Optical Fiber Array Sensor for Force Estimation and Localization in TAVI Procedure: Design, Modeling, Analysis and Validation
Abstract
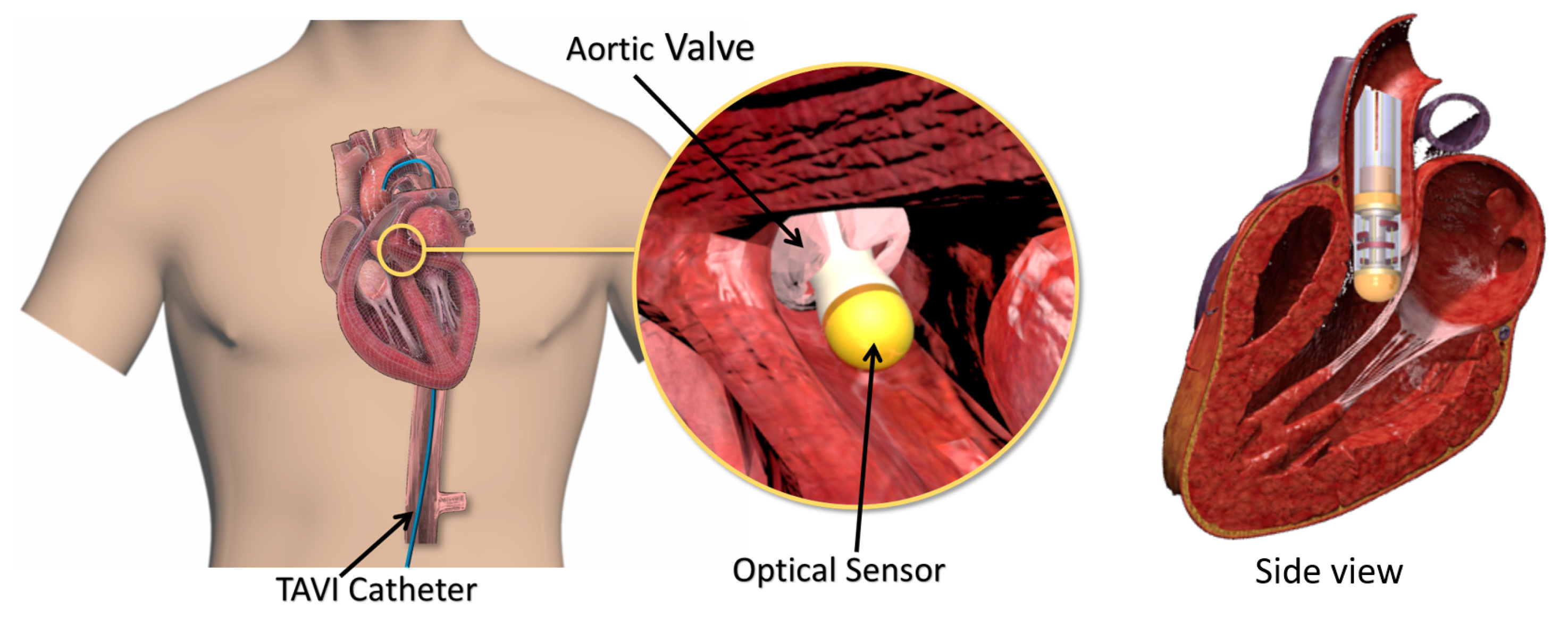
:1. Introduction
2. Design Requirements and Structural Design
3. Sensor Modeling
3.1. Sensing Principle
3.2. Finite Element Simulation
4. Validation Study
4.1. Sensor Fabrication
4.2. Experimental Setup and Validation Test
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seibold, U.; Kübler, B.; Hirzinger, G. Prototype of instrument for minimally invasive surgery with 6-axis force sensing capability. ICRA 2005, 2005, 498–503. [Google Scholar]
- Polygerinos, P.; Seneviratne, L.D.; Razavi, R.; Schaeffter, T.; Althoefer, K. Triaxial catheter-tip force sensor for MRI-guided cardiac procedures. IEEE/ASME Trans. Mechatron. 2013, 18, 386–396. [Google Scholar] [CrossRef]
- Sauerland, S.; Jaschinski, T.; Neugebauer, E.A. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst. Rev. 2010, 10, CD001546. [Google Scholar] [CrossRef]
- Yip, M.C.; Yuen, S.G.; Howe, R.D. A robust uniaxial force sensor for minimally invasive surgery. IEEE Trans. Biomed. Eng. 2010, 57, 1008–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, J.B.; Kovac, J.; Schuler, G.; Ye, J.; Cheung, A.; Kapadia, S.; Tuzcu, M.E.; Kodali, S.; Leon, M.B.; Webb, J.G. Transcatheter aortic valve implantation: Review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc. Interv. 2009, 2, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Hooshiar, A.; Sayadi, A.; Dargahi, J.; Najarian, S. Integral-free Spatial Orientation Estimation Method and Wearable Rotation Measurement Device for Robot-assisted Catheter Intervention. IEEE/ASME Trans. Mechatron. 2021. [Google Scholar] [CrossRef]
- Hooshiar, A.; Najarian, S.; Dargahi, J. Haptic Telerobotic Cardiovascular Intervention: A Review of Approaches, Methods, and Future Perspectives. IEEE Rev. Biomed. Eng. 2020, 13, 32–50. [Google Scholar] [CrossRef]
- Hooshiar, A.; Razban, M.; Bandari, N.M.; Dargahi, J. Sensing principle for real-time characterization of viscoelasticity in the beating myocardial tissue. In Proceedings of the 2017 IEEE International Conference on Computational Intelligence and Virtual Environments for Measurement Systems and Applications (CIVEMSA), Annecy, France, 26–28 June 2017; pp. 72–77. [Google Scholar]
