An Indole-Based Fluorescent Chemosensor for Detecting Zn2+ in Aqueous Media and Zebrafish
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Equipment
2.2. Synthesis of IH-Sal ((E)-N′-(2-hydroxybenzylidene)-2-(1H-indol-3-yl)acetohydrazide)
2.3. Preparation of Spectroscopic Experiments
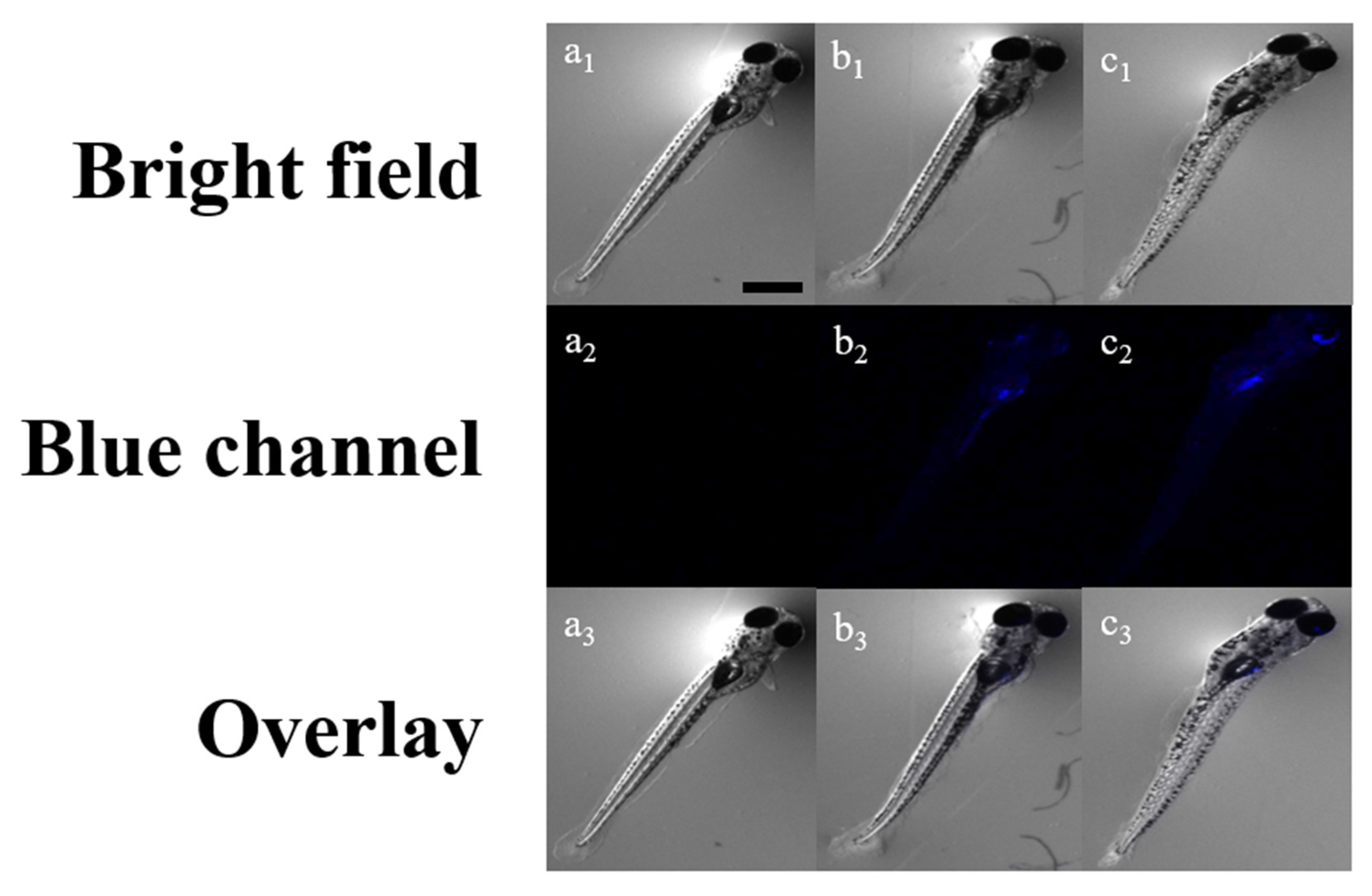
2.4. Imaging in Zebrafish
2.5. Calculations
3. Results and Discussion
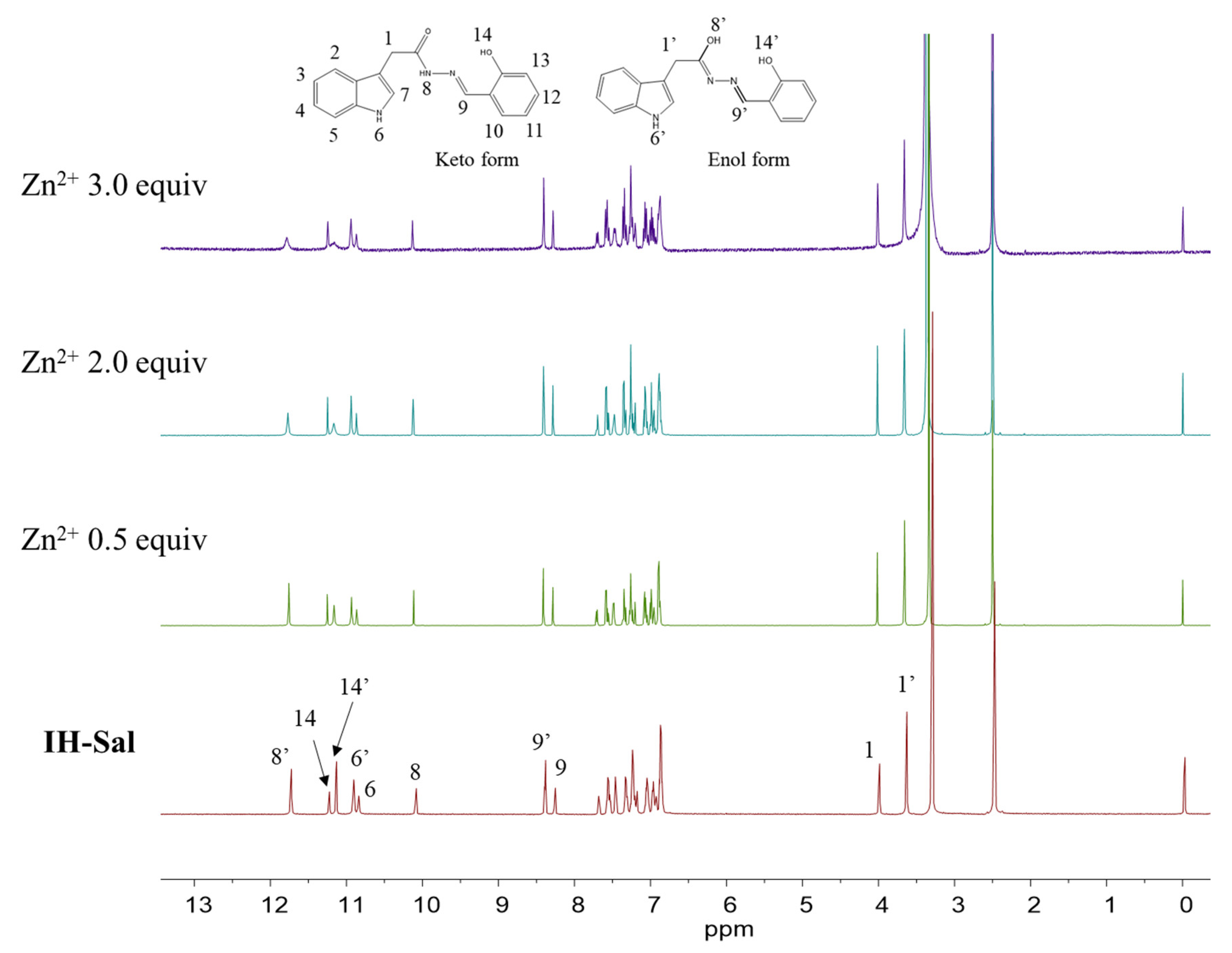
3.1. Structural Characterization of IH-Sal
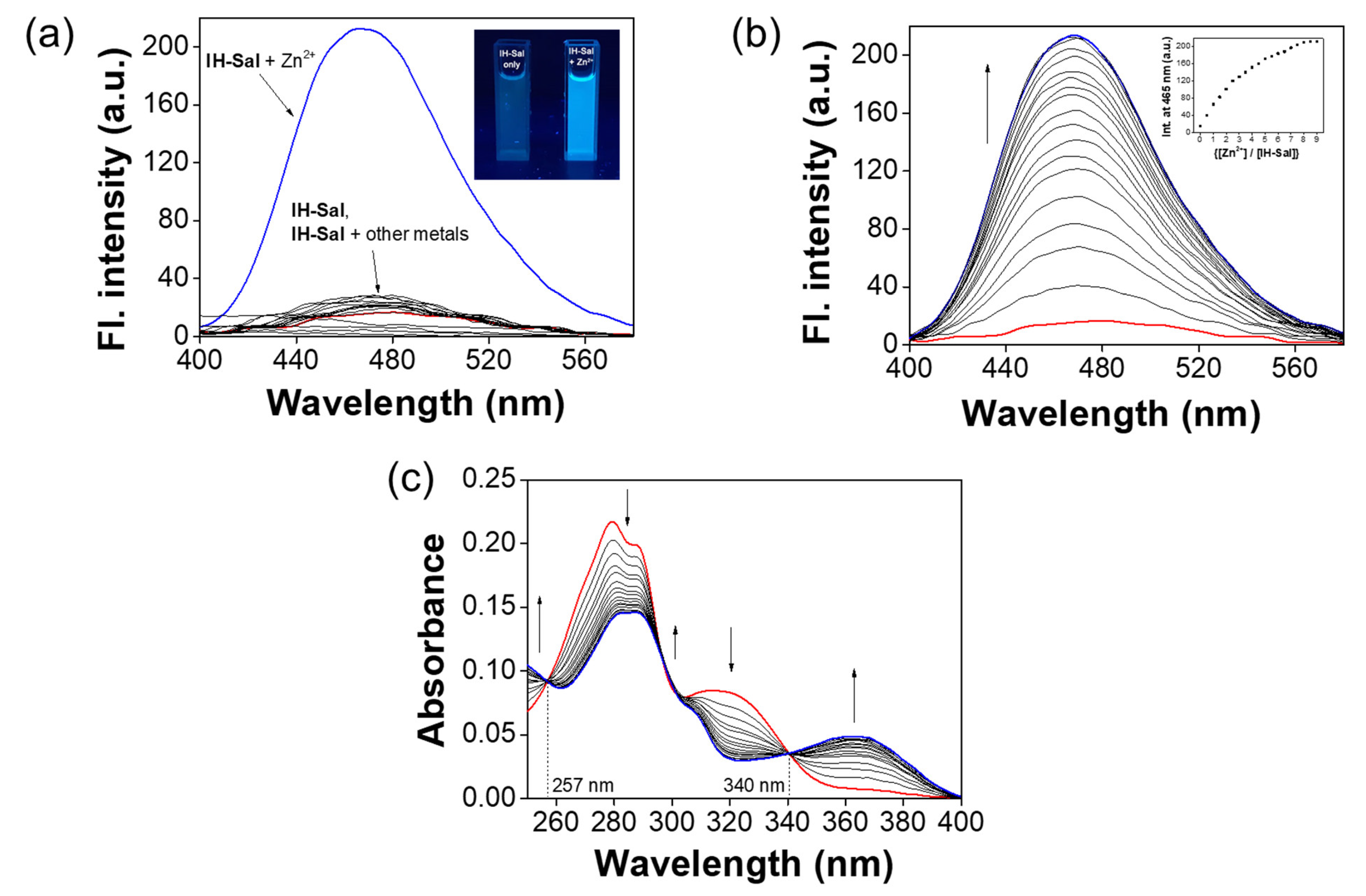
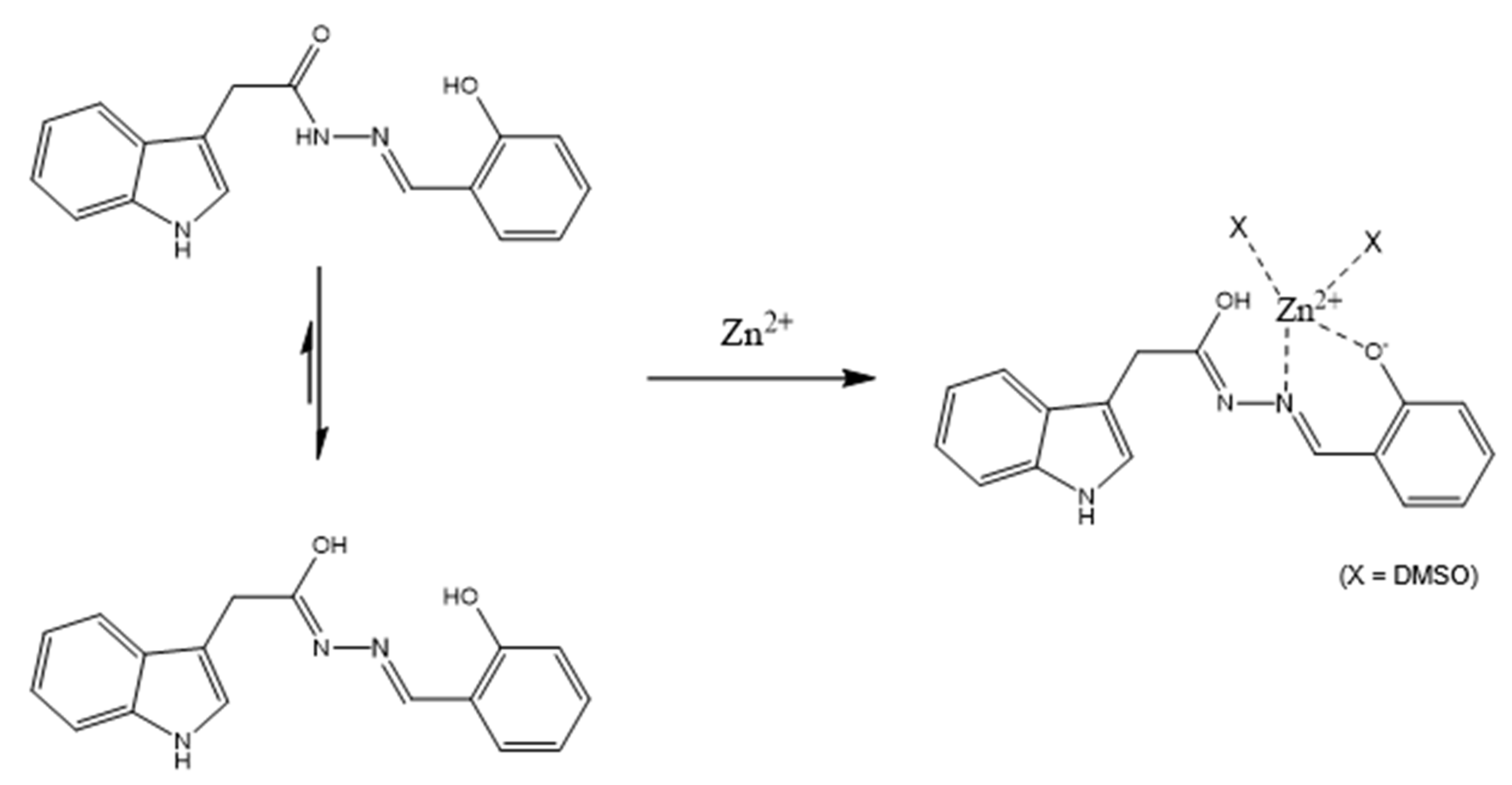
3.2. Spectroscopic Examination of IH-Sal to Zn2+
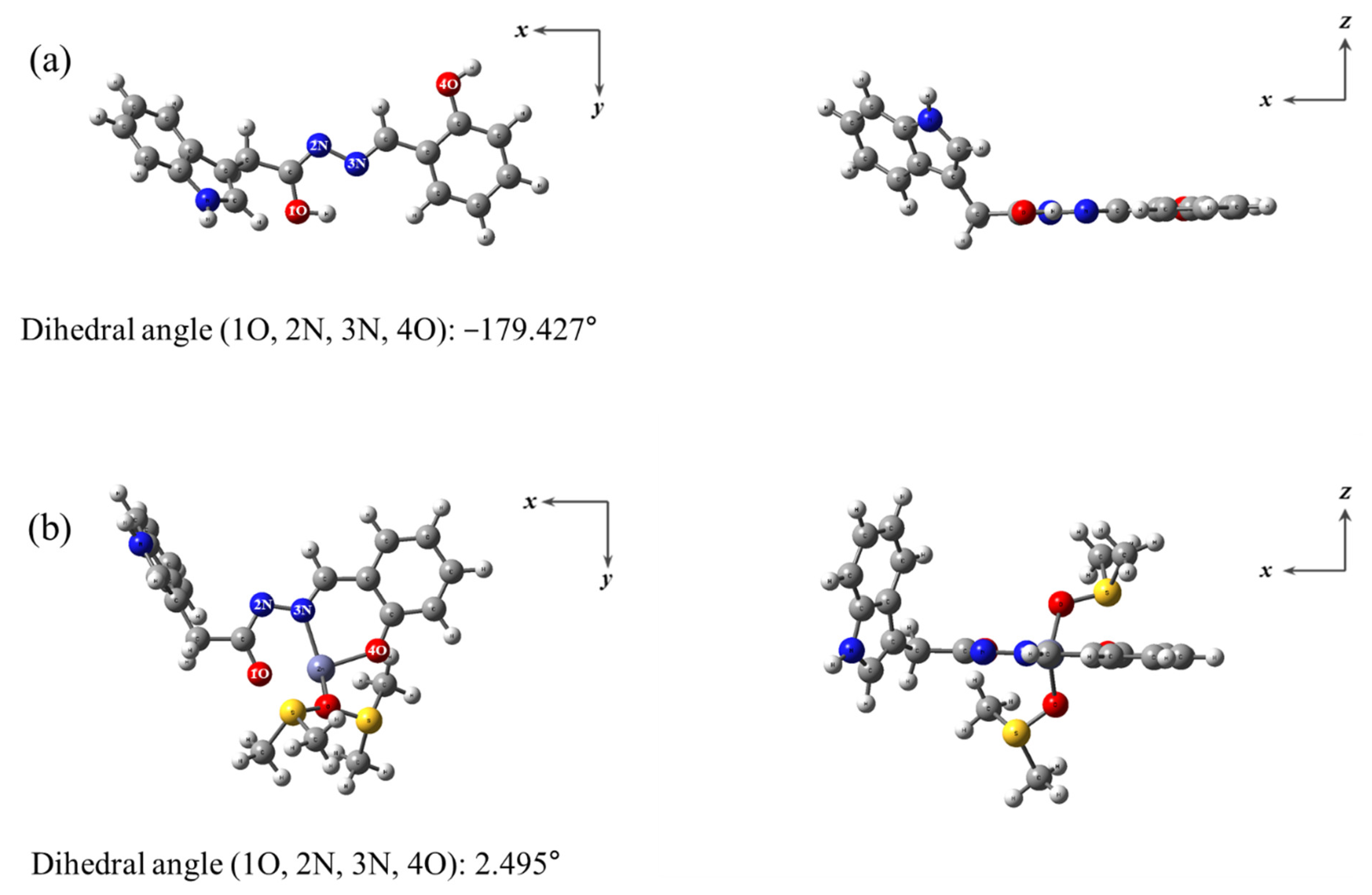
3.3. Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Lee, J.J.; Lee, S.Y.; Kim, P.G.; Kim, C. A Turn-on Fluorescent Chemosensor for Zn2+ Based on Quinoline in Aqueous Media. J. Fluoresc. 2016, 26, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, J.J.; Lee, S.Y.; Jo, T.G.; Kim, C. A highly sensitive benzimidazole-based chemosensor for the colorimetric detection of Fe(II) and Fe(III) and the fluorometric detection of Zn(II) in aqueous media. RSC Adv. 2016, 6, 61505–61515. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Han, Z.; He, W.; Guo, Z. Photoluminescence imaging of Zn2+ in living systems. Chem. Soc. Rev. 2015, 44, 4517–4546. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, P.; Maity, S.; Das, D.; Samanta, S.; Shyamal, M.; Misra, A. Proton induced green emission from AIEE active 2,2′ biquinoline hydrosol and its selective fluorescence turn-on sensing property towards Zn2+ ion in water. Sens. Actuators B Chem. 2017, 238, 1266–1276. [Google Scholar] [CrossRef]
- Goswami, S.; Manna, A.; Paul, S.; Maity, A.K.; Saha, P.; Quah, C.K.; Fun, H.K. FRET based ‘red-switch’ for Al3+ over ESIPT based ‘green-switch’ for Zn2+: Dual channel detection with live-cell imaging on a dyad platform. RSC Adv. 2014, 4, 34572–34576. [Google Scholar] [CrossRef]
