Non-Destructive Identification of Naturally Aged Alfalfa Seeds via Multispectral Imaging Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Sample
2.2. Germination Test
2.3. MSI Data Recording
2.4. Multivariate Data Analysis
3. Results
3.1. Germination and Morphologic Features of Aged and Non-Aged Seeds
3.2. Spectroscopic Analysis of Aged Seeds and Non-Aged Seeds
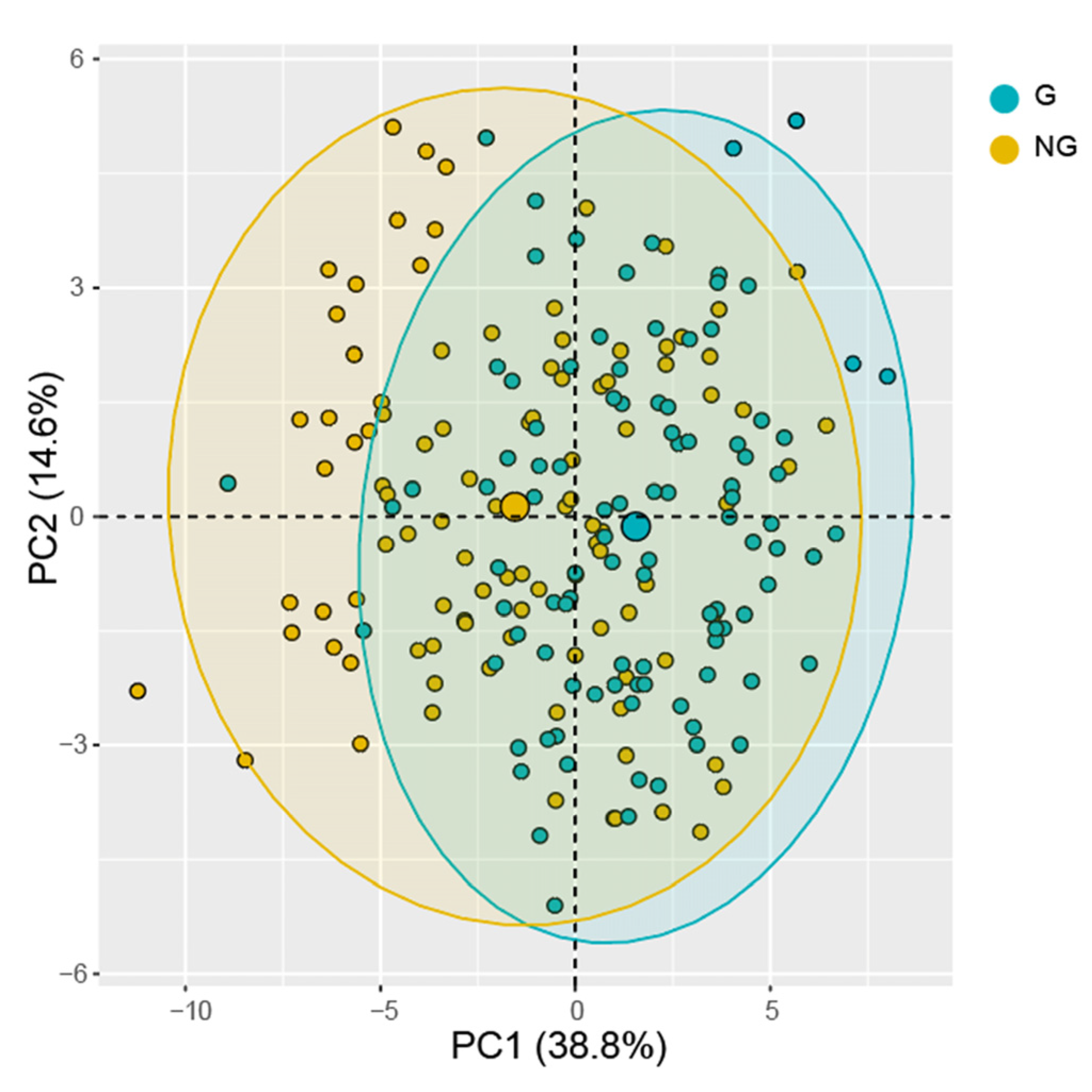
3.3. Multivariate Analysis
3.4. Multivariate Analysis of Germinated Seeds and Non-Germinated Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, F.; Cheng, H.; Chen, L.; Zhu, H.; Mao, P.; Wang, M. Influence of exogenous ascorbic acid and glutathione priming on mitochondrial structural and functional systems to alleviate aging damage in oat seeds. BMC Plant Biol. 2020, 20, 104. [Google Scholar] [CrossRef]
- Neelesh, K.; Arvind, A.; Siddiqui, M.A.; Hirdesh, K.; Asad, A. Physiological and biochemical changes during seed deterioration in aged seeds of rice (Oryza sativa L.). Am. J. Plant Physiol. 2011, 6, 28–35. [Google Scholar]
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Song, P.; Kim, G.; Song, P.; Yang, T.; Yue, X.; Gu, Y. Rapid and non-destructive detection method for water status and water distribution of rice seeds with different vigor. Int. J. Agric. Biol. Eng. 2021, 14, 231–238. [Google Scholar]
- Vujanovic, V.; Arnaud, M.; Barabé, D.; Thibeault, T. Viability testing of orchid seed and the promotion of colouration and germination. Ann. Bot. 2020, 86, 79–86. [Google Scholar] [CrossRef]
- Filho, M.J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- ElMasry, G.; ElGamal, R.; Mandour, N.; Gou, P.; Al-Rejaie, S.; Belin, E.; Rousseau, D. Emerging thermal imaging techniques for seed quality evaluation: Principles and applications. Food. Res. Int. 2020, 131, 109025. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Feng, X.; Sun, D.; Liu, F.; Bao, Y.; He, Y. Rapid and nondestructive measurement of rice seed vitality of different years using near-infrared hyperspectral imaging. Molecules 2019, 24, 2227. [Google Scholar] [CrossRef]
