New Sensor to Measure the Microencapsulated Active Compounds Released in an Aqueous Liquid Media Based in Dielectric Properties in Radiofrequency Range
Abstract
1. Introduction
2. Materials and Methods
2.1. Buffer
2.2. Reagents
2.3. Solutions of Different pH
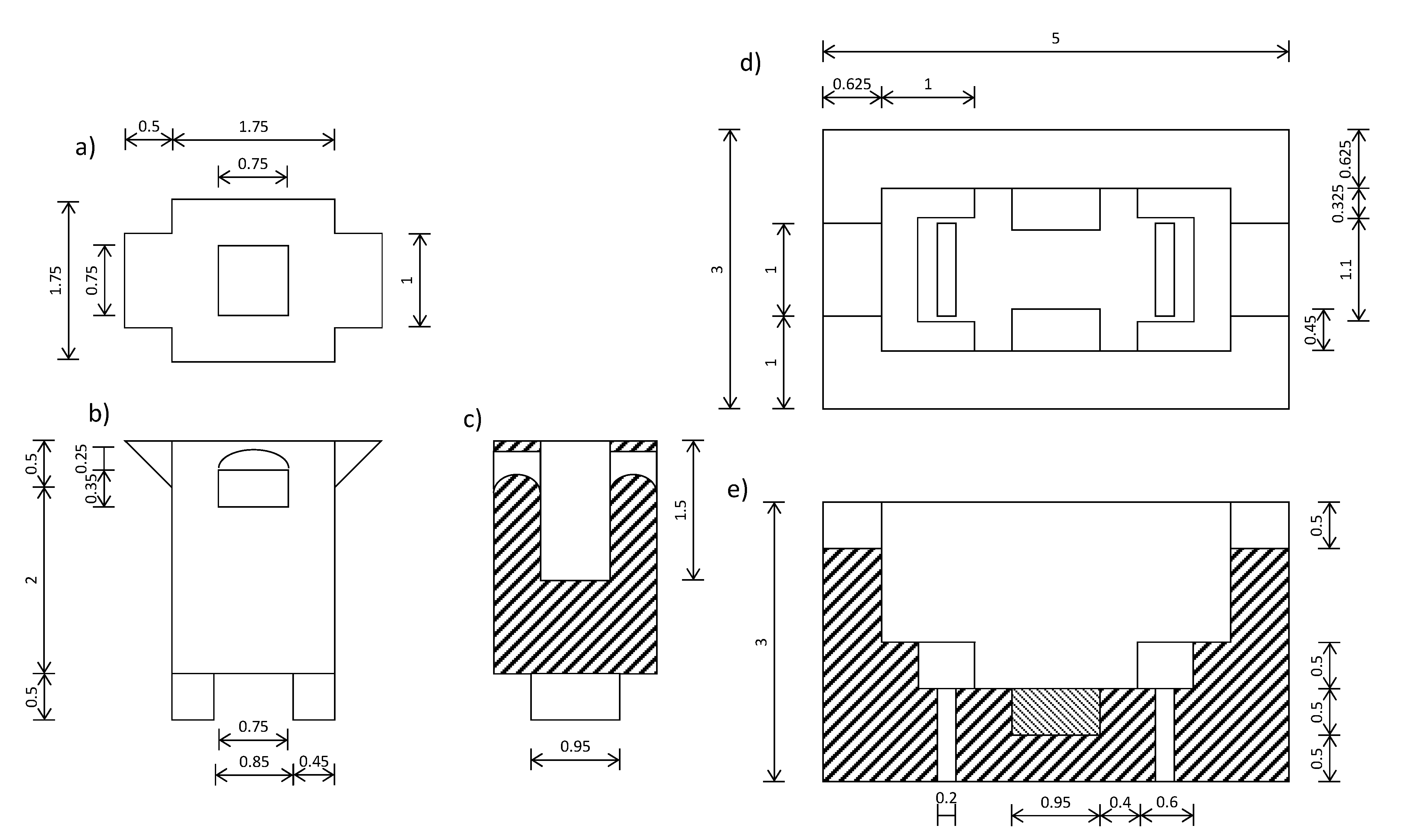
2.4. D Printing Material: Acrylonitrile Butadiene Styrene (ABS)
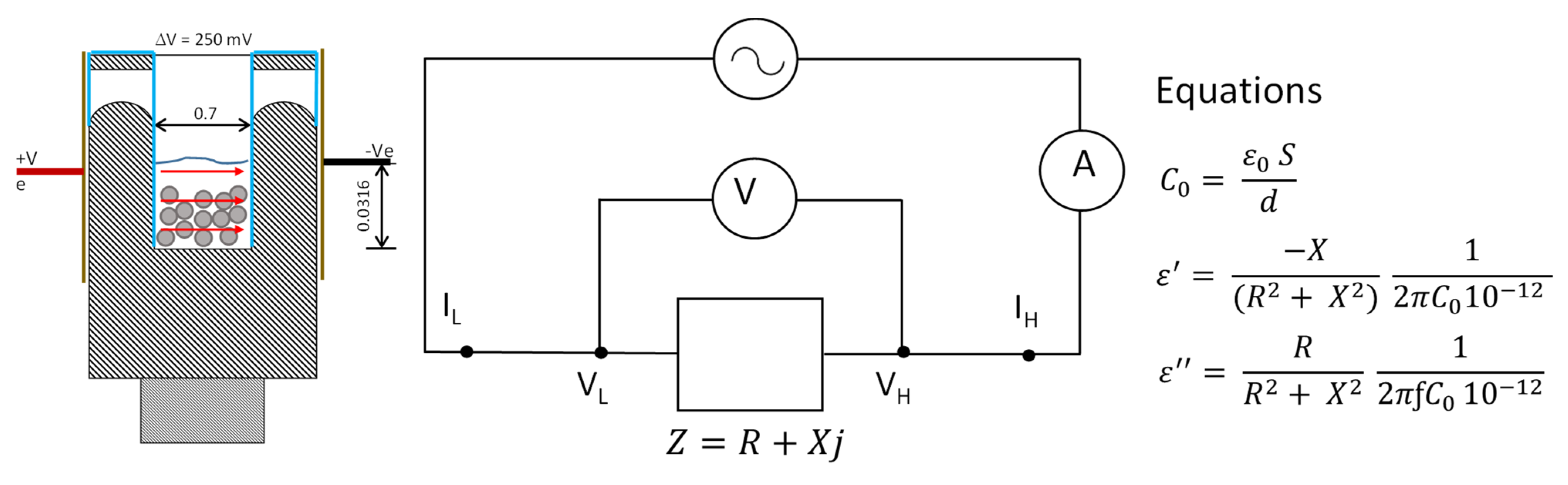
2.5. Experimental Procedure
2.6. D printing Protocol
2.7. Calcium Alginate Beads Encapsulating Iron-Protein-Succinylate and Ascorbic Acid Preparation Protocol
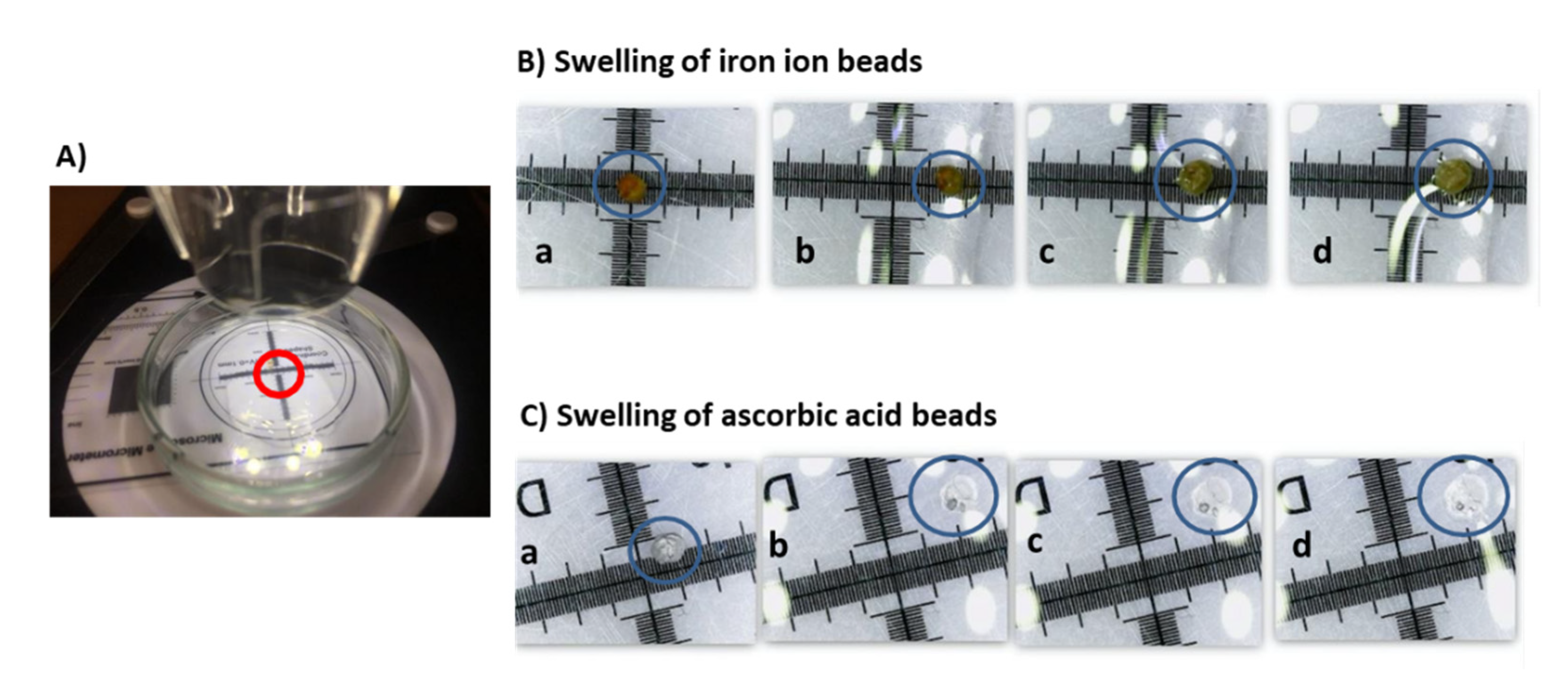
2.8. Protocol for the Determination of the Expansion Capacity of the Beads in Different Media
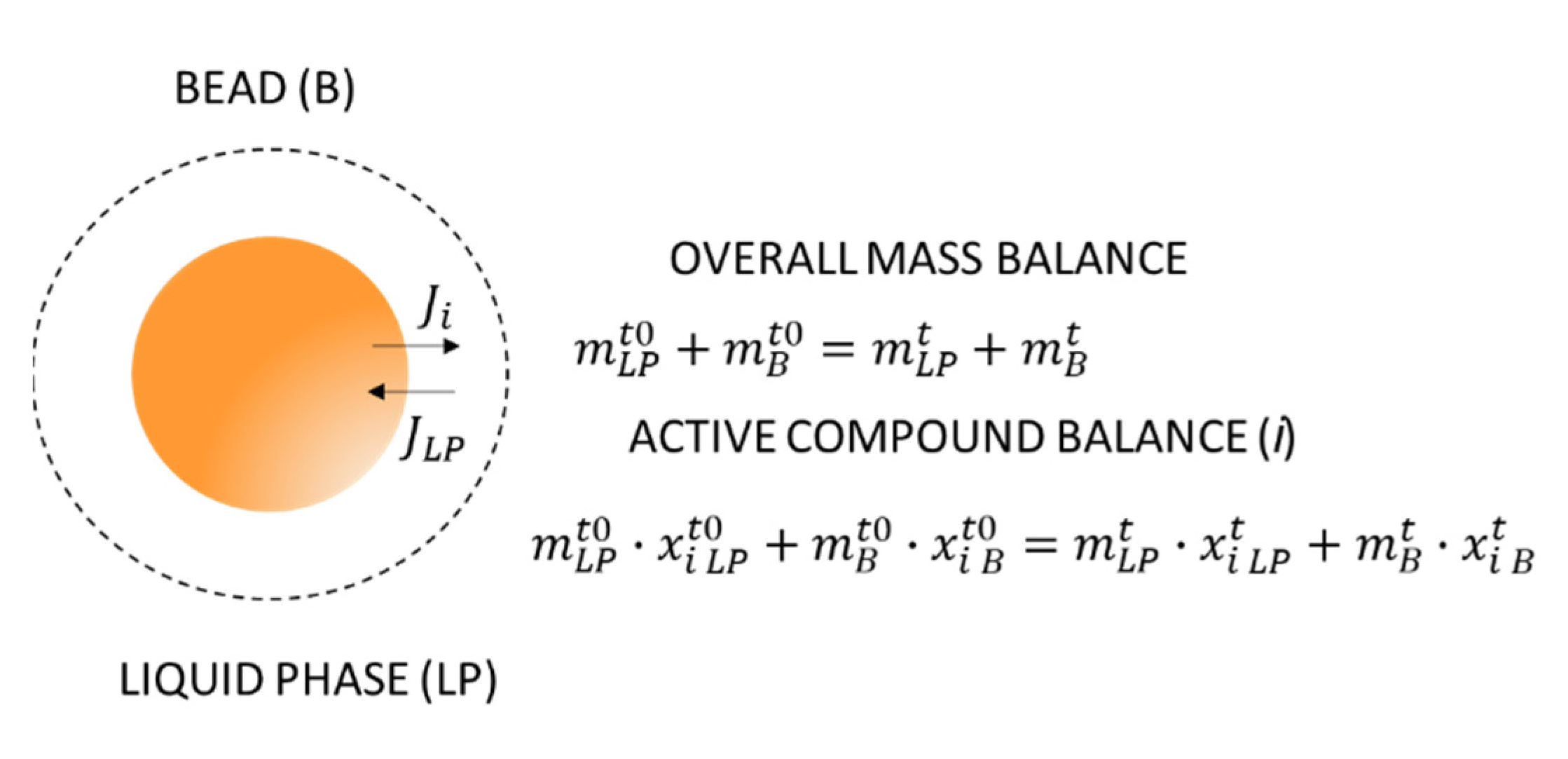
2.9. Determination of Liberation Kinetics
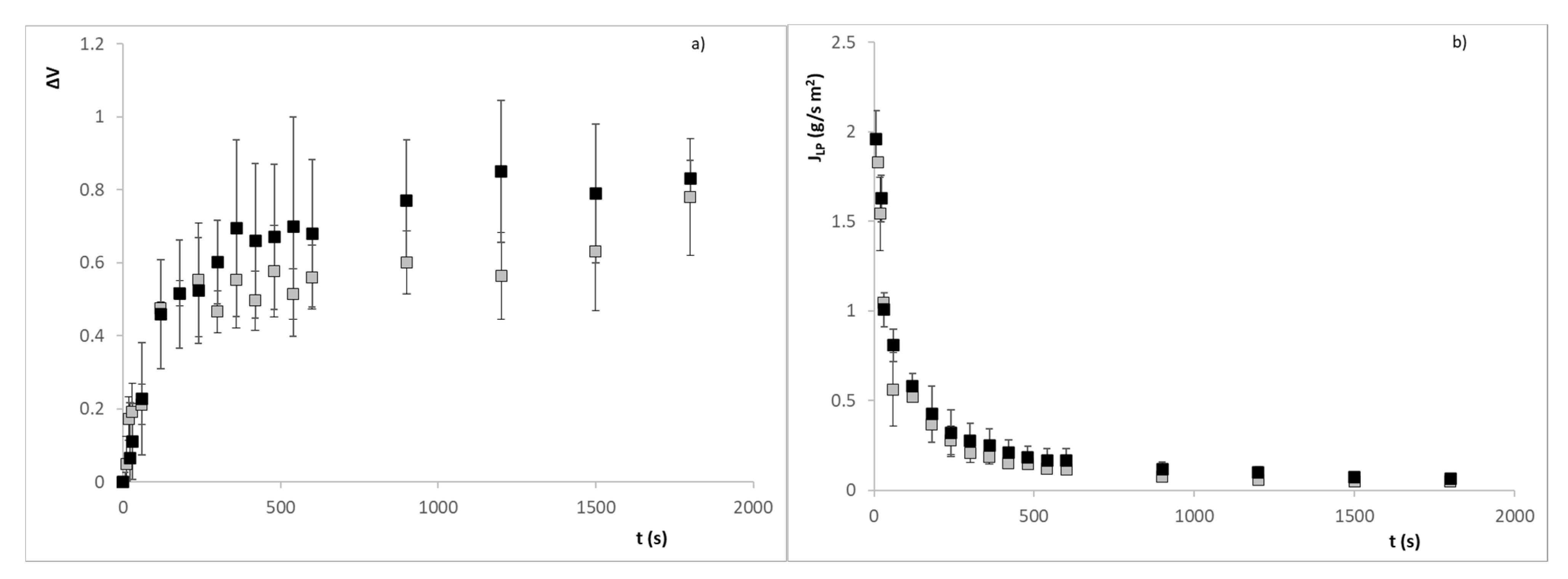
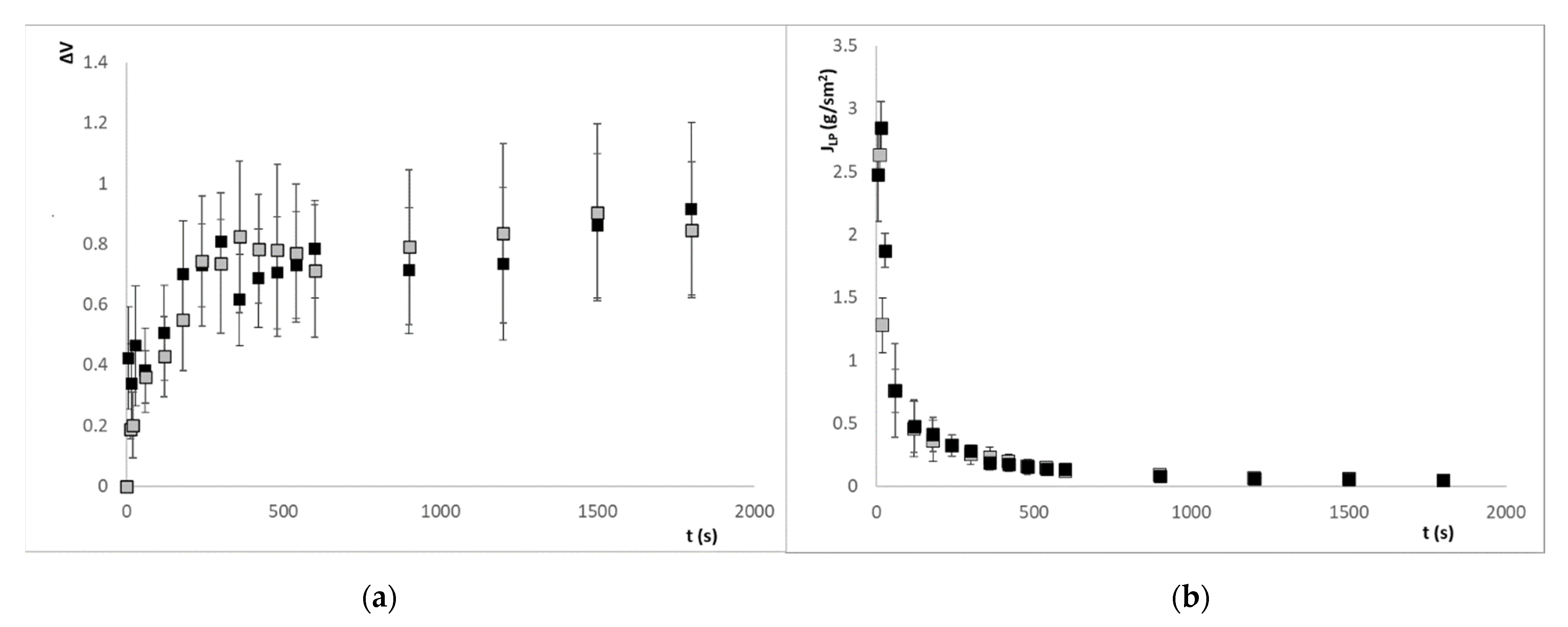
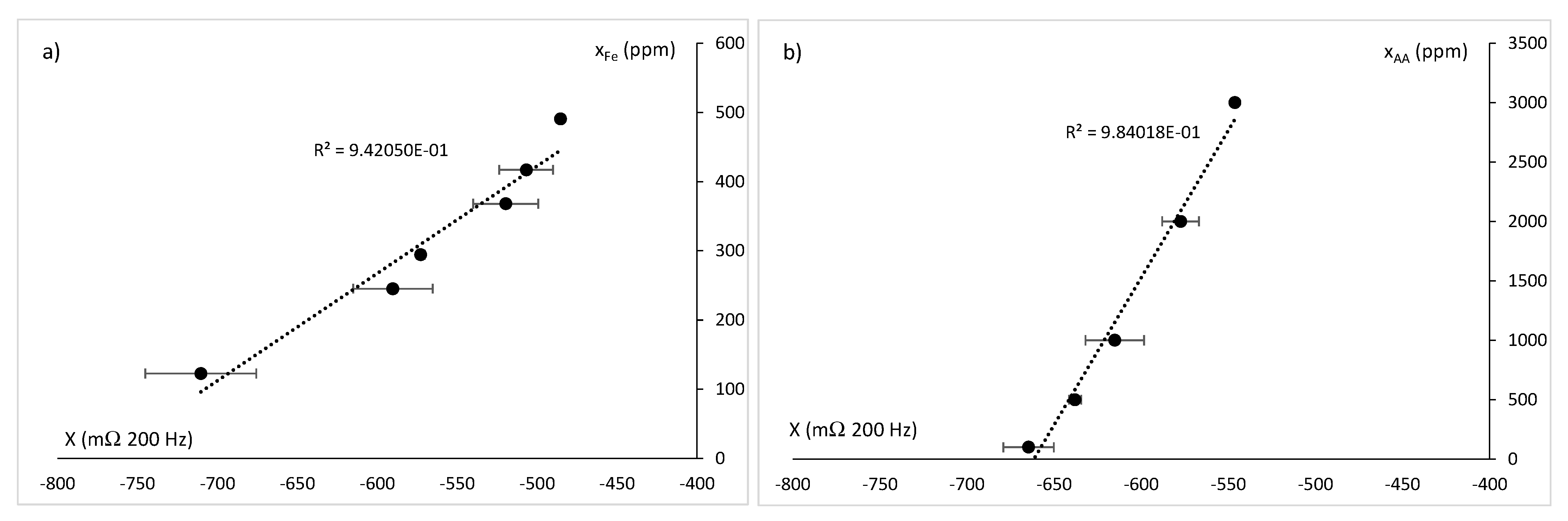
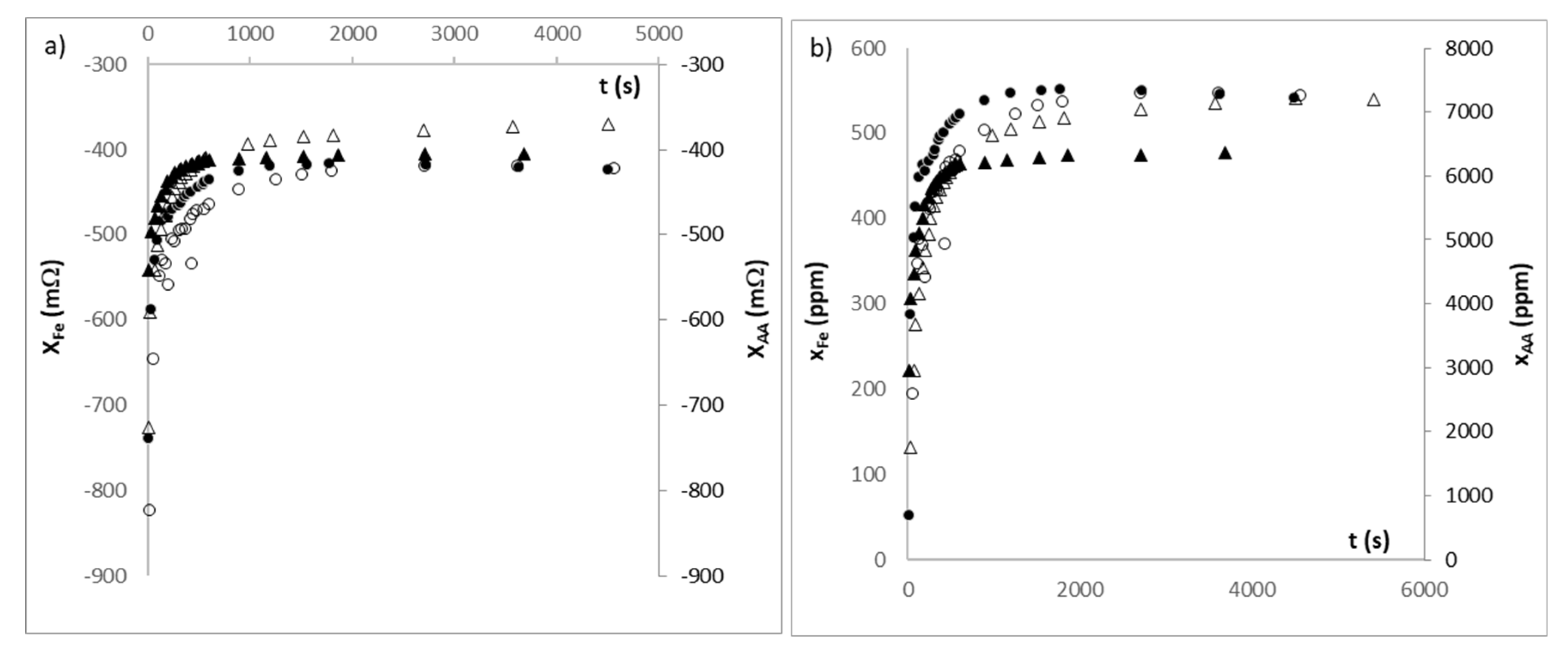
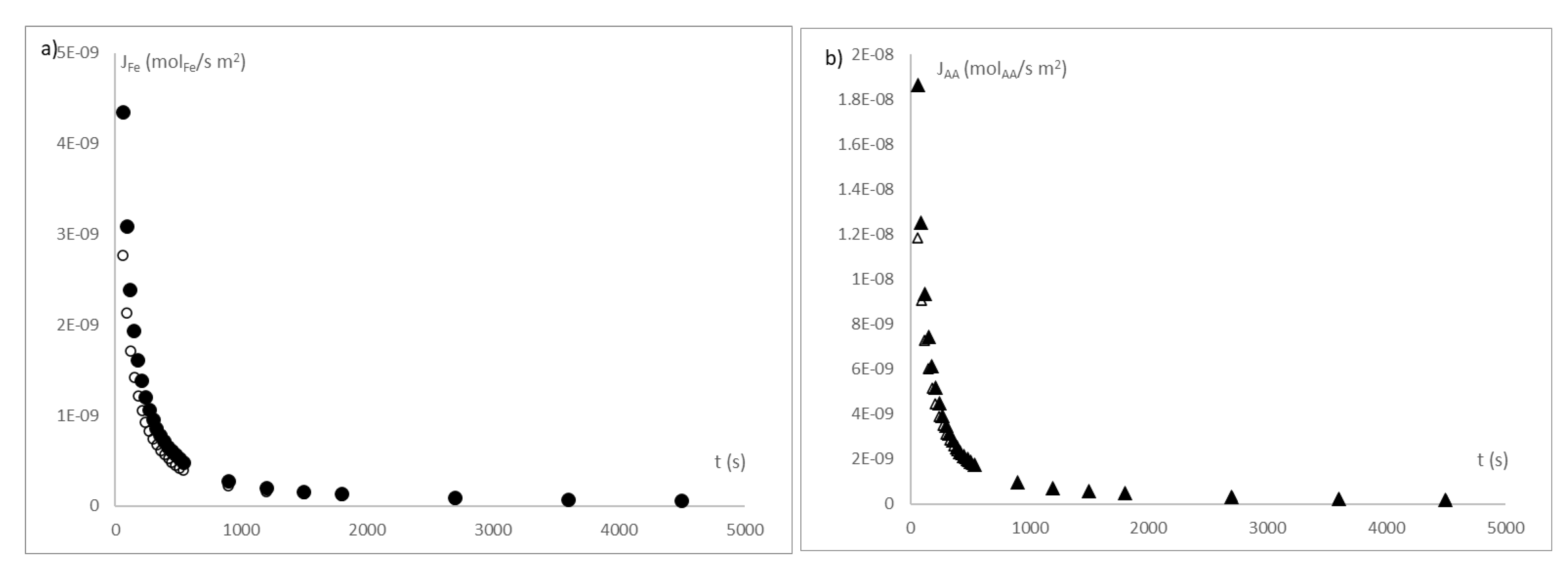
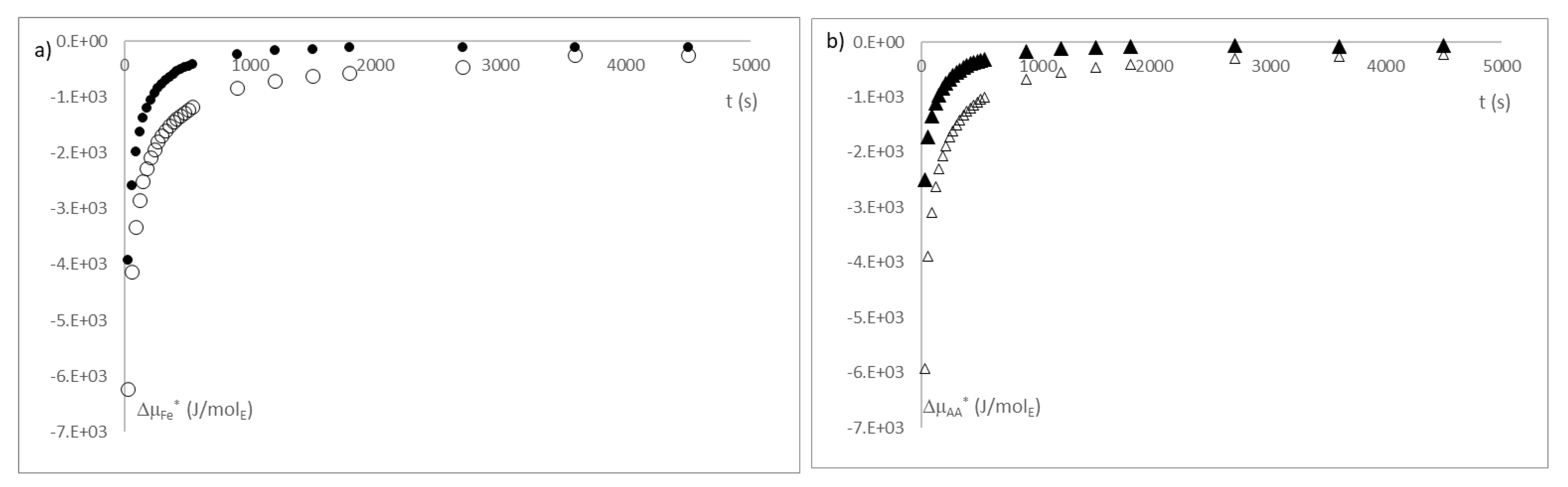
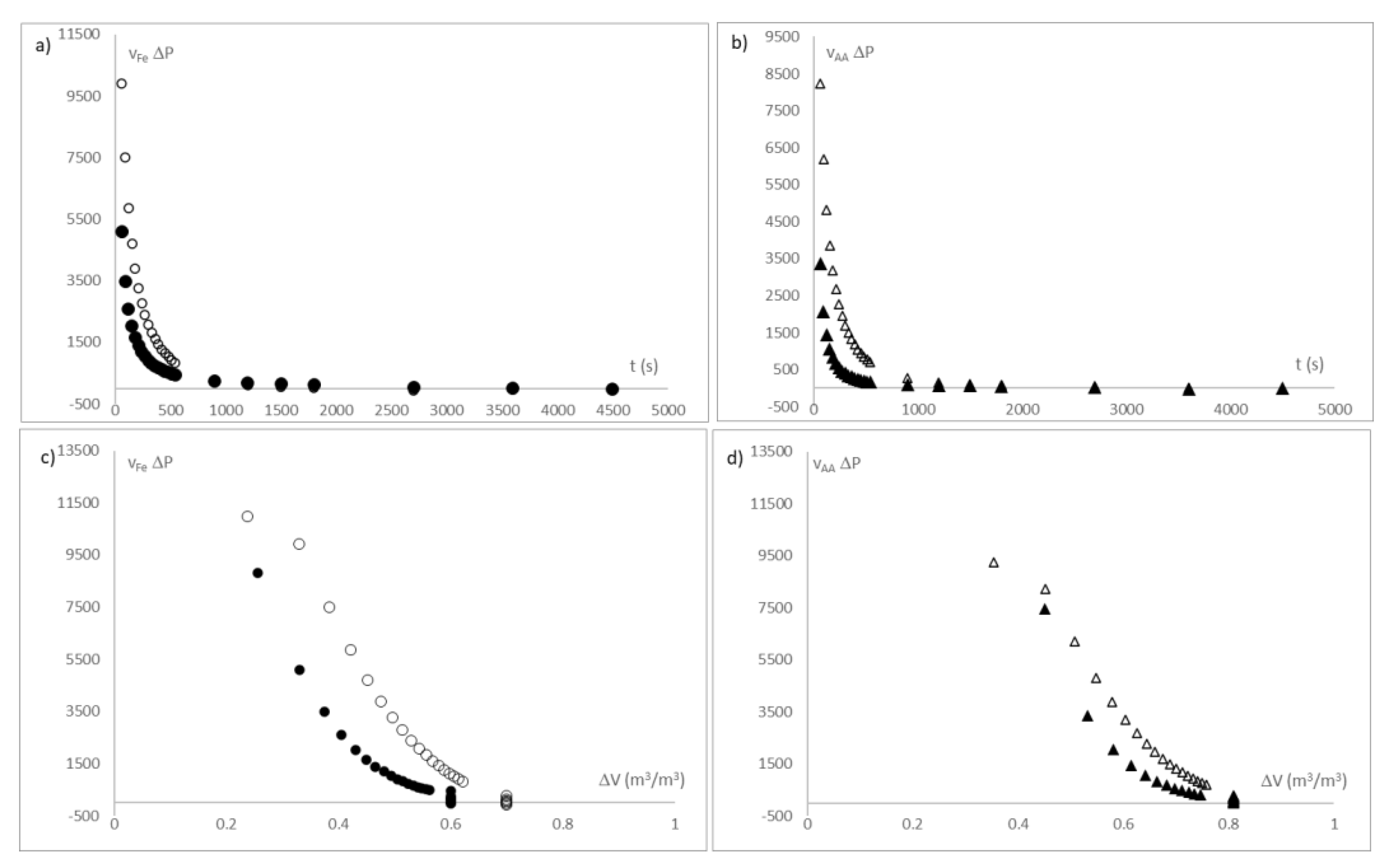
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tu, J.; Bolla, S.; Barr, J.; Miedema, J.; Li, X.; Jasti, B. Alginate Microparticles Prepared by Spray–Coagulation Method: Preparation, Drug Loading and Release Characterization. Int. J. Pharm. 2005, 303, 171–181. [Google Scholar] [CrossRef]
- Colinet, I.; Dulong, V.; Mocanu, G.; Picton, L.; Le Cerf, D. New Amphiphilic and PH-Sensitive Hydrogel for Controlled Release of a Model Poorly Water-Soluble Drug. Eur. J. Pharm. Biopharm. 