- Pandith, A.; Uddin, N.; Choi, C.H.; Kim, H.S. Highly selective imidazole-appended 9,10-N,N″-diaminomethylanthracene fluorescent probe for switch-on Zn2+ detection and switch-off H2PO4− and CN− detection in 80% aqueous DMSO, and applications to sequential logic gate operations. Sens. Actuators B Chem. 2017, 247, 840–849. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. A differentially selective sensor with fluorescence turn-on response to Zn2+ and dual-mode ratiometric response to Al3+ in aqueous media. Chem. Commun. 2012, 48, 1039–1041. [Google Scholar] [CrossRef]
- Xu, Z.; Yoon, J.; Spring, D.R. Fluorescent chemosensors for Zn2+. Chem. Soc. Rev. 2010, 39, 1996–2006. [Google Scholar] [CrossRef] [Green Version]
- Nunes, M.C.; dos Santos Carlos, F.; Fuganti, O.; Galindo, D.D.M.; De Boni, L.; Abate, G.; Nunes, F.S. Turn-on fluorescence study of a highly selective acridine-based chemosensor for Zn2+ in aqueous solutions. Inorg. Chim. Acta 2020, 499, 119191. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Maity, D.; Govindaraju, T. Reversible fluorescence sensing of Zn2+ based on pyridine-constrained bis(triazole-linked hydroxyquinoline) sensor. Supramol. Chem. 2011, 23, 703–709. [Google Scholar] [CrossRef]
- Jang, H.J.; Chae, J.B.; Jung, J.M.; So, H.; Kim, C. Colorimetric Detection of Co2+, Cu2+, and Zn2+ by a Multifunctional Chemosensor in Aqueous Solution. Bull. Korean Chem. Soc. 2019, 40, 650–657. [Google Scholar] [CrossRef]
- Sreenivasa Rao, K.; Balaji, T.; Prasada Rao, T.; Babu, Y.; Naidu, G.R.K. Determination of iron, cobalt, nickel, manganese, zinc, copper, cadmium and lead in human hair by inductively coupled plasma-atomic emission spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 1333–1338. [Google Scholar] [CrossRef]
- Antunes, G.A.; Dos Santos, H.S.; Da Silva, Y.P.; Silva, M.M.; Piatnicki, C.M.S.; Samios, D. Determination of Iron, Copper, Zinc, Aluminum, and Chromium in Biodiesel by Flame Atomic Absorption Spectrometry Using a Microemulsion Preparation Method. Energy Fuels 2017, 31, 2944–2950. [Google Scholar] [CrossRef]
