Ground-Based Hyperspectral Remote Sensing for Estimating Water Stress in Tomato Growth in Sandy Loam and Silty Loam Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Water Stress Treatment
2.3. Soil Water Content Measurement
2.4. Plant Nutrition and Pest and Diseases Management
2.5. Field Measurements
2.6. Hyperspectral Data Acquisition and Processing
Spectral Vegetative Indices
2.7. Statistical Analysis
3. Results
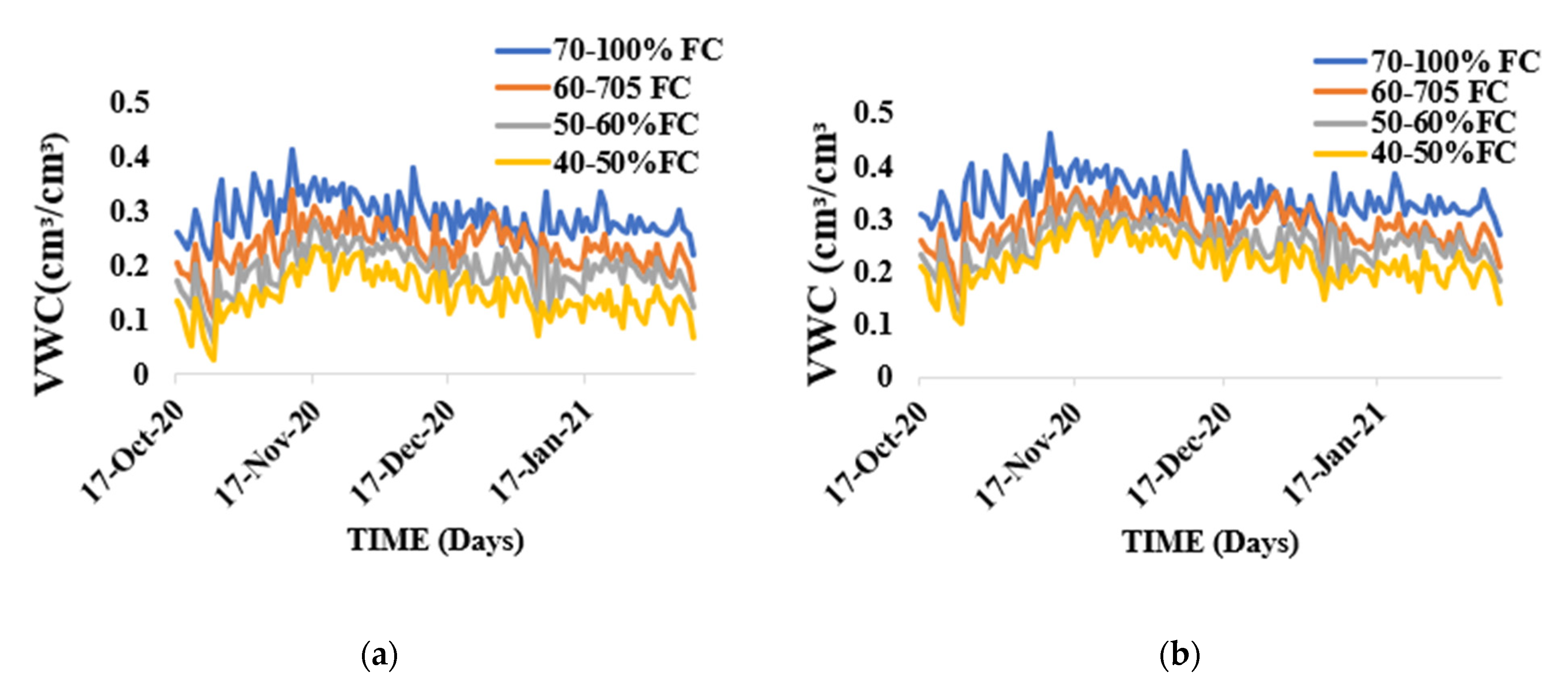
3.1. Soil Water Content Measurement
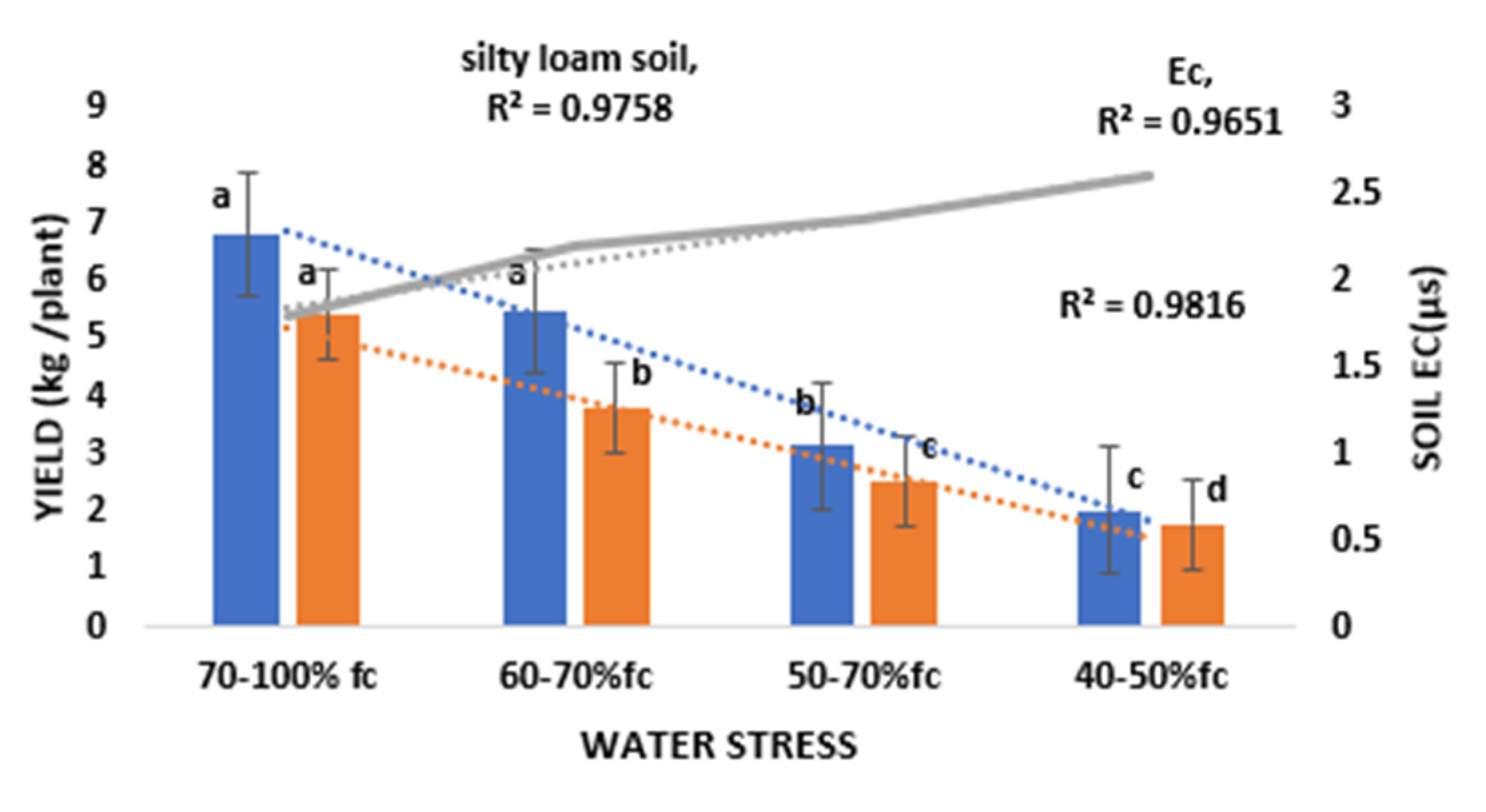
3.2. Effect of Water Stress on Electrical Conductivity (Ec) and Marketable Yield
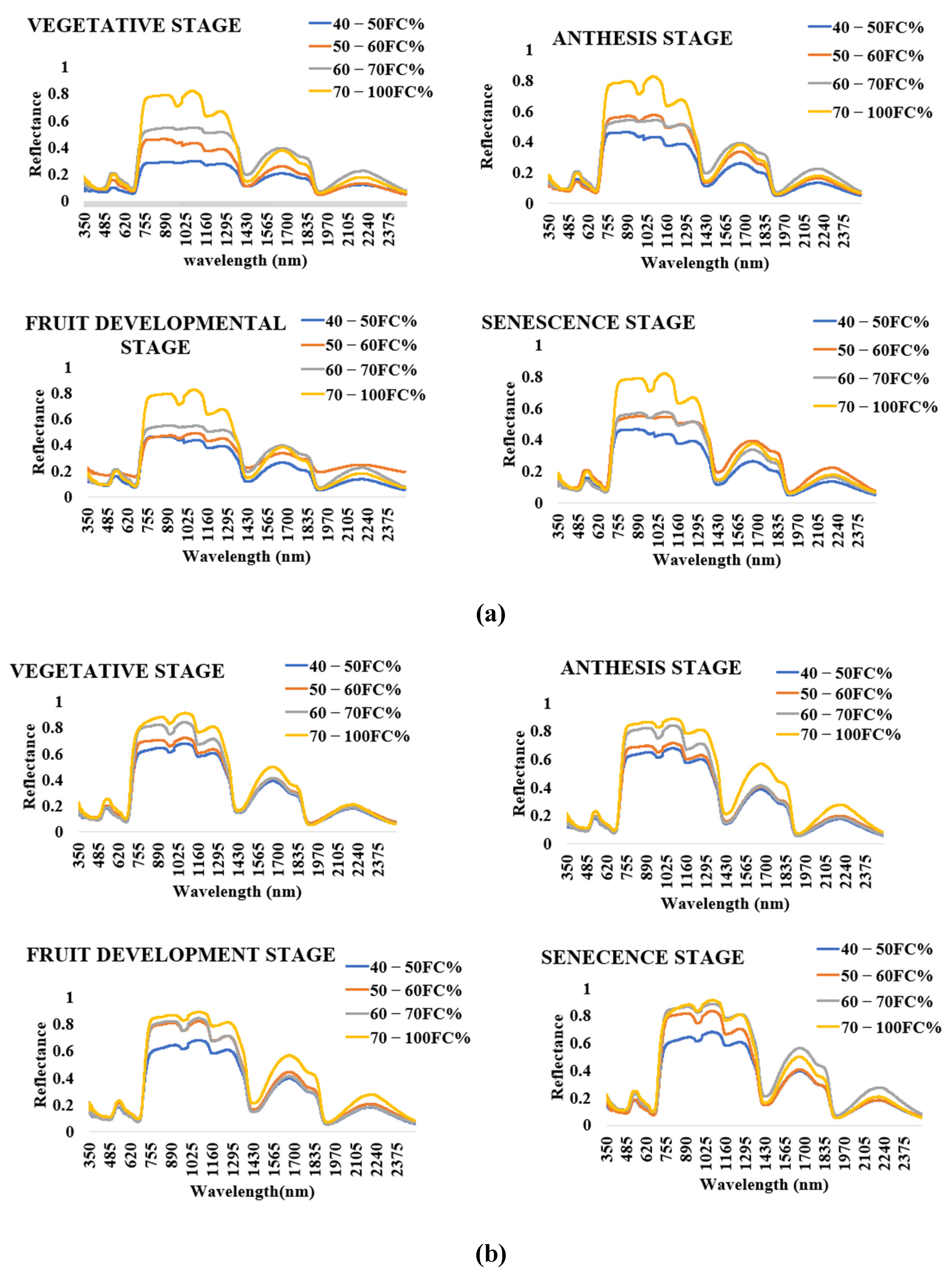
3.3. Crop Spectral Signatures
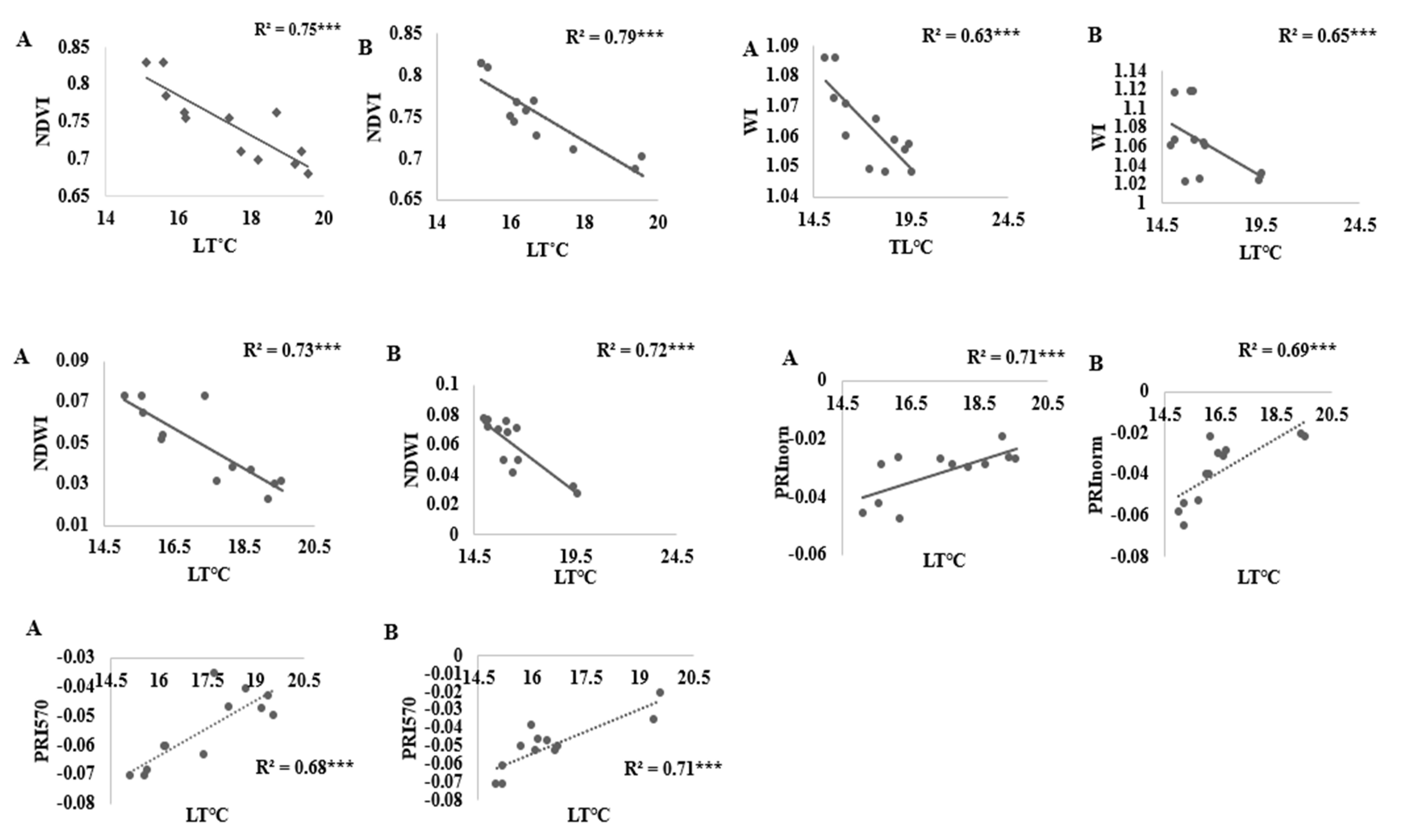
3.4. Vegetation Indices versus Water Stress Indicators and Yield
4. Discussion
4.1. Soil Water Content Measurement
4.2. Effect of Water Stress on Ec and Yield
4.3. Vegetation Indices versus Water Stress Indicators and Yield
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulla, D.J. Twenty-five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Espinoza, C.Z.; Khot, L.R.; Sankaran, S.; Jacoby, P.W. High resolution multispectral and thermal remote sensing-based water stress assessment in subsurface irrigated grapevines. Remote Sens. 2017, 9, 961. [Google Scholar] [CrossRef] [Green Version]
- Zinkernagel, J.; Maestre-Valero, J.F.; Seresti, S.Y.; Intrigliolo, D.S. New technologies and practical approaches to improve irrigation management of open field vegetable crops. Agric. Water Manag. 2020, 242, 106404. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Sensitivity of spectral vegetation indices for monitoring water stress in tomato plants. Comput. Electron. Agric. 2019, 163, 104860. [Google Scholar] [CrossRef]
- Ustin, S..; Darling, D.; Kefauver, S.; Greenberg, J.; Cheng, Y.; Whiting, M. Remotely sensed estimates of crop water demand. Int. Soc. Opt. Eng. 2004, 5544, 230–240. [Google Scholar] [CrossRef]
- Gao, B. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Osroosh, Y.; Peters, R.T.; Campbell, C.S.; Zhang, Q. Automatic irrigation scheduling of apple tress using therietical crop water stress index with and innovative dynamic threshold. Comp. Electron. Agric. 2015, 118, 193–203. [Google Scholar] [CrossRef]
- Katsoulas, N.; Elvanidi, A.; Ferentinos, K.P.; Bartzanas, T.; Kittas, C. Crop reflectance monitoring as a tool for water stress detection in greenhouses: A review. Biosyst. Eng. 2016, 151, 374–398. [Google Scholar] [CrossRef]
- Zhang, C.; Filella, I.; Liu, D.; Ogaya, R.; Llusià, J.; Asensio, D.; Peñuelas, J. Photochemical Reflectance Index (PRI) for Detecting Responses of Diurnal and Seasonal Photosynthetic Activity to Experimental Drought and Warming in a Mediterranean Shrubland. Remote Sens. 2017, 9, 1189. [Google Scholar] [CrossRef] [Green Version]