- Bandari, N.; Dargahi, J.; Packirisamy, M. Tactile Sensors for Minimally Invasive Surgery: A Review of the State-of-the-art, Applications, and Perspectives. IEEE Access 2020, 8, 7682–7708. [Google Scholar] [CrossRef]
- Fontanelli, G.A.; Buonocore, L.R.; Ficuciello, F.; Villani, L.; Siciliano, B. An External Force Sensing System for Minimally Invasive Robotic Surgery. IEEE/ASME Trans. Mechatron. 2020, 25, 1543–1554. [Google Scholar] [CrossRef]
- Kumar, N.; Wirekoh, J.; Saba, S.; Riviere, C.N.; Park, Y.L. Soft Miniaturized Actuation and Sensing Units for Dynamic Force Control of Cardiac Ablation Catheters. Soft Robot. 2020, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ju, F.; Wei, X.; Wang, D.; Wang, Y. A Piezoelectric Tactile Sensor for Tissue Stiffness Detection with Arbitrary Contact Angle. Sensors 2020, 20, 6607. [Google Scholar] [CrossRef]
- Lee, J.i.; Lee, S.; Oh, H.M.; Cho, B.R.; Seo, K.H.; Kim, M.Y. 3D Contact Position Estimation of Image-Based Areal Soft Tactile Sensor with Printed Array Markers and Image Sensors. Sensors 2020, 20, 3796. [Google Scholar] [CrossRef]
- Mohammadi, A.; Xu, Y.; Tan, Y.; Choong, P.; Oetomo, D. Magnetic-based soft tactile sensors with deformable continuous force transfer medium for resolving contact locations in robotic grasping and manipulation. Sensors 2019, 19, 4925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koike, R.; Sakaino, S.; Tsuji, T. Hysteresis compensation in force/torque sensors using time series information. Sensors 2019, 19, 4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzun, D.; Ulgen, O.; Kocaturk, O. Optical Force Sensor with Enhanced Resolution for MRI Guided Biopsy. IEEE Sens. J. 2020, 20, 9202–9208. [Google Scholar] [CrossRef]
- Ahmadi, R.; Packirisamy, M.; Dargahi, J. Innovative optical microsystem for static and dynamic tissue diagnosis in minimally invasive surgical operations. J. Biomed. Opt. 2012, 17, 0814161–0814168. [Google Scholar] [CrossRef] [PubMed]
- Hooshiar, A.; Bandari, N.M.; Dargahi, J. Image-based estimation of contact forces on catheters for robot-assisted cardiovascular intervention. In Proceedings of the Hamlyn Symposium on Medical Robotics 2018, London, UK, 21–29 June 2018; pp. 119–120. [Google Scholar]