2009, 73, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Rizzello, L.; Bayer, I.S.; Pompa, P.P.; Cingolani, R.; Athanassiou, A. Controlled Antiseptic Release by Alginate Polymer Films and Beads. Carbohydr. Polym. 2013, 92, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Miere, F.; Teusdea, A.C.; Laslo, V.; Fritea, L.; Moldovan, L.; Costea, T.; Uivarosan, D.; Vicas, S.I.; Pallag, A. Natural Polymeric Beads for Encapsulation of Stellaria Media Extract with Antioxidant Properties. Mater. Plast. 2019, 56, 671–679. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Jamshidian, M.; Salmieri, S.; Desjardins, N.; Sahraoui, A.; Lacroix, M. Effect of Cellulose Nanocrystals on Thyme Essential Oil Release from Alginate Beads: Study of Antimicrobial Activity against Listeria Innocua and Ground Meat Shelf Life in Combination with Gamma Irradiation. Cellulose 2019, 26, 5247–5265. [Google Scholar] [CrossRef]
- Najafi-Soulari, S.; Shekarchizadeh, H.; Kadivar, M. Encapsulation Optimization of Lemon Balm Antioxidants in Calcium Alginate Hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 1631–1644. [Google Scholar] [CrossRef]
- Orozco-Villafuerte, J.; Escobar-Rojas, A.; Buendía-González, L.; García-Morales, C.; Hernandez-Jaimes, C.; Alvarez-Ramirez, J. Evaluation of the Protection and Release Rate of Bougainvillea (Bougainvillea Spectabilis) Extracts Encapsulated in Alginate Beads. J. Dispers. Sci. Technol. 2019, 40, 1065–1074. [Google Scholar] [CrossRef]
- Apoorva, A.; Rameshbabu, A.P.; Dasgupta, S.; Dhara, S.; Padmavati, M. Novel PH-Sensitive Alginate Hydrogel Delivery System Reinforced with Gum Tragacanth for Intestinal Targeting of Nutraceuticals. Int. J. Biol. Macromol. 2020, 147, 675–687. [Google Scholar] [CrossRef]