- Kandpal, L.M.; Lohumi, S.; Kim, S.M.; Kang, J.S.; Cho, B.K. Near-infrared hyperspectral imaging system coupled with multivariate methods to predict viability and vigor in muskmelon seeds. Sens. Actuators B Chem. 2016, 229, 534–544. [Google Scholar] [CrossRef]
- Qiu, G.; Lü, E.; Lu, H.; Xu, S.; Zeng, F.; Shui, Q. Single-kernel FT-NIR spectroscopy for detecting supersweet corn (Zea mays L.) seed viability with multivariate data analysis. Sensors 2018, 18, 1010. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, F.A.; Cichy, K.A.; Sprague, C.; Goffnett, A.; Lu, R.; Kelly, J.D. Forecast of canned black bean texture (Phaseolus vulgaris L.) from intact dry seeds using visible/near infrared spectroscopy and hyperspectral imaging data. J. Sci. Food Agric. 2018, 98, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, A.; Lohumi, S.; Lee, W.; Cho, B.K. Comparative nondestructive measurement of corn seed viability using Fourier transform near-infrared (FT-NIR) and Raman spectroscopy. Sens. Actuators B Chem. 2016, 224, 500–506. [Google Scholar] [CrossRef]
- Tigabu, M.; Odén, P.C. Rapid and non-destructive analysis of vigour of Pinus patula seeds using single seed near infrared transmittance spectra and multivariate analysis. Seed Sci. Technol. 2004, 32, 593–606. [Google Scholar] [CrossRef]
- Yang, D.F.; Yin, S.X.; Jiang, L.; Gao, S.R. Research on maize vigor intelligent detection based on near infrared spectroscopy. J. Nucl. Agric. Sci. 2013, 27, 957–961. [Google Scholar]