- Jolaei, M.; Hooshiar, A.; Dargahi, J.; Packirisamy, M. Toward Task Autonomy in Robotic Cardiac Ablation: Learning-based Kinematic Control of Soft Tendon-driven Catheters. Soft Robot. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Jolaei, M.; Hooshiar, A.; Sayadi, A.; Dargahi, J.; Packirisamy, M. Sensor-free Force Control of Tendon-driven Ablation Catheters through Position Control and Contact Modeling. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 5248–5251. [Google Scholar]
- Hooshiar, A.; Payami, A.; Dargahi, J.; Najarian, S. Magnetostriction-based force feedback for robot-assisted cardiovascular surgery using smart magnetorheological elastomers. Mech. Syst. Signal Process. 2021, 161, 107918. [Google Scholar] [CrossRef]
- Hooshiar, A.; Alkhalaf, A.; Dargahi, J. Development and Assessment of a Stiffness Display System for Minimally Invasive Surgery based on Smart Magneto-rheological Elastomers. Mater. Sci. Eng. C 2020, 108, 110409. [Google Scholar] [CrossRef]
- Alkhalaf, A.; Hooshiar, A.; Dargahi, J. Composite magnetorheological elastomers for tactile displays: Enhanced MReffect through Bi-layer composition. Compos. Part B Eng. 2020, 190, 107888. [Google Scholar] [CrossRef]
- Ozioko, O.; Navaraj, W.; Hersh, M.; Dahiya, R. Tacsac: A wearable haptic device with capacitive touch-sensing capability for tactile display. Sensors 2020, 20, 4780. [Google Scholar] [CrossRef]
- Konstantinova, J.; Jiang, A.; Althoefer, K.; Dasgupta, P.; Nanayakkara, T. Implementation of tactile sensing for palpation in robot-assisted minimally invasive surgery: A review. IEEE Sens. J. 2014, 14, 2490–2501. [Google Scholar] [CrossRef] [Green Version]
- Polygerinos, P.; Ataollahi, A.; Schaeffter, T.; Razavi, R.; Seneviratne, L.D.; Althoefer, K. MRI-compatible intensity-modulated force sensor for cardiac catheterization procedures. IEEE Trans. Biomed. Eng. 2011, 58, 721–726. [Google Scholar] [CrossRef]
- Noh, Y.; Liu, H.; Sareh, S.; Chathuranga, D.; Wurdemann, H.; Rhode, K.; Althoefer, K. Image-based optical miniaturized three-axis force sensor for cardiac catheterization. IEEE Sens. J 2016, 16, 7924–7932. [Google Scholar] [CrossRef]