- Gholamian, S.; Nourani, M.; Bakhshi, N. Formation and Characterization of Calcium Alginate Hydrogel Beads Filled with Cumin Seeds Essential Oil. Food Chem. 2021, 338, 128143. [Google Scholar] [CrossRef]
- Hoseyni, S.Z.; Jafari, S.M.; Shahiri Tabarestani, H.; Ghorbani, M.; Assadpour, E.; Sabaghi, M. Release of Catechin from Azivash Gum-Polyvinyl Alcohol Electrospun Nanofibers in Simulated Food and Digestion Media. Food Hydrocoll. 2021, 112, 106366. [Google Scholar] [CrossRef]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of Biological Active Phenolic Compounds Extracted from Wine Wastes in Alginate-Chitosan Microbeads. J. Microencapsul. 2018, 35, 229–240. [Google Scholar] [CrossRef] [PubMed]
- De Benoist, B.; Cogswell, M.; Egli, I.; McLean, E. Worldwide Prevalence of Anaemia 1993–2005; WHO Global Database of Anaemia: Geneva, Switzerland, 2008. [Google Scholar]
- Charles, C.V. Iron Deficiency Anemia: A Public Health Problem of Global Proportions. Public Health—Methodol. Environ. Syst. 2012, 2012, 109. [Google Scholar]
- Anderson, G.J.; Frazer, D.M.; McLaren, G.D. Iron Absorption and Metabolism. Curr. Opin. Gastroenterol. 2009, 25, 129–135. [Google Scholar] [CrossRef]
- Sermini, C.G.; Acevedo, M.J.; Arredondo, M. Biomarkers of Metabolism and Iron Nutrition. Rev. Peru. Med. Exp. Salud Publica 2017, 34, 690–698. [Google Scholar] [CrossRef]
- Sharp, P.; Srai, S.K. Molecular Mechanisms Involved in Intestinal Iron Absorption. World J. Gastroenterol. 2007, 13, 4716. [Google Scholar] [CrossRef]