- Pang, L.; Wang, J.; Men, S.; Yan, L.; Xiao, J. Hyperspectral imaging coupled with multivariate methods for seedvitality estimation and forecast for quercus variabilis. Spectrochim. Acta A 2021, 245, 118888. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, S.; Wu, J.; Zhang, C.; Xu, F.; Yang, X.; Li, J. Application of long-wave near infrared hyperspectral imaging for determination of moisture content of single maize seed. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119666. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Martinez, E.; Diezma, B. Application of hyperspectral imaging in the assessment of drought and salt stress in magneto-primed triticale seeds. Plant 2021, 10, 835. [Google Scholar] [CrossRef]
- Hao, L.; Liu, Z.; Heng, S.; Rao, Z.; Ji, H. Identification of soybean varieties based on hyperspectral imaging technology and one-dimensional convolutional neural network. J. Food Process. Eng. 2021, 11, e13767. [Google Scholar]
- Hu, X.; Yang, L.; Zhang, Z.; Wang, Y. Differentiation of alfalfa and sweet clover seeds via multispectral imaging. Seed Sci. Technol. 2020, 48, 83–99. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.; Lu, X.; Chen, W.; Yang, J.; Zheng, L. Nondestructive determination of transgenic Bacillus thuringiensis rice seeds (Oryza sativa L.) using multispectral imaging and chemometric methods. Food Chem. 2014, 153, 87–93. [Google Scholar] [CrossRef]
- Olesen, M.H.; Nikneshan, P.; Shrestha, S.; Tadayyon, A.; Deleuran, L.C.; Boelt, B.; Gislum, R. Viability prediction of Ricinus cummunis L. seeds using multispectral imaging. Sensor 2015, 15, 4592–4604. [Google Scholar] [CrossRef] [PubMed]
- Baek, I.; Kusumaningrum, D.; Kandpal, L.M.; Lohumi, S.; Mo, C.; Kim, M.S.; Cho, B. Rapid measurement of soybean seed viability using kernel-based multispectral image analysis. Sensor 2019, 19, 271. [Google Scholar] [CrossRef]
- Rego, C.H.Q.; Franca-Silva, F.; Gomes-Junior, F.G.; Moraes, M.H.D.; de Medeiros, A.D.; Da Silva, C.B. Using multispectral imaging for detecting seed-borne fungi in cowpea. Agriculture 2020, 10, 361. [Google Scholar] [CrossRef]