- Li, W.T.; Wu, G.Y.; Qu, W.J.; Li, Q.; Lou, J.C.; Lin, Q.; Yao, H.; Zhang, Y.M.; Wei, T.B. A colorimetric and reversible fluorescent chemosensor for Ag+ in aqueous solution and its application in IMPLICATION logic gate. Sens. Actuators B Chem. 2017, 239, 671–678. [Google Scholar] [CrossRef]
- Yuan, C.; Li, S.; Wu, Y.; Lu, L.; Zhu, M. Zn(II)-selective and sensitive fluorescent chemosensor based on steric constrains and inhibition of ESIPT. Sens. Actuators B Chem. 2017, 242, 1035–1042. [Google Scholar] [CrossRef]
- Patra, C.; Bhanja, A.K.; Sen, C.; Ojha, D.; Chattopadhyay, D.; Mahapatra, A.; Sinha, C. Vanillinyl thioether Schiff base as a turn-on fluorescence sensor to Zn2+ ion with living cell imaging. Sens. Actuators B Chem. 2016, 228, 287–294. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Fang, H.; Zhu, W.; He, J.X.; Yao, H.; Wei, T.B.; Lin, Q.; Qu, W.J. Ratiometric fluorescent sensor based oxazolo-phenazine derivatives for detect hypochlorite via oxidation reaction and its application in environmental samples. Dyes Pigment. 2020, 172, 107765. [Google Scholar] [CrossRef]
- Lee, D.Y.; Singh, N.; Jang, D.O. A benzimidazole-based single molecular multianalyte fluorescent probe for the simultaneous analysis of Cu2+ and Fe3+. Tetrahedron Lett. 2010, 51, 1103–1106. [Google Scholar] [CrossRef]
- Jung, H.J.; Singh, N.; Lee, D.Y.; Jang, D.O. Single sensor for multiple analytes: Chromogenic detection of I− and fluorescent detection of Fe3+. Tetrahedron Lett. 2010, 51, 3962–3965. [Google Scholar] [CrossRef]
- Goswami, S.; Das, S.; Aich, K.; Sarkar, D.; Mondal, T.K.; Quah, C.K.; Fun, H.K. CHEF induced highly selective and sensitive turn-on fluorogenic and colorimetric sensor for Fe3+. Dalt. Trans. 2013, 42, 15113–15119. [Google Scholar] [CrossRef]
- Goswami, S.; Aich, K.; Das, A.K.; Manna, A.; Das, S. A naphthalimide-quinoline based probe for selective, fluorescence ratiometric sensing of trivalent ions. RSC Adv. 2013, 3, 2412–2416. [Google Scholar] [CrossRef]