- Durán, E.; Villalobos, C.; Churio, O.; Pizarro, F.; Valenzuela, C. Encapsulación de Hierro: Otra Estrategia Para La Prevención o Tratamiento de La Anemia Por Deficiencia de Hierro. Rev. Chil. Nutr. 2017, 44, 234–243. [Google Scholar] [CrossRef][Green Version]
- Santagapita, P.R.; Mazzobre, M.F.; Buera, M.P. Invertase Stability in Alginate Beads: Effect of Trehalose and Chitosan Inclusion and of Drying Methods. Food Res. Int. 2012, 47, 321–330. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Aguirre Calvo, T.R.; Castro-Giráldez, M.; Fito, P.J.; Santagapita, P.R. Alginate Beads Containing Lactase: Stability and Microstructure. Biomacromolecules 2017, 18, 1785–1792. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giráldez, M.; Fito, P.J.; Santagapita, P.R. Encapsulation of Lactase in Ca(II)-Alginate Beads: Effect of Stabilizers and Drying Methods. Food Res. Int. 2017, 100, 296–303. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with Chitosan by Spray Drying for Industry Applications—A Review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Busch, V.M.; Santagapita, P.R. Stability and Release of an Encapsulated Solvent-Free Lycopene Extract in Alginate-Based Beads. LWT 2017, 77, 406–412. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Molino, S.; Perullini, M.; Rufián-Henares, J.A.; Santagapita, P.R. Effect of in Vitro Digestion-Fermentation of Ca(II)-Alginate Beads Containing Sugar and Biopolymers over Global Antioxidant Response and Short Chain Fatty Acids Production. Food Chem. 2020, 333, 127483. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giráldez, M.; Fito, P.J.; Perullini, M.; Santagapita, P.R. Gums Induced Microstructure Stability in Ca(II)-Alginate Beads Containing Lactase Analyzed by SAXS. Carbohydr. Polym. 