- Vresak, M.; Olesen, M.H.; Gislum, R.; Bavec, F.; Jorgensen, J.R. The use of image-spectroscopy technology as a diagnostic method for seed health testing and variety identification. PLoS ONE 2016, 11, e01520113. [Google Scholar] [CrossRef]
- Lei, X.; Liu, W.; Zhao, J.; You, M.; Xiong, C.; Xiong, Y.; Xiong, Y.; Yu, Q.; Bai, S.; Ma, X. Comparative physiological and proteomic analysis reveals different involvement of proteins during artificial aging of Siberian wildrye seeds. Plant 2020, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Pornpanomchai, C.; Jongsriwattanaporn, S.; Pattanakul, T.; Suriyun, W. Image analysis on color and texture for chili (Capsicum frutescence) seed germination. Sci. Eng. Health Stud. 2020, 14, 169–183. [Google Scholar]
- Shetty, N.; Olesen, M.H.; Gislum, R.; Deleuran, L.C.; Boelt, B. Use of partial least squares discriminant analysis on visible-near infrared multispectral image data to examine germination ability and germ length in spinach seeds. J. Chem. 2012, 26, 462–466. [Google Scholar] [CrossRef]
- United States Department of Agriculture. 2017 census of agriculture united states summa and state data. NASS 2019, 8, 515–526. [Google Scholar]
- National Livestock Station. China Grassland Statistical Yearbook 2017; China Agriculture Press: Beijing, China, 2018; pp. 100–113. [Google Scholar]
- Shi, S.; Nan, L.; Smith, K. The current status, problems, and prospects of alfalfa (Medicago sativa L.) Breeding in China. Agronomy 2017, 7, 1. [Google Scholar] [CrossRef]
- Hu, X.; Yang, L.; Zhang, Z. Non-destructive identification of single hard seed via multispectral imaging analysis in six legume species. Plant Methods 2020, 16, 116. [Google Scholar] [CrossRef]
- ElMasry, G.; Mandour, N.; Al-Rejaie, S.; Belin, E.; Rousseau, D. Recent applications of multispectral imaging in seed phenotyping and quality monitoring an overview. Sensor 2019, 19, 1090. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhu, X.; Zhu, Y.; Gan, J. Robust SVM with adaptive graph learning. World Wide Web 2020, 23, 1945–1968. [Google Scholar] [CrossRef]
