. 2016, 49, 684–690. [Google Scholar] [CrossRef]
- Rahman, J.; Tang, Q.; Monda, M.; Miles, J.; McCarthy, I. Gait assessment as a functional outcome measure in total knee arthroplasty: A cross-sectional study. BMC Musculoskelet. Disord. 2015, 16, 66. [Google Scholar] [CrossRef] [Green Version]
- Ismailidis, P.; Hegglin, L.; Egloff, C.; Pagenstert, G.; Kernen, R.; Eckardt, A.; Ilchmann, T.; Nuesch, C.; Mundermann, A. Side to side kinematic gait differences within patients and spatiotemporal and kinematic gait differences between patients with severe knee osteoarthritis and controls measured with inertial sensors. Gait Posture 2021, 84, 24–30. [Google Scholar] [CrossRef]
- Ismailidis, P.; Nuesch, C.; Kaufmann, M.; Clauss, M.; Pagenstert, G.; Eckardt, A.; Ilchmann, T.; Mundermann, A. Measuring gait kinematics in patients with severe hip osteoarthritis using wearable sensors. Gait Posture 2020, 81, 49–55. [Google Scholar] [CrossRef]
- Ismailidis, P.; Egloff, C.; Hegglin, L.; Pagenstert, G.; Kernen, R.; Eckardt, A.; Ilchmann, T.; Mundermann, A.; Nuesch, C. Kinematic changes in patients with severe knee osteoarthritis are a result of reduced walking speed rather than disease severity. Gait Posture 2020, 79, 256–261. [Google Scholar] [CrossRef]
- Ismailidis, P.; Kaufmann, M.; Clauss, M.; Pagenstert, G.; Eckardt, A.; Ilchmann, T.; Mundermann, A.; Nuesch, C. Kinematic changes in severe hip osteoarthritis measured at matched gait speeds. J. Orthop. Res. 2021, 39, 1253–1261. [Google Scholar] [CrossRef]
- Lebleu, J.; Fonkoue, L.; Bandolo, E.; Fossoh, H.; Mahaudens, P.; Cornu, O.; Detrembleur, C. Lower limb kinematics improvement after genicular nerve blockade in patients with knee osteoarthritis: A milestone study using inertial sensors. BMC Musculoskelet. Disord. 2020, 21, 822. [Google Scholar] [CrossRef]
- Teufl, W.; Taetz, B.; Miezal, M.; Lorenz, M.; Pietschmann, J.; Jollenbeck, T.; Frohlich, M.; Bleser, G. Towards an Inertial Sensor-Based Wearable Feedback System for Patients after Total Hip Arthroplasty: Validity and Applicability for Gait Classification with Gait Kinematics-Based Features. Sensors 2019, 19, 5006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, D.; Vap, A.; Queen, R.M. Effect of end-stage hip, knee, and ankle osteoarthritis on walking mechanics. Gait Posture 2015, 42, 373–379. [Google Scholar] [CrossRef]
- Pataky, T.C. One-dimensional statistical parametric mapping in Python. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Nilsdotter, A.K.; Lohmander, L.S.; Klassbo, M.; Roos, E.M. Hip disability and osteoarthritis outcome score (HOOS)—Validity and responsiveness in total hip replacement. BMC Musculoskelet. Disord. 2003, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellgren, J.H.; Lawrence, J.S. Atlas of Standard Radiographs; University of Manchester, Blackwell: Oxford, UK, 1963. [Google Scholar]
- Nüesch, C.; Roos, E.; Pagenstert, G.; Mündermann, A. Measuring joint kinematics of treadmill walking and running: Comparison between an inertial sensor based system and a camera-based system. J. Biomech. 2017, 57, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seel, T.; Raisch, J.; Schauer, T. IMU-based joint angle measurement for gait analysis. Sensors 2014, 14, 6891–6909. [Google Scholar] [CrossRef] [Green Version]
- Schicketmueller, A.; Rose, G.; Hofmann, M. Feasibility of a Sensor-Based Gait Event Detection Algorithm for Triggering Functional Electrical Stimulation during Robot-Assisted Gait Training. Sensors 2019, 19, 4804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donath, L.; Faude, O.; Lichtenstein, E.; Nüesch, C.; Mündermann, A. Validity and reliability of a portable gait analysis system for measuring spatiotemporal gait characteristics: Comparison to an instrumented treadmill. J. Neuroeng. Rehabil. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donath, L.; Faude, O.; Lichtenstein, E.; Pagenstert, G.; Nuesch, C.; Mundermann, A. Mobile inertial sensor based gait analysis: Validity and reliability of spatiotemporal gait characteristics in healthy seniors. Gait Posture 2016, 49, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Eitzen, I.; Fernandes, L.; Nordsletten, L.; Risberg, M.A. Sagittal plane gait characteristics in hip osteoarthritis patients with mild to moderate symptoms compared to healthy controls: A cross-sectional study. BMC Musculoskelet. Disord. 2012, 13, 258. [Google Scholar] [CrossRef]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Vector field statistical analysis of kinematic and force trajectories. J. Biomech. 2013, 46, 2394–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favre, J.; Jolles, B.M. Gait analysis of patients with knee osteoarthritis highlights a pathological mechanical pathway and provides a basis for therapeutic interventions. EFORT Open Rev. 2016, 1, 368–374. [Google Scholar] [CrossRef]