- Gamon, J.; Peñuelas, J.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Hamouda, Y.A.; Shaghaleh, H.; Sheteiwy, M.; Guoa, X.; Elshaikha, N.; Khand, N.; Oumaroue, A.; Rahima, S. Impact of alternative wetting and soil drying and soil clay content on the morphological and physiological traits of rice roots and their relationships to yield and nutrient use-efficiency. Agric. Water Manag. 2019, 233, 3–9. [Google Scholar]
- Streher, A.S.; Torres, R.D.S.; Cerdeira-Morellato, L.P.; Freire-Silva, T.S. Accuracy and limitations for spectroscopic prediction of leaf traits in seasonally dry tropical environments. Remote Sens. Environ. 2020, 244, 111828. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Huang, G.; Xu, X.; Huang, Q. Effects of water stress on processing tomatoes yield, quality and water use efficiency with plastic mulched drip irrigation in sandy soil of the Hetao irrigation district. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Neményi, A.; Bocs, A.; Pék, Z.; Helyes, L. Physiological factors and their relationship with the productivity of processing tomato under different water supplies. Water 2019, 11, 586. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhou, G. Estimation of vegetation water content Indices:using hyperspectral vegetation Indicators, a comparison of crop water Treatments, in response to water stress Maize, for summer. BMC Ecol. 2019, 19, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.J.; Zhang, Y.H.; Han, Z.M.; Liu, X.Y.; Jian, Y.F.; Hu, C.G.; Dian, Y.Y. Hyperspectral sensing of photosynthesis, stomatal conductance, and transpiration for citrus tree under drought condition. BioRxiv 2021. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Ustin, S.L. Modeling canopy water content for carbon estimates from MODIS data at land EOS validation sites. Int. Geosci. Remote Sens. 2001, 1, 342–344. [Google Scholar]
- Wang, X.; Zhao, C.; Guo, N.; Li, Y.; Jian, S.; Yu, K. Determining the canopy water stress for spring wheat using Canopy Hyperspectral Reflectance Data in Loess Plateau Semiarid Regions. Lett. Spectrosc. 2015, 48, 492–498. [Google Scholar] [CrossRef]
- Rosales, M.A.; Ruiz, J.M.; Hernandez, J.; Soriano, T.; Castilla, N.; Romero, L. Antioxidant content and ascorbate metabolism in cherry tomato exocarp in relation to temperature and solar radiation. J. Sci. Food Agric. 2006, 86, 1545–1551. [Google Scholar] [CrossRef]
- Hazaymeh, K.; Hassan, Q.K. Remote sensing of agricultural drought monitoring: A state of art review. AIMS Environ. Sci. 2016, 3, 604–630. [Google Scholar] [CrossRef]