- Zhao, G.; Guo, B.; Wei, G.; Guang, S.; Gu, Z.; Xu, H. A novel dual-channel Schiff base fluorescent chemo-sensor for Zn2+ and Ca2+ recognition: Synthesis, mechanism and application. Dyes Pigment. 2019, 170, 107614. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Jung, S.H.; Hyun, T.K.; Feng, M.; Kim, J.Y.; Lee, J.H.; Lee, H.; Kim, J.S.; Kang, C.; et al. Highly selective fluorescence imaging of zinc distribution in HeLa cells and Arabidopsis using a naphthalene-based fluorescent probe. Chem. Commun. 2015, 51, 7463–7465. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Dai, X.; Zhong, K.; Wu, D.; Wen, X. A novel 2,5-diphenyl-1,3,4-oxadiazole derived fluorescent sensor for highly selective and ratiometric recognition of Zn2+ in water through switching on ESIPT. Sens. Actuators B Chem. 2014, 203, 557–564. [Google Scholar] [CrossRef]
- Maity, D.; Raj, A.; Karthigeyan, D.; Kundu, T.K.; Govindaraju, T. Reaction-based probes for Co(II) and Cu(I) with dual output modes: Fluorescence live cell imaging. RSC Adv. 2013, 3, 16788–16794. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. Naphthaldehyde-urea/thiourea conjugates as turn-on fluorescent probes for Al3+ based on restricted C=N isomerization. Eur. J. Inorg. Chem. 2011, 2011, 5479–5485. [Google Scholar] [CrossRef]
- Hosseini, M.; Vaezi, Z.; Ganjali, M.R.; Faridbod, F.; Abkenar, S.D.; Alizadeh, K.; Salavati-Niasari, M. Fluorescence “turn-on” chemosensor for the selective detection of zinc ion based on Schiff-base derivative. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 978–982. [Google Scholar] [CrossRef]
- Sil, A.; Maity, A.; Giri, D.; Patra, S.K. A phenylene-vinylene terpyridine conjugate fluorescent probe for distinguishing Cd2+ from Zn2+ with high sensitivity and selectivity. Sens. Actuators B Chem. 2016, 226, 403–411. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; He, J. A “turn-on” fluorescent chemosensor for the detection of Zn(II) in aqueous solution at neutral pH and its application in live cells imaging. Talanta 2016, 153, 381–385. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, S.; Singh, K.; Sharma, P.R.; Kaur, T. Indole-based chemosensor for Hg2+ and Cu2+ Ions: Applications in molecular switches and live cell imaging. Dalt. Trans. 2011, 40, 10818–10821. [Google Scholar] [CrossRef] [PubMed]