- Ardestani, M.M.; Wimmer, M.A. Can a linear combination of gait principal component vectors identify hip OA stages? J. Biomech. 2016, 49, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Pau, M.; Leban, B.; Deidda, M.; Sorrentino, M.; Arippa, F.; Marongiu, G. Lower Limb Kinematics in Individuals with Hip Osteoarthritis during Gait: A Focus on Adaptative Strategies and Interlimb Symmetry. Bioengineering 2021, 8, 47. [Google Scholar] [CrossRef]
- Maly, M.R.; Costigan, P.A.; Olney, S.J. Mechanical factors relate to pain in knee osteoarthritis. Clin. Biomech. (Bristol Avon) 2008, 23, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Astephen Wilson, J.L.; Deluzio, K.J.; Dunbar, M.J.; Caldwell, G.E.; Hubley-Kozey, C.L. The association between knee joint biomechanics and neuromuscular control and moderate knee osteoarthritis radiographic and pain severity. Osteoarthr. Cartil. 2011, 19, 186–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensalma, F.; Mezghani, N.; Ouakrim, Y.; Fuentes, A.; Choinière, M.; Bureau, N.J.; Durand, M.; Hagemeister, N. A multivariate relationship between the kinematic and clinical parameters of knee osteoarthritis population. Biomed. Eng. OnLine 2019, 18, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenlund, S.; Holsgaard-Larsen, A.; Overgaard, S.; Jensen, C. The gait deviation index is associated with hip muscle strength and patient-reported outcome in patients with severe hip osteoarthritis: A cross-sectional study. PLoS ONE 2016, 11, e0153177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tateuchi, H.; Akiyama, H.; Goto, K.; So, K.; Kuroda, Y.; Ichihashi, N. Gait- and Posture-Related Factors Associated With Changes in Hip Pain and Physical Function in Patients With Secondary Hip Osteoarthritis: A Prospective Cohort Study. Arch. Phys. Med. Rehabil. 2019, 100, 2053–2062. [Google Scholar] [CrossRef]






| Parameter | Patients with Knee OA | Patients with Hip OA | Asymptomatic Controls |
|---|---|---|---|
| N | 29 | 30 | 54 |
| Sex (female/male) | 16/13 | 18/12 | 23/31 |
| Age (years) | 67.2 (8.5) | 64.9 (11.6) | 66.4 (7.9) |
| Body height (m) | 1.70 (0.10) | 1.72 (0.08) | 1.70 (0.09) |
| Body mass (kg) | 80.6 (14.4) 1 | 80.6 (12.8) 1 | 73.1 (13.5) |
| Body mass index (kg/m2) | 27.9 (3.9) 1 | 27.1 (3.1) 1 | 25.3 (4.0) |
| KOOS | |||
| Symptoms | 50.1 (19.9) 1 | 95.4 (6.1) | |
| Pain | 47.5 (17.7) 1 | 97.6 (3.9) | |
| ADL | 52.5 (21.9) 1 | 98.2 (3.0) | |
| Sport/rec | 28.2 (19.8) 1 | 94.9 (9.1) | |
| QOL | 25.6 (19.0) 1 | 95.5 (8.8) | |
| HOOS | |||
| Symptoms | 48.3 (16.8) 1 | 96.9 (5.6) | |
| Pain | 50.9 (14.9) 1 | 98.2 (4.4) | |
| ADL | 53.9 (17.3) 1 | 98.6 (3.5) | |
| Sport/rec | 36.1 (20.0) 1 | 98.0 (5.0) | |
| QOL | 27.8 (16.4) 1 | 97.3 (7.2) |
| Criteria | Patients with Knee OA | Patients with Hip OA | Asymptomatic Controls |
|---|---|---|---|
| Inclusion criteria |
|
|
|
| Exclusion criteria |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nüesch, C.; Ismailidis, P.; Koch, D.; Pagenstert, G.; Ilchmann, T.; Eckardt, A.; Stoffel, K.; Egloff, C.; Mündermann, A. Assessing Site Specificity of Osteoarthritic Gait Kinematics with Wearable Sensors and Their Association with Patient Reported Outcome Measures (PROMs): Knee versus Hip Osteoarthritis. Sensors 2021, 21, 5363. https://doi.org/10.3390/s21165363
Nüesch C, Ismailidis P, Koch D, Pagenstert G, Ilchmann T, Eckardt A, Stoffel K, Egloff C, Mündermann A. Assessing Site Specificity of Osteoarthritic Gait Kinematics with Wearable Sensors and Their Association with Patient Reported Outcome Measures (PROMs): Knee versus Hip Osteoarthritis. Sensors. 2021; 21(16):5363. https://doi.org/10.3390/s21165363
Chicago/Turabian StyleNüesch, Corina, Petros Ismailidis, David Koch, Geert Pagenstert, Thomas Ilchmann, Anke Eckardt, Karl Stoffel, Christian Egloff, and Annegret Mündermann. 2021. "Assessing Site Specificity of Osteoarthritic Gait Kinematics with Wearable Sensors and Their Association with Patient Reported Outcome Measures (PROMs): Knee versus Hip Osteoarthritis" Sensors 21, no. 16: 5363. https://doi.org/10.3390/s21165363
APA StyleNüesch, C., Ismailidis, P., Koch, D., Pagenstert, G., Ilchmann, T., Eckardt, A., Stoffel, K., Egloff, C., & Mündermann, A. (2021). Assessing Site Specificity of Osteoarthritic Gait Kinematics with Wearable Sensors and Their Association with Patient Reported Outcome Measures (PROMs): Knee versus Hip Osteoarthritis. Sensors, 21(16), 5363. https://doi.org/10.3390/s21165363