- Honkavaara, E.; Saari, H.; Kaivosoja, J.; Pölönen, I.; Hakala, T.; Litkey, P.; Mäkynen, J.; Pesonen, L. Processing and assessment of spectrometric, stereoscopic imagery collected using a lightweight UAV spectral camera for precision agriculture. Remote Sens. 2013, 5, 5006–5039. [Google Scholar] [CrossRef] [Green Version]
- Blanke, M.; Cooke, D. Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regul. 2004, 42, 153–160. [Google Scholar] [CrossRef]
- Singh, M.C.; Singh, K.G.; Singh, J. Indirect method for measurement of leaf area and leaf area index of soilless cucumber crop. Adv. Plants Agric. Res. 2019, 8, 188–191. [Google Scholar] [CrossRef] [Green Version]
- Danner, M.; Locherer, M.; Hank, T.; Richter, K. Spectral Sampling with the ASD FieldSpec 4—Theory, Measurement, Problems, Interpretation; EnMAP Field Guides Technical Report; EnMAP: Potsdam, Germany, 2015. [Google Scholar]
- Sánchez-Ruiz, S.; Piles, M.; Sánchez, N.; Martínez-Fernández, J.; Vall-llossera, M.; Camps, A. Combining SMOS with visible and near/shortwave/thermal infrared satellite data for high resolution soil moisture estimates. J. Hidrol. 2014, 516, 273–283. [Google Scholar] [CrossRef]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and Future Perspectives of Multi- Hyperspectral Thermal Infrared Remote Sensing for Crop Water-Stress Detection: A Review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.; Baker, J.; Arkebauer, T.J. Leaf radiative properties and the leaf energy budget. In Micrometeorology in Agricultural Systems; Agronomy Monograph, American Society of Agronomy; Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2005. [Google Scholar]
- Roujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res Lett. 2005. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Gitelson, A.; Arkebauer, T. MODIS-based corn grain yield estimation model incorporating crop phenology information. Remote Sens. Env. 2013, 131, 215–231. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Chen, D.; Huang, J.; Jackson, T.J. Vegetation water content estimation for corn and soybeans using spectral indices derived from MODIS near- and short-wave infrared bands. Remote Sens. Environ. 2005, 98, 225–236. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Z.; Andersen, M.; Hu, T. Tomato yield and water use efficiency—coupling effects between growth stage specific soil water deficits. Soil Plant Sci. 2015, 65, 460–469. [Google Scholar] [CrossRef]
- Liu, J.; Hu, T.; Feng, P.; Wang, L.; Yang, S. Tomato yield and water use efficiency change with various soil moisture and potassium levels during different growth stages. PLoS ONE 2019, 14, e0213643. [Google Scholar] [CrossRef] [PubMed]
- Mzid, N.; Cantore, V.; De-Mastro, G.; Albrizio, R.; Sellami, M.; Todorovic, M. The Application of Ground Based and Satellite Remote Sensing for Estimation of Bio-Physiological Parameters of Wheat Grown Under Different Water Regimes. Water 2020, 12, 2095. [Google Scholar] [CrossRef]