- Mashraqui, S.H.; Ghorpade, S.S.; Tripathi, S.; Britto, S. A new indole incorporated chemosensor exhibiting selective colorimetric and fluorescence ratiometric signaling of fluoride. Tetrahedron Lett. 2012, 53, 765–768. [Google Scholar] [CrossRef]
- Rathikrishnan, K.R.; Indirapriyadharshini, V.K.; Ramakrishna, S.; Murugan, R. 4,7-Diaryl indole-based fluorescent chemosensor for iodide ions. Tetrahedron 2011, 67, 4025–4030. [Google Scholar] [CrossRef]
- Tümay, S.O.; Okutan, E.; Sengul, I.F.; Özcan, E.; Kandemir, H.; Doruk, T.; Çetin, M.; Çoşut, B. Naked-eye fluorescent sensor for Cu(II) based on indole conjugate BODIPY dye. Polyhedron 2016, 117, 161–171. [Google Scholar] [CrossRef]
- Son, Y.A.; Gwon, S.Y.; Kim, S.H. Characteristics of Guajazulene Based Chemosensor Toward CN− and F− Anions. Mol. Cryst. Liq. Cryst. 2014, 600, 189–195. [Google Scholar] [CrossRef]
- Wu, H.H.; Sun, Y.L.; Wan, C.F.; Yang, S.T.; Chen, S.J.; Hu, C.H.; Wu, A.T. Highly selective and sensitive fluorescent chemosensor for Hg2+ in aqueous solution. Tetrahedron Lett. 2012, 53, 1169–1172. [Google Scholar] [CrossRef]
- Choi, Y.W.; Lee, J.J.; Nam, E.; Lim, M.H.; Kim, C. A fluorescent chemosensor for Al3+ based on julolidine and tryptophan moieties. Tetrahedron 2016, 72, 1998–2005. [Google Scholar] [CrossRef]
- Liu, J.R.; Miao, H.; Deng, D.Q.; Vaziri, N.D.; Li, P.; Zhao, Y.Y. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell. Mol. Life Sci. 2021, 78, 909–922. [Google Scholar] [CrossRef]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Papadimitriou, N.; Gunter, M.J.; Murphy, N.; Gicquiau, A.; Achaintre, D.; Brezina, S.; Gumpenberger, T.; Baierl, A.; Ose, J.; Geijsen, A.J.M.R.; et al. Circulating tryptophan metabolites and risk of colon cancer: Results from case-control and prospective cohort studies. Int. J. Cancer 2021, 1–11. [Google Scholar] [CrossRef]