2018, 179, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Santagapita, P.R.; Mazzobre, M.F.; Buera, M.P. Formulation and Drying of Alginate Beads for Controlled Release and Stabilization of Invertase. Biomacromolecules 2011, 12, 3147–3155. [Google Scholar] [CrossRef]
- Rahman, M.S.; Labuza, T.P. Water activity and food preservation. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; pp. 487–506. [Google Scholar]
- Velázquez-Varela, J.; Castro-Giráldez, M.; Cuibus, L.; Tomas-Egea, J.A.; Socaciu, C.; Fito, P.J. Study of the Cheese Salting Process by Dielectric Properties at Microwave Frequencies. J. Food Eng. 2018, 224, 121–128. [Google Scholar] [CrossRef]
- Tomas-Egea, J.A.; Traffano-Schiffo, M.V.; Castro-Giráldez, M.; Fito, P.J. Hot Air and Microwave Combined Drying of Potato Monitored by Infrared Thermography. Appl. Sci. 2021, 11, 1730. [Google Scholar] [CrossRef]
- Talens, C.; Castro-Giráldez, M.; Fito, P.J. A Thermodynamic Model for Hot Air Microwave Drying of Orange Peel. J. Food Eng. 2016, 175, 33–42. [Google Scholar] [CrossRef]
- Cuibus, L.; Castro-Giráldez, M.; Fito, P.J.; Fabbri, A. Application of Infrared Thermography and Dielectric Spectroscopy for Controlling Freezing Process of Raw Potato. Innov. Food Sci. Emerg. Technol. 2014, 24, 80–87. [Google Scholar] [CrossRef]
- Castro-Giráldez, M.; Balaguer, N.; Hinarejos, E.; Fito, P.J. Thermodynamic Approach of Meat Freezing Process. Innov. Food Sci. Emerg. Technol. 2014, 23, 138–145. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Schick, C. Kauzmann Paradox and the Crystallization of Glass-Forming Melts. J. Non-Cryst. Solids 2018, 501, 21–35. [Google Scholar] [CrossRef]