- Rouse, J.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTSThird ERTS Symposium. NASA SP 1973, 351 I, 309–317. [Google Scholar]
- Zeng, R.; Xu, L.-H.; Gao, S.; Ni, X.; Chen, C.-L.; Chen, J.-C.; DAI, F. One-step reverse transcription loop-mediated isothermal amplification assay for rapid detection of melon yellow spot virus. Eur. J. Plant Pathol. 2016, 145, 119–124. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Niu, Y.; Han, W. Mapping Maize Water Stress Based on UAV Multispectral Remote Sensing. Remote Sens. 2019, 11, 605. [Google Scholar] [CrossRef] [Green Version]
- Fischlin, A.; Midgley, G.F.; Price, J.T.; Leemans, R.; Gopal, B.; Turley, C.; Rounsevell, M.D.A.; Dube, O.P.; Tarazona, J.; Velichko, A.A. Climate Change Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Ecosyst. Their Prop. Goods Serv. 2007, 3, 121–134. [Google Scholar]
- Magney, T.S.; Vierling, L.A.; Eitel, J.U.; Huggins, D.R.; Garrity, S.R. Response of high frequency Photochemical Reflectance Index (PRI) measurements to environmental conditions in wheat. Remote Sens. Environ. 2016, 173, 84–97. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies. A review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Wong, C.Y.S.; Gamon, J.A. Three causes of variation in the photochemical reflectance index (PRI) in evergreen conifers. New Phytol. 2015, 206, 187–195. [Google Scholar] [CrossRef]
- Zhang, H.; Han, M.; Chavez, J.L.; Lan, Y. Improvement in estimation of soil water deficit by integrating airborne imagery data into a soil water balance model. Int. J. Agric. Biol. Eng. 2017, 10, 37–46. [Google Scholar]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.S.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Peñuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef] [Green Version]
- Massonnet, E..; Serge, R.; Erwin, D.; Regnard, J. Stomatal regulation of photosynthesis in apple leaves: Evidence for different water-use strategies between two cultivars Catherine. Ann. Bot. 2007, 100, 1347–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrol, N.; Ramos, P.; Reigosa, M. Phenotypic plasticity and acclimation to water deficits in velvet-grass: A long-term greenhouse experiment. Changes in leaf morphology, photosynthesis and stress induced metabolites. J. Plant Physiol. 2000, 157, 383–393. [Google Scholar] [CrossRef]
- Afzal, A.; Duiker, S.W.; Watson, J.E.; Luthe, D. Leaf thickness and electrical capacitance as measures of plant water status. Trans. ASABE 2017, 60, 1063–1074. [Google Scholar] [CrossRef]
- Tahar, B.; Abdellah, A.; Abdul, K.; Al-Shoaibi, A.; Ali, M. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J. Taibah Univ. Sci. 2010, 3, 39–48. [Google Scholar]