- Li, T.; Shi, C.; Ren, H. Three-dimensional catheter distal force sensing for cardiac ablation based on fiber Bragg grating. IEEE/ASME Trans. Mechatron. 2018, 23, 2316–2327. [Google Scholar] [CrossRef]
- Li, T.; Pan, A.; Ren, H. A High-Resolution Tri-axial Catheter-tip Force Sensor with Miniature Flexure and Suspended Optical Fibers. IEEE Trans. Ind. Electron. 2019, 67, 5101–5111. [Google Scholar] [CrossRef]
- Bandari, N.M.; Hooshiar, A.; Packirisamy, M.; Dargahi, J. Optical Fiber Array Sensor for Lateral and Circumferential Force Measurement Suitable for Minimally Invasive Surgery: Design, Modeling and Analysis. In Specialty Optical Fibers; Optical Society of America: Washington, DC, USA, 2016; p. JTu4A-44. [Google Scholar]
- Bandari, N.; Dargahi, J.; Packirisamy, M. Miniaturized optical force sensor for minimally invasive surgery with learning-based nonlinear calibration. IEEE Sens. J. 2020, 20, 3579–3592. [Google Scholar] [CrossRef]
- Gauthier, R.C.; Ross, C. Theoretical and experimental considerations for a single-mode fiber-optic bend-type sensor. Appl. Opt. 1997, 36, 6264–6273. [Google Scholar] [CrossRef] [PubMed]
- Bandari, N.M.; Hooshair, A.; Packirisamy, M.; Dargahi, J. Bending-based formulation of light intensity modulation for miniaturization of optical tactile sensors. In Optical Sensors; Optical Society of America: Washington, DC, USA, 2018; p. SeM2E-3. [Google Scholar]
- Bandari, N.; Dargahi, J.; Packirisamy, M. Validation of a Variable Bending Radius Sensing Principle for Optical-fiber Tactile Sensors. In Proceedings of the 2019 Photonics North (PN), Quebec City, QC, Canada, 21–23 May 2019. [Google Scholar]
- Spotts, M.F.; Shoup, T.E.; Hornberger, L.E. Design of Machine Elelments; Pearson Education: London, UK, 2006. [Google Scholar]
- Bandari, N.; Dargahi, J.; Packirisamy, M. Image-based Optical-fiber Force Sensor for Minimally Invasive Surgery with ex-vivo Validation. J. Electrochem. Soc. 2020, 167, 127504. [Google Scholar] [CrossRef]
- Bandari, N.; Dargahi, J.; Packirisamy, M. Camera-Based Optical-Fiber Tactile Sensor for Intraoperative Grasping Force Measurement. In Proceedings of the 237th ECS Meeting with the 18th International Meeting on Chemical Sensors (IMCS 2020), Montreal, QC, Canada, 10–14 May 2020. [Google Scholar]