- Lurstwut, B.; Pornpanomchai, C. Image analysis based on color, shape and texture for rice seed (Oryza sativa L.) germination evaluation. Agric. Nat. Resour. 2017, 51, 383–389. [Google Scholar] [CrossRef]
- Devos, O.; Ruckebusch, C.; Durand, A.; Duponchel, L.; Huvenne, J.P. Support vector machines (SVM) in near infrared (NIR) spectroscopy: Focus on parameters optimization and model interpretation. Chemom. Intell. Lab. Syst. 2009, 96, 27–33. [Google Scholar] [CrossRef]
- Tang, Y.; Dananjayan, S.; Hou, C.; Guo, Q.; Luo, S.; He, Y. A survey on the 5G network and its impact on agriculture: Challenges and opportunities. Comput. Electron. Agric. 2021, 180, 105895. [Google Scholar] [CrossRef]
- Boelt, B.; Shrestha, S.; Salimi, Z.; Jorgensen, J.R.; Nicolaisen, M.; Carstensen, J.M. Multispectral imaging-a new tool in seed quality assessment? Seed Sci. Res. 2018, 28, 222–228. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Hu, X. Cultivar discrimination of single alfalfa (Medicago sativa L.) seed via multispectral imaging combined with multivariate analysis. Sensor 2020, 20, 6575. [Google Scholar] [CrossRef]
- Shrestha, S.; Deleuran, L.C.; Gislum, R. Classification of different tomato seed cultivars by multispectral visible-near infrared spectroscopy and chemometrics. J. Spectr. Imaging 2016, 5, a1. [Google Scholar] [CrossRef]
- ElMasry, G.; Mandour, N.; Wagner, M.; Demilly, D.; Verdier, J.; Belin, E.; Rousseau, D. Utilization of computer vision and multispectral imaging techniques for classification of cowpea (Vigna unguiculata) seeds. Plant Methods 2019, 15, 24. [Google Scholar] [CrossRef]
- Salimi, Z.; Boelt, B. Classification of processing damage in sugar beet (Beta vulgaris) seeds by multispectral image analysis. Sensor 2019, 19, 2360. [Google Scholar] [CrossRef]