- Hellmuth, O.; Schmelzer, J.W.P.; Feistel, R. Ice-Crystal Nucleation in Water: Thermodynamic Driving Force and Surface Tension. Part I: Theoretical Foundation. Entropy 2020, 22, 50. [Google Scholar] [CrossRef]
- Berezovskiy, Y.M.; Korolev, I.A.; Sarantsev, T.A. Calculation of Heat Capacity in Meat during Its Freezing Considering Phase Change. Theory Pract. Meat Process. 2020, 5, 22–26. [Google Scholar] [CrossRef]
- Roos, Y.H. Glass Transition and Re-Crystallization Phenomena of Frozen Materials and Their Effect on Frozen Food Quality. Foods 2021, 10, 447. [Google Scholar] [CrossRef]
- Tomas-Egea, J.A.; Fito, P.J.; Castro-Giráldez, M. Analysis of Apple Candying by Microwave Spectroscopy. Foods 2019, 8, 316. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giráldez, M.; Colom, R.J.; Talens, P.; Fito, P.J. New Methodology to Analyze the Dielectric Properties in Radiofrequency and Microwave Ranges in Chicken Meat during Postmortem Time. J. Food Eng. 2021, 292, 110350. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giráldez, M.; Herrero, V.; Colom, R.J.; Fito, P.J. Development of a Non-Destructive Detection System of Deep Pectoral Myopathy in Poultry by Dielectric Spectroscopy. J. Food Eng. 2018, 237, 137–145. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giráldez, M.; Colom, R.J.; Fito, P.J. Development of a Spectrophotometric System to Detect White Striping Physiopathy in Whole Chicken Carcasses. Sensors 2017, 17, 1024. [Google Scholar] [CrossRef]