- Shi, D.T.; Zhang, B.; Yang, Y.X.; Guan, C.C.; He, X.P.; Li, Y.C.; Chen, G.R.; Chen, K. Bis-triazolyl indoleamines as unique “off-approach-on” chemosensors for copper and fluorine. Analyst 2013, 138, 2808–2811. [Google Scholar] [CrossRef]
- Juanjuan, S.; Linlin, W.; Yangfeng, H. Colorimetric, turn-on fluorescence detection of fluoride ions using simple indole-based receptors in living cells. Anal. Methods 2019, 11, 2585–2590. [Google Scholar] [CrossRef]
- Wang, Q.; Li, D.; Rao, N.; Zhang, Y.; Le, Y.; Liu, L.; Huang, L.; Yan, L. Development of indole-based fluorescent probe for detection of fluoride and cell imaging of HepG2. Dyes Pigment. 2021, 188, 109166. [Google Scholar] [CrossRef]
- Rattanopas, S.; Piyanuch, P.; Wisansin, K.; Charoenpanich, A.; Sirirak, J.; Phutdhawong, W.; Wanichacheva, N. Indole-based fluorescent sensors for selective sensing of Fe2+ and Fe3+ in aqueous buffer systems and their applications in living cells. J. Photochem. Photobiol. A Chem. 2019, 377, 138–148. [Google Scholar] [CrossRef]
- Chang, Y.; Li, B.; Mei, H.; Yang, L.; Xu, K.; Pang, X. Indole-based colori/fluorimetric probe for selective detection of Cu2+ and application in living cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117631. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Watanabe, Y.; Yamamoto, Y. Development of ratiometric fluorescent probe for zinc ion based on indole fluorophore. Tetrahedron Lett. 2009, 50, 1345–1347. [Google Scholar] [CrossRef]
- Xu, T.; Duan, H.; Wang, X.; Meng, X.; Bu, J. Fluorescence sensors for Zn2+ based on conjugated indole Schiff base. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Tripathi, A.; Rana, M.; Kishore Goswami, S.; Pathak, A.; Chowdhury, P. “Turn on/off” proton transfer based fluorescent sensor for selective detection of environmentally hazardous metal ions (Zn2+, Pb2+) in aqueous media. J. Lumin. 2015, 165, 46–55. [Google Scholar] [CrossRef]