- Weng, H.; Tian, Y.; Wu, N.; Li, X.; Yang, B.; Huang, Y.; Ye, D.; Wu, R. Development of a low-cost narrow band multispectral imaging system coupled with chemometric analysis for rapid detection of rice false smut in rice seed. Sensor 2020, 20, 1209. [Google Scholar] [CrossRef] [PubMed]
- Martins Bianchini, V.D.J.; Mascarin, G.M.; Aparecida Santos Silva, L.C.; Arthur, V.; Carstensen, J.M.; Boelt, B.; Da Silva, C.B. Multispectral and X-ray images for characterization of Jatropha curcas L. seed quality. Plant Methods 2021, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Franca-Silva, F.; Queiroz Rego, C.H.; Guilhien Gomes-Junior, F.; Duarte De Moraes, M.H.; de Medeiros, A.D.; Da Silva, C.B. Detection of drechslera avenae (eidam) Sharif in black oat seeds (Avena strigosa) using multispectral imaging. Sensor 2020, 20, 3343. [Google Scholar] [CrossRef] [PubMed]
- Witjes, H.; Rijpkema, M.; van der Graaf, M.; Melssen, W.; Heerschap, A.; Buydens, L. Multispectral magnetic resonance image analysis using principal component and linear discriminant analysis. J. Magn. Reson. Imaging 2003, 17, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.K.; Gislum, R.; Jørgensen, J.R.; Boelt, B. The use of multispectral imaging and single seed and bulk near-infrared spectroscopy to characterize seed covering structures: Methods and applications in seed testing and research. Agriculture 2021, 11, 301. [Google Scholar] [CrossRef]
- Al-Turki, T.A.; Baskin, C.C. Determination of seed viability of eight wild saudi arabian species by germination and X-ray tests. Saudi J. Biol. Sci. 2017, 24, 822–829. [Google Scholar] [CrossRef][Green Version]
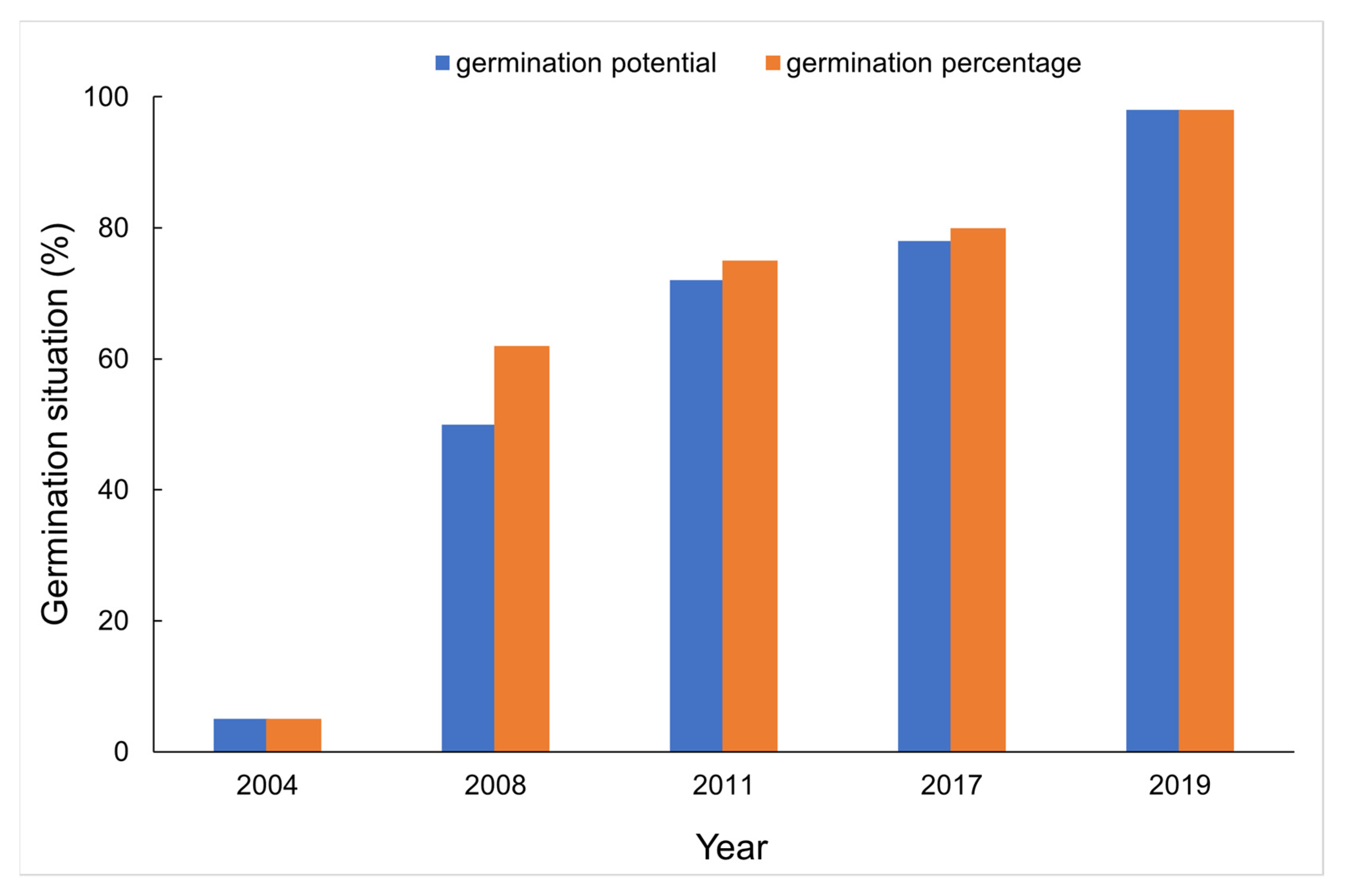
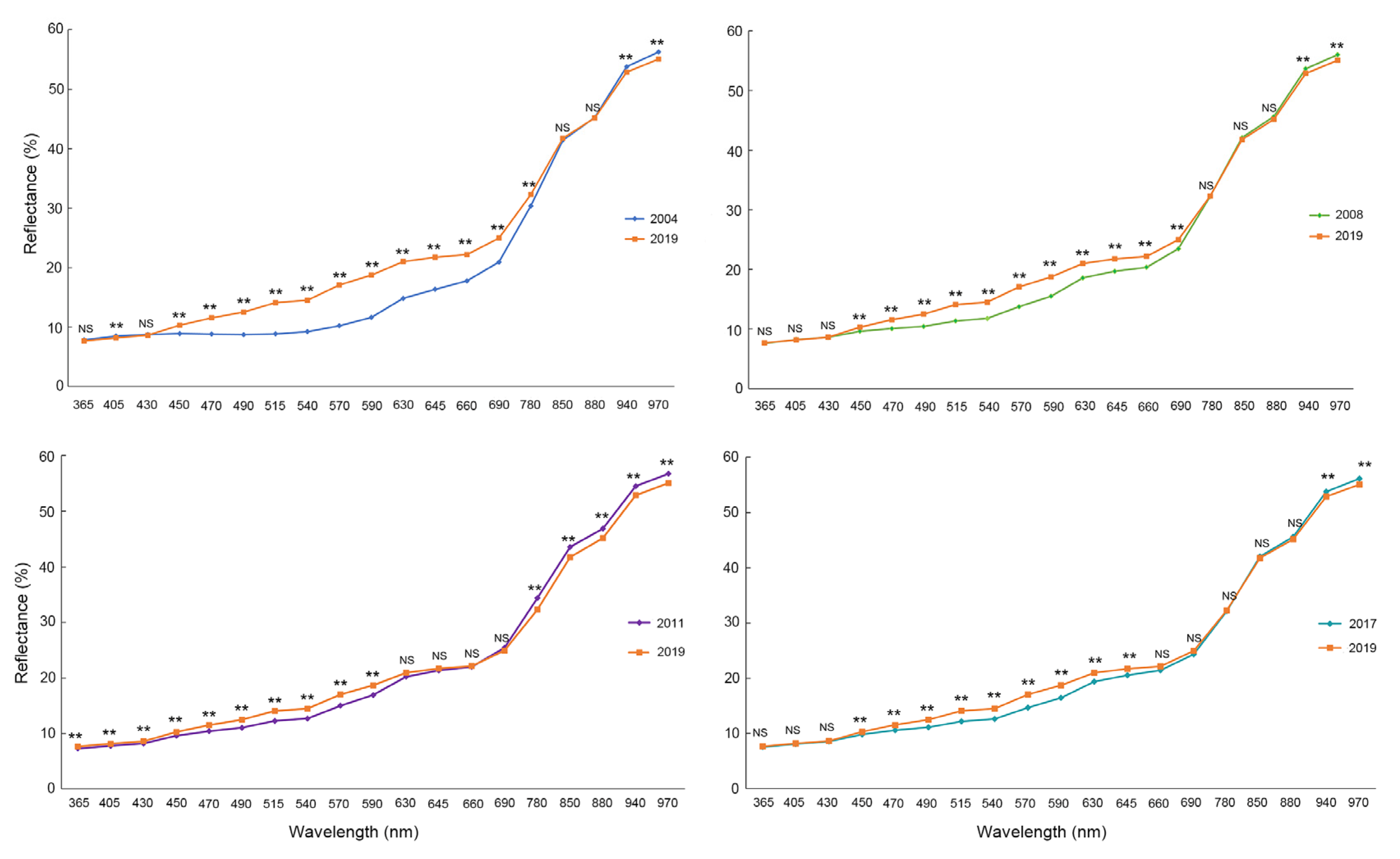

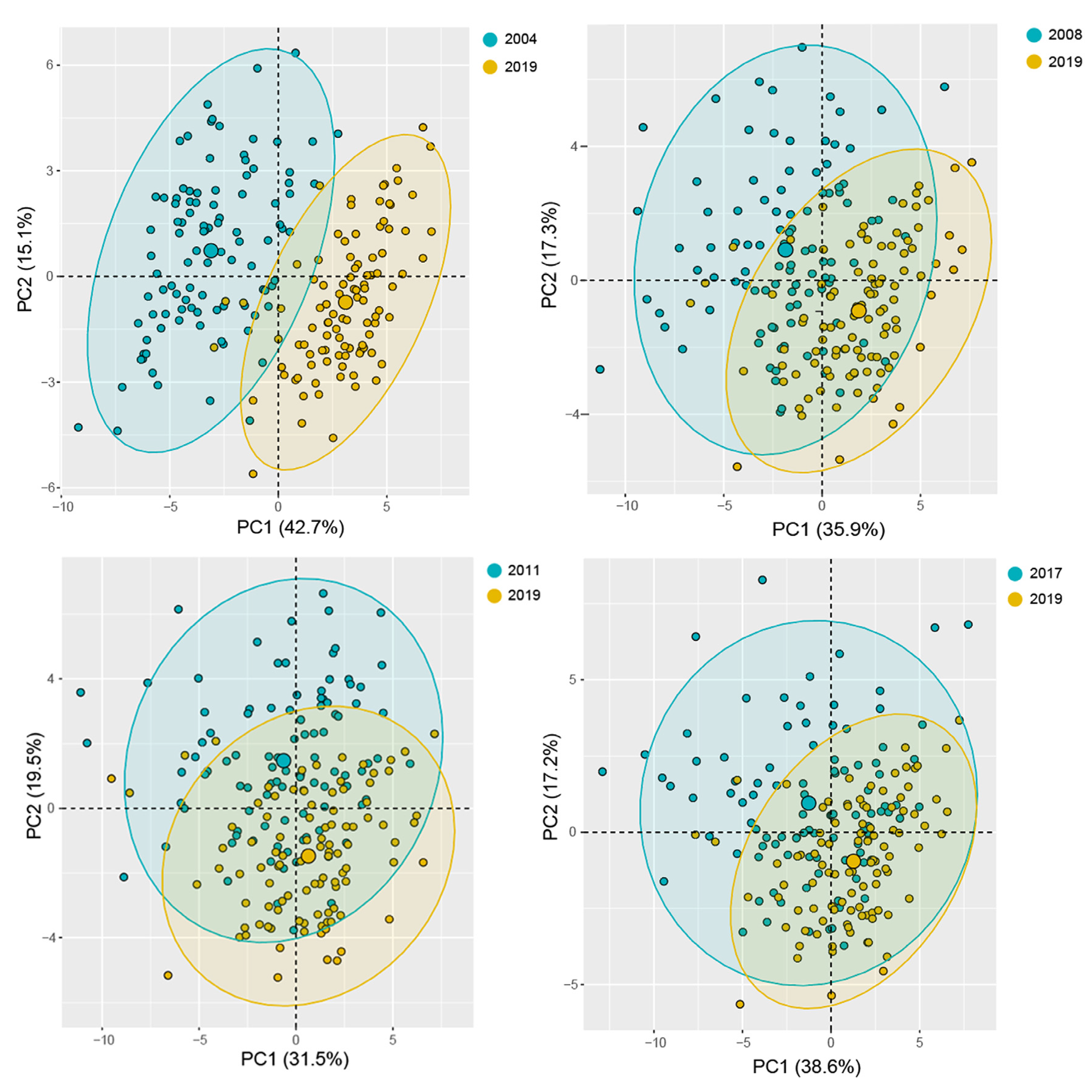
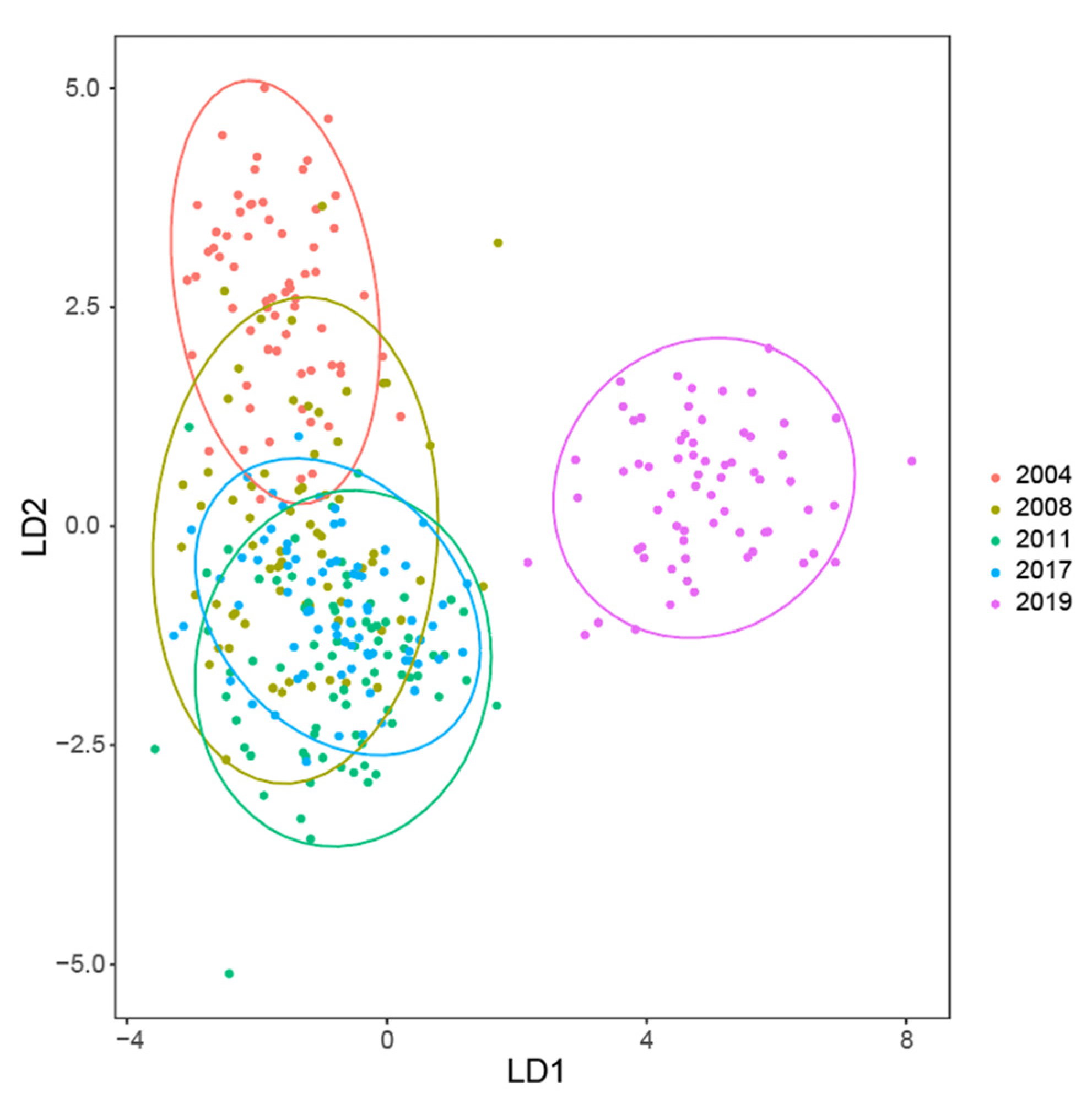

| Feature | 2019 (CK) | 2004 | 2008 | 2011 | 2017 |
|---|---|---|---|---|---|
| Area (mm2) | 2.85 ± 0.42 | 3.32 ± 0.51 ** | 3.27 ± 0.58 ** | 3.25 ± 0.41 ** | 3.41 ± 0.62 ** |