- Zhang, L.; Lyng, J.G.; Brunton, N.P. The Effect of Fat, Water and Salt on the Thermal and Dielectric Properties of Meat Batter and Its Temperature Following Microwave or Radio Frequency Heating. J. Food Eng. 2007, 80, 142–151. [Google Scholar] [CrossRef]
- Castro-Giráldez, M.; Botella, P.; Toldrá, F.; Fito, P. Low-Frequency Dielectric Spectrum to Determine Pork Meat Quality. Innov. Food Sci. Emerg. Technol. 2010, 11, 376–386. [Google Scholar] [CrossRef]
- Trabelsi, S.; Roelvink, J.; Russell, R.B. Investigating the Influence of Aging on Radiofrequency Dielectric Properties of Chicken Meat. J. Microw. Power Electromagn. Energy 2014, 48, 215–220. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giráldez, M.; Colom, R.J.; Fito, P.J. Innovative Photonic System in Radiofrequency and Microwave Range to Determine Chicken Meat Quality. J. Food Eng. 2018, 239, 1–7. [Google Scholar] [CrossRef]
- Brunton, N.P.; Lyng, J.G.; Zhang, L.; Jacquier, J.C. The Use of Dielectric Properties and Other Physical Analyses for Assessing Protein Denaturation in Beef Biceps Femoris Muscle during Cooking from 5 to 85 °C. Meat Sci. 2006, 72, 236–244. [Google Scholar] [CrossRef]
- Shang, L.; Guo, W.; Nelson, S.O. Apple Variety Identification Based on Dielectric Spectra and Chemometric Methods. Food Anal. Methods 2015, 8, 1042–1052. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giráldez, M.; Colom, R.J.; Fito, P.J. New Spectrophotometric System to Segregate Tissues in Mandarin Fruit. Food Bioprocess Technol. 2018, 11, 399–406. [Google Scholar] [CrossRef]
- Deladino, L.; Anbinder, P.S.; Navarro, A.S.; Martino, M.N. Encapsulation of Natural Antioxidants Extracted from Ilex Paraguariensis. Carbohydrate Polymers. 2008, 71, 126–134. [Google Scholar] [CrossRef]