- Taghvaeian, S.; Chavez, J.L.; Hansen, N.C. Infrared Thermometry to Estimate Crop Water Stress Index and Water Use of Irrigated Maize in Northeastern Colorado. Remote Sens. 2012, 4, 3619–3637. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.H.; Kuan, N.; Ibrahim, A.; Ismail, B.; Majid, M.R.; Ramli, R.; Mansor, N. Determination of soil water content using time domain reflectometer (TDR) for clayey soil. In Proceedings of the Advances in Civil Engineering and Science Technology, Penang, Malaysia, 5–6 September 2018; pp. 1–5. [Google Scholar]
- Cao, Z.; Wang, Q.; Zheng, C. Best hyperspectral indices for tracing leaf water status as determined from leaf dehydration experiments. Ecol. Indic. 2015, 54, 96–100. [Google Scholar] [CrossRef]
- Pieczynski, M.; Marczewski, W.; Hennig, J.; Dolata, J.; Bielewicz, D.; Piontek, P.; Wyrzykowska, A.; Krusiewicz, D.; Strzelczyk-Zyta, D.; Konokpa-Postupolska, D.; et al. Down-regulation of CBP80 gene expression as a strategy to engineer a drought-toletant potato. Plant Biotechnol. J. 2013, 11, 459–469. [Google Scholar] [CrossRef]
- Bai, H.; Purcell, L.C. Aerial canopy temperature differences between fast- and slow-wilting soya bean genotypes. J. Agron. Crop Sci. 2018, 204, 243–251. [Google Scholar] [CrossRef]
- Bausch, W.; Diker, K.; Khosla, R.; Paris, J. Estimating corn nitrogen status using ground—Based and satellite multispectral data. Remote Sens. Model. Ecosyst. Sustain. 2004, 5544, 489–498. [Google Scholar] [CrossRef]
- Blanco, F.; Folegatti, M.V. A new method for estimating the leaf area index of cucumber and tomato plants. Hortic. Bras. 2003, 21, 666–669. [Google Scholar] [CrossRef]
- Berni, J.A.; Zarco-Tejada, P.J.; Suárez, L.; Fereres, E. Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. Geosci. Remote Sens. IEEE Trans. 2009, 47, 722–738. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.C.; Lin, C.P.; Wang, K.; Lin, C.; Sheng-Ngui, Y.J. Improved TDR Method for Quality Control of Soil-Nailing Works. J. Geotech. Geoenviron. Eng. 2016, 142, 06015011. [Google Scholar] [CrossRef]
- Tanriverdi, C. Using TDR in the agricultural water management. KSUJ Sci. Eng. 2005, 2, 108–115. [Google Scholar]
- Degirmenci, H.; Gonen, E.; Boyaci, S.; Drip, S.; Components, Y.; Resources, W.; Location, T. A comparison of the gravimetric and TDR methods in terms of determining the soil water content of the corn plant. Ser. A Agron. 2016, 59, 152–158. [Google Scholar]
- Topp, G. Electromagnetic determination of soil water content: Measurements in coaxial transmission lines. Water Rersour. Res. 1980, 16, 574–582. [Google Scholar] [CrossRef] [Green Version]