| Length (mm) | 2.39 ± 0.25 | 2.6 ± 0.24 ** | 2.62 ± 0.29 ** | 2.56 ± 0.22 ** | 2.68 ± 0.27 ** |
| Width (mm) | 1.59 ± 0.12 | 1.69 ± 0.15 ** | 1.65 ± 0.17 ** | 1.68 ± 0.12 ** | 1.7 ± 0.15 ** |
| RatioWidthLength | 0.67 ± 0.07 | 0.65 ± 0.06 | 0.64 ± 0.06 ** | 0.66 ± 0.06 | 0.64 ± 0.05 ** |
| Compactness Circle | 0.65 ± 0.07 | 0.63 ± 0.06 * | 0.61 ± 0.07 ** | 0.64 ± 0.06 | 0.61 ± 0.05 ** |
| Compactness Ellipse | 0.99 ± 0.01 | 0.99 ± 0.01 * | 0.98 ± 0.01 ** | 0.99 ± 0.01 | 0.99 ± 0.01 ** |
| BetaShape_a | 1.53 ± 0.13 | 1.5 ± 0.14 | 1.46 ± 0.14 ** | 1.51 ± 0.13 | 1.47 ± 0.11 ** |
| BetaShape_b | 1.46 ± 0.14 | 1.42 ± 0.13 | 1.39 ± 0.12 ** | 1.45 ± 0.11 | 1.4 ± 0.11 ** |
| Vertical Skewness | −0.04 ± 0.03 | −0.04 ± 0.03 | −0.04 ± 0.03 | −0.03 ± 0.03 | −0.04 ± 0.03 |
| CIELab L* | 48.62 ± 3.39 | 35 ± 4.37 ** | 42.94 ± 4.63 ** | 45.23 ± 4.74 ** | 44.71 ± 4.91 ** |
| CIELab A* | 9.67 ± 1.77 | 17.21 ± 1.99 ** | 14.19 ± 2.79 ** | 13.61 ± 3.18 ** | 13.01 ± 2.62 ** |
| CIELab B* | 31.34 ± 2.46 | 21.94 ± 4.83 ** | 28.33 ± 3.42 ** | 31.34 ± 3.12 | 29.67 ± 2.87 ** |
| Saturation | 32.64 ± 2.21 | 28.44 ± 4.36 ** | 31.84 ± 2.71 * | 34.27 ± 2.8 ** | 32.47 ± 2.41 |
| Hue | 1.27 ± 0.06 | 0.91 ± 0.08 ** | 1.11 ± 0.1 ** | 1.16 ± 0.1 ** | 1.16 ± 0.09 * |
| Model | Index | 2004 vs. 2019 | 2008 vs. 2019 | 2011 vs. 2019 | 2017 vs. 2019 | G vs. NG |
|---|---|---|---|---|---|---|
| SVM | Accuracy (%) | 99.3 | 91.3 | 90.9 | 87.4 | 72.0 |
| Sensitivity (%) | 99.6 | 94.0 | 93.0 | 87.6 | 69.5 | |
| Specificity (%) | 99.0 | 88.8 | 88.8 | 87.3 | 74.4 | |
| RF | Accuracy (%) | 99.3 | 89.6 | 85.5 | 84.6 | 69.7 |
| Sensitivity (%) | 99.7 | 92.3 | 86.7 | 85.6 | 66.5 | |
| Specificity (%) | 98.0 | 86.6 | 84.3 | 83.7 | 72.8 | |
| LDA | Accuracy (%) | 100.0 | 100.0 | 100.0 | 99.8 | 97.6 |
| Sensitivity (%) | 100.0 | 100.0 | 100.0 | 99.7 | 96.5 | |
| Specificity (%) | 100.0 | 100.0 | 100.0 | 100.0 | 98.7 |