- Li, L.; Dang, Y.Q.; Li, H.W.; Wang, B.; Wu, Y. Fluorescent chemosensor based on Schiff base for selective detection of zinc(II) in aqueous solution. Tetrahedron Lett. 2010, 51, 618–621. [Google Scholar] [CrossRef]
- Dutta, K.; Deka, R.C.; Das, D.K. A new fluorescent and electrochemical Zn2+ ion sensor based on Schiff base derived from benzil and L-tryptophan. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 124–129. [Google Scholar] [CrossRef]
- Maurya, M.R.; Kumar, N. Chloromethylated polystyrene cross-linked with divinylbenzene and grafted with vanadium(IV) and vanadium(V) complexes having ONO donor ligand for the catalytic activity. J. Mol. Catal. A Chem. 2014, 383–384, 172–181. [Google Scholar] [CrossRef]
- Kang, J.H.; Han, J.; Lee, H.; Lim, M.H.; Kim, K.T.; Kim, C. A water-soluble fluorescence chemosensor for the sequential detection of Zn2+ and pyrophosphate in living cells and zebrafish. Dyes Pigment. 2018, 152, 131–138. [Google Scholar] [CrossRef]
- Hwang, S.M.; Yun, D.; Lee, H.; Kim, M.; Lim, M.H.; Kim, K.T.; Kim, C. Relay detection of Zn2+ and S2− by a quinoline-based fluorescent chemosensor in aqueous media and zebrafish. Dyes Pigment. 2019, 165, 264–272. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Klamt, A.; Moya, C.; Palomar, J. A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J. Chem. Theory Comput. 2015, 11, 4220–4225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Xue, W. Highly Selective Recognition of Zinc Ion by Salicylaldehyde-2-phenylacetylhydrazone. Available online: https://core.ac.uk/download/pdf/41434139.pdf (accessed on 1 July 2021).
- Dai, Z.; Ding, Z.; He, J. Cationic Fluorescent Probe Based on Tetraphenyl Ethylene Structure. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?CC=CN&NR=112724040A&KC=A&FT=D&ND=3&date=20210430&DB=EPODOC&locale=en_EP (accessed on 1 July 2021).