- Matema, L.E. Adding solid fertilizers to soil in pot experiments. Protocols.io 2019, 1–16. [Google Scholar] [CrossRef]
- Sun, Z.J.; Young, G.D. Saline clayey soil moisture measurement using time domain reflectometry. In Proceedings of the TDR 2001 Symposium, Evanston, IL, USA, 5–7 September 2001; pp. 5–7. [Google Scholar]
- Hoseini-Izadi, O.; Pommerenke, D. Measurement of Dielectric Constant and Cross-Sectional Variations of a Wire. IEEE Trans. Instrum. Meas. 2018, 67, 1409–1416. [Google Scholar] [CrossRef]
- Cataldo, A.; De-Benedetto, E.; Cannazza, G.; Monti, G.; Piuzzi, E. TDR-based monitoring of rising damp through the embedding of wire-like sensing elements in building structures. Measurement 2017, 98, 355–360. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Molnár, K.; Vígh, R.; Nagy, J.; Dobos, A. Relationships between stomatal behaviour, spectral traits and water use and productivity of green peas (Pisum sativum L.) in dry seasons. Acta Physiol. Plant. 2015, 37, 34. [Google Scholar] [CrossRef]
- Mohamed, F.; EL-Aziz, A.B.D. The Effect of Silicon on Minimizing the Implications of Water Stress on Tomato Plants. Environ. Biodivers. Soil Secur. 2020, 4, 137–148. [Google Scholar]
- Nemeskéri, E.; Molnár, K.; Pék, Z.; Helyes, L. Effect of water supply on the water use-related physiological traits and yield of snap beans in dry seasons. Irrig. Sci. 2018, 36, 143–158. [Google Scholar] [CrossRef]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of deficit irrigation on biomass, yield, water productivity and fruit quality of processing tomato under semi-arid Mediterranean climate conditions. Sci. Hortic. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Pék, Z.; Szuvandzsiev, P.; Daood, H.; Neményi, A.; Helyes, L. Effect of irrigation on yield parameters and antioxidant profiles of processing cherry tomato. Open Life Sci. 2014, 9, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Helyes, L.; Szuvandzsiev, P.; Neményi, A.; Pék, Z.; Lugasi, A. Different water supply and stomatal conductance correlates with yield quantity and quality parameters. Acta Hortic. 2013, 971, 119–126. [Google Scholar] [CrossRef]
- Takács, S.; Pék, Z.; Csányi, D.; Daood, H.G.; Szuvandzsiev, P.; Palotás, G.; Helyes, L. Influence of Water Stress Levels on the Yield and Lycopene Content of Tomato. Water 2020, 12, 2165. [Google Scholar] [CrossRef]
- Lu, J.; Shao, G.; Cui, J.; Wang, X.; Keabetswe, L. Yield, fruit quality and water use efficiency of tomato for processing under regulated deficit irrigation: A meta-analysis. Agric. Water Manag. 2019, 222, 30. [Google Scholar] [CrossRef]
- Chakroun, H.; Mouillot, F.; Hamdi, A. Regional equivalent water thickness modeling from remote sensing across a tree cover/LAI gradient in Mediterranean forests of Northern Tunisia. Remote Sens. 2015, 7, 1937–1961. [Google Scholar] [CrossRef] [Green Version]