| Model | Data | 2004 vs. 2019 | 2008 vs. 2019 | 2011 vs. 2019 | 2017 vs. 2019 | G vs. NG |
|---|---|---|---|---|---|---|
| LDA | morphological | 99.2 | 87.7 | 82.2 | 79.7 | 71.7 |
| spectral | 99.4 | 99.4 | 99.3 | 98.4 | 73.1 | |
| morphological+spectral | 100 | 100 | 100 | 99.8 | 97.6 | |
| SVM | morphological | 99.2 | 82.8 | 79.9 | 76.8 | 69.0 |
| spectral | 98.8 | 89.8 | 87.7 | 87.3 | 68.4 | |
| morphological+spectral | 99.3 | 91.3 | 90.9 | 87.4 | 72.0 | |
| RF | morphological | 98.6 | 84.8 | 82.4 | 76.8 | 74.5 |
| spectral | 95.1 | 82.2 | 78.6 | 71.6 | 64.8 | |
| morphological+spectral | 99.3 | 89.6 | 85.5 | 84.6 | 69.7 |
| Sample | Non-Germinated Seeds | Non-Germinated Seeds Predicted by nCDA | Correctly Predicted Seeds | Accuracy of Predicting Non-Germinated Seeds (%) |
|---|---|---|---|---|
| 2004 | 95 | 96 | 95 | 99 |
| 2008 | 38 | 36 | 29 | 76 |
| 2011 | 25 | 19 | 19 | 76 |
| 2017 | 20 | 15 | 15 | 75 |
| 2019 | 2 | 2 | 2 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, H.; Song, R.; He, X.; Mao, P.; Jia, S. Non-Destructive Identification of Naturally Aged Alfalfa Seeds via Multispectral Imaging Analysis. Sensors 2021, 21, 5804. https://doi.org/10.3390/s21175804
Wang X, Zhang H, Song R, He X, Mao P, Jia S. Non-Destructive Identification of Naturally Aged Alfalfa Seeds via Multispectral Imaging Analysis. Sensors. 2021; 21(17):5804. https://doi.org/10.3390/s21175804
Chicago/Turabian StyleWang, Xuemeng, Han Zhang, Rui Song, Xin He, Peisheng Mao, and Shangang Jia. 2021. "Non-Destructive Identification of Naturally Aged Alfalfa Seeds via Multispectral Imaging Analysis" Sensors 21, no. 17: 5804. https://doi.org/10.3390/s21175804
APA StyleWang, X., Zhang, H., Song, R., He, X., Mao, P., & Jia, S. (2021). Non-Destructive Identification of Naturally Aged Alfalfa Seeds via Multispectral Imaging Analysis. Sensors, 21(17), 5804. https://doi.org/10.3390/s21175804