- Castro-Giráldez, M.; Fito, P.J.; Fito, P. Non-Equilibrium Thermodynamic Approach to Analyze the Pork Meat (Longissimus Dorsi) Salting Process. J. Food Eng. 2010, 99, 24–30. [Google Scholar] [CrossRef]
- Gambár, K.; Márkus, F. On the Global Symmetry of Thermodynamics and Onsager’s Reciprocity Relations. J. Non-Equilib. Thermodyn. 1993, 18, 51–58. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomas-Egea, J.A.; Fito, P.J.; Colom, R.J.; Castro-Giraldez, M. New Sensor to Measure the Microencapsulated Active Compounds Released in an Aqueous Liquid Media Based in Dielectric Properties in Radiofrequency Range. Sensors 2021, 21, 5781. https://doi.org/10.3390/s21175781
Tomas-Egea JA, Fito PJ, Colom RJ, Castro-Giraldez M. New Sensor to Measure the Microencapsulated Active Compounds Released in an Aqueous Liquid Media Based in Dielectric Properties in Radiofrequency Range. Sensors. 2021; 21(17):5781. https://doi.org/10.3390/s21175781
Chicago/Turabian StyleTomas-Egea, Juan Angel, Pedro J. Fito, Ricardo J. Colom, and Marta Castro-Giraldez. 2021. "New Sensor to Measure the Microencapsulated Active Compounds Released in an Aqueous Liquid Media Based in Dielectric Properties in Radiofrequency Range" Sensors 21, no. 17: 5781. https://doi.org/10.3390/s21175781
APA StyleTomas-Egea, J. A., Fito, P. J., Colom, R. J., & Castro-Giraldez, M. (2021). New Sensor to Measure the Microencapsulated Active Compounds Released in an Aqueous Liquid Media Based in Dielectric Properties in Radiofrequency Range. Sensors, 21(17), 5781. https://doi.org/10.3390/s21175781