- Wittamperuma, I.; Hafeez, M.; Pakparvar, M.; Louis, J. Remote-sensing-based biophysical models for estimating LAI of irrigated crops in Murry darling basin. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2012, 34, 367–373. [Google Scholar]
- Tittebrand, A.; Spank, U. Comparison of satellite and ground-based NDVI above different land-use types. Theor. Appl. Clim. 2009, 98, 171–186. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.; Gao, X.; Ferreira, L. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]

| Parameters | Sandy Loam Soil | Silt Loam Soil |
|---|---|---|
| PH | 5.64 ± 0.12 | 5.30 ± 0.20 |
| O.M (g/kg) | 15.91 ± 1.3 | 22.97 ± 1.79 |
| Total N (g/kg) | 1.23 ± 0.013 | 1.518 ± 0.021 |
| Total P (g/kg) | 0.88 ± 0.21 | 0.865 ± 0.32 |
| Total K (g/kg) | 9.30 ± 0.025 | 19.59 ± 0.05 |
| Alkalized N(mg/kg) | 450.28 ± 1.9 | 72.71 ± 2.38 |
| Avail. P (mg/kg) | 195.72 ± 2.43 | 28.25 ± 1.72 |
| Avail. K (mg/kg) | 428.43 ± 42.3 | 85.50 ± 20.1 |
| Sand (%) | 47.6 ± 0.06 | 43.53 ± 0.34 |
| Clay (%) | 17.3 ± 1.54 | 16.53 ± 1.5 |
| Silt (%) | 35.1 ± 0.3 | 39.93 ± 0.67 |
| Bulk density (gcm−3) | 1.34 ± 0.11 | 1.32 ± 0.07 |
| Field capacity (%) | 0.21 ± 0.01 | 31 ± 1.53 |
| WP (%) | 0.09 ± 0.13 | 19 ± 0.19 |
| Saturation point (%) | 0.48 ± 0.15 | 45.73 ± 0.252 |
| Index | Formula | References |
|---|---|---|
| Greenness vegetation index Normalized difference vegetation index (NDVI) | [35,36] | |
| Modified normalized difference vegetation index(mNDVI750) | [6] | |
| Renormalized difference vegetative index (RDVI) | [28] | |
| Green chlorophyll index (CLgreen) | [29] | |
| Water content vegetative index Water index (WI) | [31] | |
| Simple ratio water index (SRWI) | [17] | |
| Normalized difference water index | [6] | |
| Normalized difference water index centered at 1640 nm (NDWI1640) | [32] | |
| Normalized difference water index centered at NDWI2130 | [32] | |
| Xanthophyll pigment Photochemical reflective index (PRI570) | [10] | |
| Normalized photochemical reflective index (PRInorm) | [10] |
| Treatment (% FC) | Duration (Days) | Irrigation Water Applied (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soil A | Soil B | 70–100 | 60–70 | 50–60 | 40–50 | |||||
| Soil A | Soil B | Soil A | Soil B | Soil A | Soil B | Soil A | Soil B | |||
| Vegetative stage | 30 | 32 | 50 | 55 | 32.5 | 35.8 | 27.5 | 30.3 | 22.5 | 24.8 |
| Anthesis stage | 40 | 40 | 60.1 | 65 | 39.1 | 42.3 | 33.1 | 23.3 | 27.1 | 29.3 |
| Fruit expansion stage | 50 | 50 | 70 | 73 | 45.5 | 47.5 | 38.5 | 40.2 | 31.5 | 32.9 |
| Senescence stage | 30 | 31 | 45.8 | 50 | 29.8 | 32.5 | 25.2 | 27.5 | 20.6 | 22.5 |
| Total | 150 | 153 | 225.9 | 243 | 146.9 | 158.1 | 124.3 | 121.3 | 101.7 | 109.5 |