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric inverstigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Banerjee, D.; Koll, A.; Mandal, A.; Filarowski, A.; Fitzmaurice, D.; Das, R.; Mukherjee, S. Excited state intermolecular proton transfer and caging of salicylidine-3,4,7-methyl amine in cyclodextrins. J. Photochem. Photobiol. A Chem. 2005, 175, 94–99. [Google Scholar] [CrossRef]
- Chae, J.B.; Yun, D.; Kim, S.; Lee, H.; Kim, M.; Lim, M.H.; Kim, K.T.; Kim, C. Fluorescent determination of zinc by a quinoline-based chemosensor in aqueous media and zebrafish. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.G.; Lee, J.J.; Nam, E.; Bok, K.H.; Lim, M.H.; Kim, C. A highly selective fluorescent sensor for the detection of Al3+ and CN− in aqueous solution: Biological applications and DFT calculations. New J. Chem. 2016, 40, 8918–8927. [Google Scholar] [CrossRef]
- Choi, Y.W.; You, G.R.; Lee, J.J.; Kim, C. Turn-on fluorescent chemosensor for selective detection of Zn2+ in an aqueous solution: Experimental and theoretical studies. Inorg. Chem. Commun. 2016, 63, 35–38. [Google Scholar] [CrossRef]

| Sample | Zn2+ Added (μM) | Zn2+ Found (μM) | Recovery (%) | R.S.D. (n = 3) (%) |
|---|---|---|---|---|
| Drinking water | 0.0 | 0.0 | - | - |
| 10.0 | 10.0 | 100.01 | 1.58 | |
| Tap water | 0.0 | 0.0 | - | - |
| 10.0 | 10.1 | 101.00 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, D.; So, H.; Park, S.; Lee, H.; Chae, J.B.; Kim, J.; Kim, K.-T.; Kim, C. An Indole-Based Fluorescent Chemosensor for Detecting Zn2+ in Aqueous Media and Zebrafish. Sensors 2021, 21, 5591. https://doi.org/10.3390/s21165591
Choe D, So H, Park S, Lee H, Chae JB, Kim J, Kim K-T, Kim C. An Indole-Based Fluorescent Chemosensor for Detecting Zn2+ in Aqueous Media and Zebrafish. Sensors. 2021; 21(16):5591. https://doi.org/10.3390/s21165591
Chicago/Turabian StyleChoe, Donghwan, Haeri So, Soyoung Park, Hangyul Lee, Ju Byeong Chae, Jiwon Kim, Ki-Tae Kim, and Cheal Kim. 2021. "An Indole-Based Fluorescent Chemosensor for Detecting Zn2+ in Aqueous Media and Zebrafish" Sensors 21, no. 16: 5591. https://doi.org/10.3390/s21165591