- Kakani, V.; Cui, X.; Ma, M.; Kim, H. Vision-Based Tactile Sensor Mechanism for the Estimation of Contact Position and Force Distribution Using Deep Learning. Sensors 2021, 21, 1920. [Google Scholar] [CrossRef]



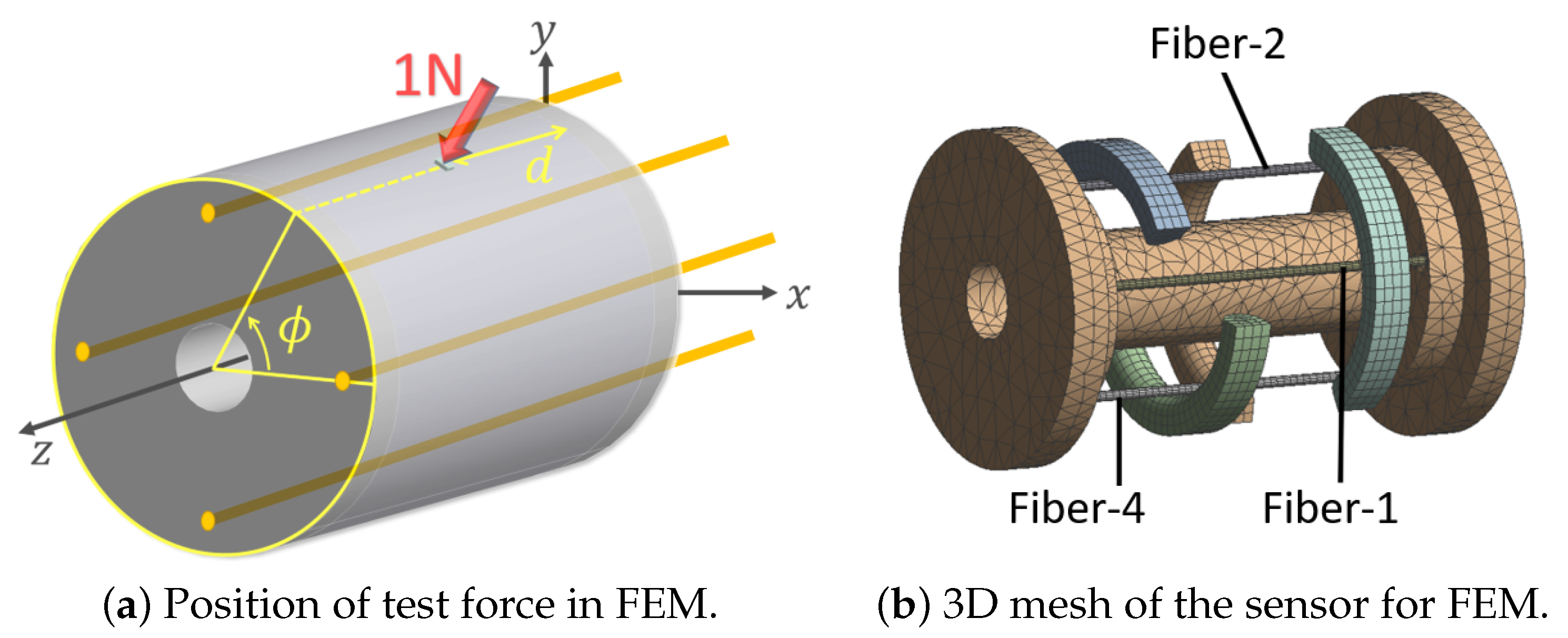





| Dimensions | Value | Mechanical Properties | Value | |
|---|---|---|---|---|
| Outer diameter | 6 mm | Hyperelastic | ||
| Flexible shell | Length | 10 mm | Two-term Mooney–Rivlin [31] | |
| Thickness | 0.5 mm | |||
| Rigid Substrate | Tip diameter | 6 mm | Elastic modulus | 0.5 GPa |
| Shaft diameter | 2 mm | Poisson’s ratio | 0.43 | |
| Optical fiber | Outer diameter | 0.250 mm | Elastic modulus | 16.5 GPa |
| Free length | 8 mm | Poisson’s ratio | 0.2 |
| Parameter | Values |
|---|---|
| d | mm |
| from to 180° with 20° step-size |
| Fiber No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Maximum deflection (mm) | 0.0788 | 0.0788 | 0.136 | 0.136 |
| −60° | −40° | −20° | 0° | 20° | 40° | 60° | |
|---|---|---|---|---|---|---|---|
| d = 2 mm | 3 ± 3 | 12 ± 4 | 71 ± 5 | 100 (Maximum) | 74 ± 4 | 10 ± 5 | 0 ± 5 |
| d = 4 mm | 2 ± 4 | 11 ± 5 | 51 ± 3 | 72 ± 6 | 48 ± 6 | 7 ± 5 | 0 ± 4 |
| d = 6 mm | 2 ± 6 | 6 ± 4 | 43 ± 5 | 61 ± 7 | 45 ± 4 | 6 ± 5 | 0 ± 3 |
| d = 8 mm | 1 ± 2 | 4 ± 3 | 23 ± 3 | 40 ± 4 | 24 ± 4 | 4 ± 4 | 0 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandari, N.; Dargahi, J.; Packirisamy, M. Optical Fiber Array Sensor for Force Estimation and Localization in TAVI Procedure: Design, Modeling, Analysis and Validation. Sensors 2021, 21, 5377. https://doi.org/10.3390/s21165377
Bandari N, Dargahi J, Packirisamy M. Optical Fiber Array Sensor for Force Estimation and Localization in TAVI Procedure: Design, Modeling, Analysis and Validation. Sensors. 2021; 21(16):5377. https://doi.org/10.3390/s21165377
Chicago/Turabian StyleBandari, Naghmeh, Javad Dargahi, and Muthukumaran Packirisamy. 2021. "Optical Fiber Array Sensor for Force Estimation and Localization in TAVI Procedure: Design, Modeling, Analysis and Validation" Sensors 21, no. 16: 5377. https://doi.org/10.3390/s21165377
APA StyleBandari, N., Dargahi, J., & Packirisamy, M. (2021). Optical Fiber Array Sensor for Force Estimation and Localization in TAVI Procedure: Design, Modeling, Analysis and Validation. Sensors, 21(16), 5377. https://doi.org/10.3390/s21165377