| Vegetative Indices | Water Stress Indicators in Sandy Loam Soil | Water Stress Indicators Silty Loam Soil | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LT (℃) | RLWC (%) | LAI (m2/m2) | LCC (mg/g) | Yield (kg/plant) | LT (℃) | RLWC (%) | LAI (m2/m2) | LCC (mg/g) | Yield (kg/plant) | |
| Greenness vegetation index NDVI | 0.75 *** | 0.59 *** | 0.80 *** | 0.88 *** | 0.72 *** | 0.79 *** | 0.67 *** | 0.70 *** | 0.87 *** | 0.74 *** |
| RDVI | 0.61 *** | 0.52 *** | 0.63 *** | 0.71 *** | 0.80 *** | 0.68 *** | 0.77 *** | 0.66 *** | 0.65 *** | 0.68 *** |
| CLgreen | 0.57 *** | 0.45 ** | 0.47 ** | 0.62 *** | 0.61 *** | 0.59 *** | 0.36 * | 0.22 * | 0.60 *** | 0.71 *** |
| Water content vegetative index WI | 0.63 *** | 0.64 *** | 0.53 *** | 0.41 ** | 0.51 ** | 0.64 *** | 0.67 *** | 0.57 *** | 0.52 *** | 0.57 *** |
| NDWI | 0.73 *** | 0.72 *** | 0.52 *** | 0.83 *** | 0.74 *** | 0.72 *** | 0.75 *** | 0.55 *** | 0.87 *** | 0.79 *** |
| NDWI1640 | 0.64 *** | 0.61 *** | 0.58 *** | 0.75 *** | 0.67 *** | 0.67 *** | 0.54 *** | 0.52 *** | 0.7 *** | 0.71 *** |
| NDWI2130 | 0.51 ** | 0.37 * | 0.57 ** | 0.21 * | 0.49 ** | 0.67 *** | 0.46 * | 0.37 * | 0.44 * | 0.51 ** |
| Xanthophyll pigment PRI570 | 0.68 *** | 0.74 *** | 0.45 * | 0.55 *** | 0.70 *** | 0.71 *** | 0.70 *** | 0.37 * | 0.62 *** | 0.66 *** |
| PRInorm | 0.71 *** | 0.73 *** | 0.58 ** | 0.45 * | 0.70 *** | 0.69 *** | 0.69 *** | 0.54 ** | 0.54 ** | 0.61 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alordzinu, K.E.; Li, J.; Lan, Y.; Appiah, S.A.; AL Aasmi, A.; Wang, H.; Liao, J.; Sam-Amoah, L.K.; Qiao, S. Ground-Based Hyperspectral Remote Sensing for Estimating Water Stress in Tomato Growth in Sandy Loam and Silty Loam Soils. Sensors 2021, 21, 5705. https://doi.org/10.3390/s21175705
Alordzinu KE, Li J, Lan Y, Appiah SA, AL Aasmi A, Wang H, Liao J, Sam-Amoah LK, Qiao S. Ground-Based Hyperspectral Remote Sensing for Estimating Water Stress in Tomato Growth in Sandy Loam and Silty Loam Soils. Sensors. 2021; 21(17):5705. https://doi.org/10.3390/s21175705
Chicago/Turabian StyleAlordzinu, Kelvin Edom, Jiuhao Li, Yubin Lan, Sadick Amoakohene Appiah, Alaa AL Aasmi, Hao Wang, Juan Liao, Livingstone Kobina Sam-Amoah, and Songyang Qiao. 2021. "Ground-Based Hyperspectral Remote Sensing for Estimating Water Stress in Tomato Growth in Sandy Loam and Silty Loam Soils" Sensors 21, no. 17: 5705. https://doi.org/10.3390/s21175705
APA StyleAlordzinu, K. E., Li, J., Lan, Y., Appiah, S. A., AL Aasmi, A., Wang, H., Liao, J., Sam-Amoah, L. K., & Qiao, S. (2021). Ground-Based Hyperspectral Remote Sensing for Estimating Water Stress in Tomato Growth in Sandy Loam and Silty Loam Soils. Sensors, 21(17), 5705. https://doi.org/10.3390/s21175705







