Carbon Nanotube Wearable Sensors for Health Diagnostics
Abstract
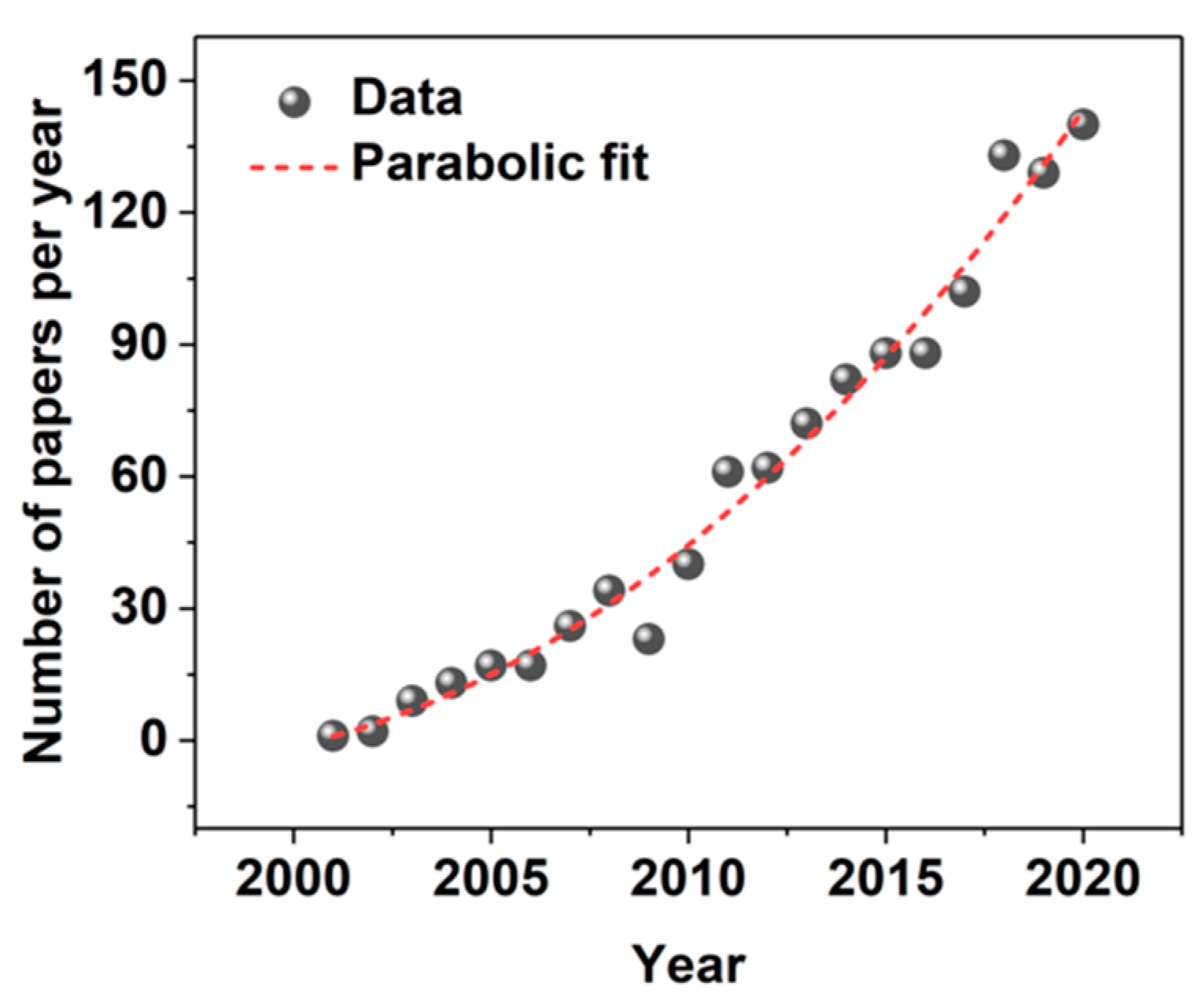
:1. Introduction
2. Formation of Wearable Sensors from CNTs
3. The Opportunities Offered by Wearable Sensors from CNTs
3.1. Glucose Level
3.2. ECG
3.3. EEG
3.4. EMG
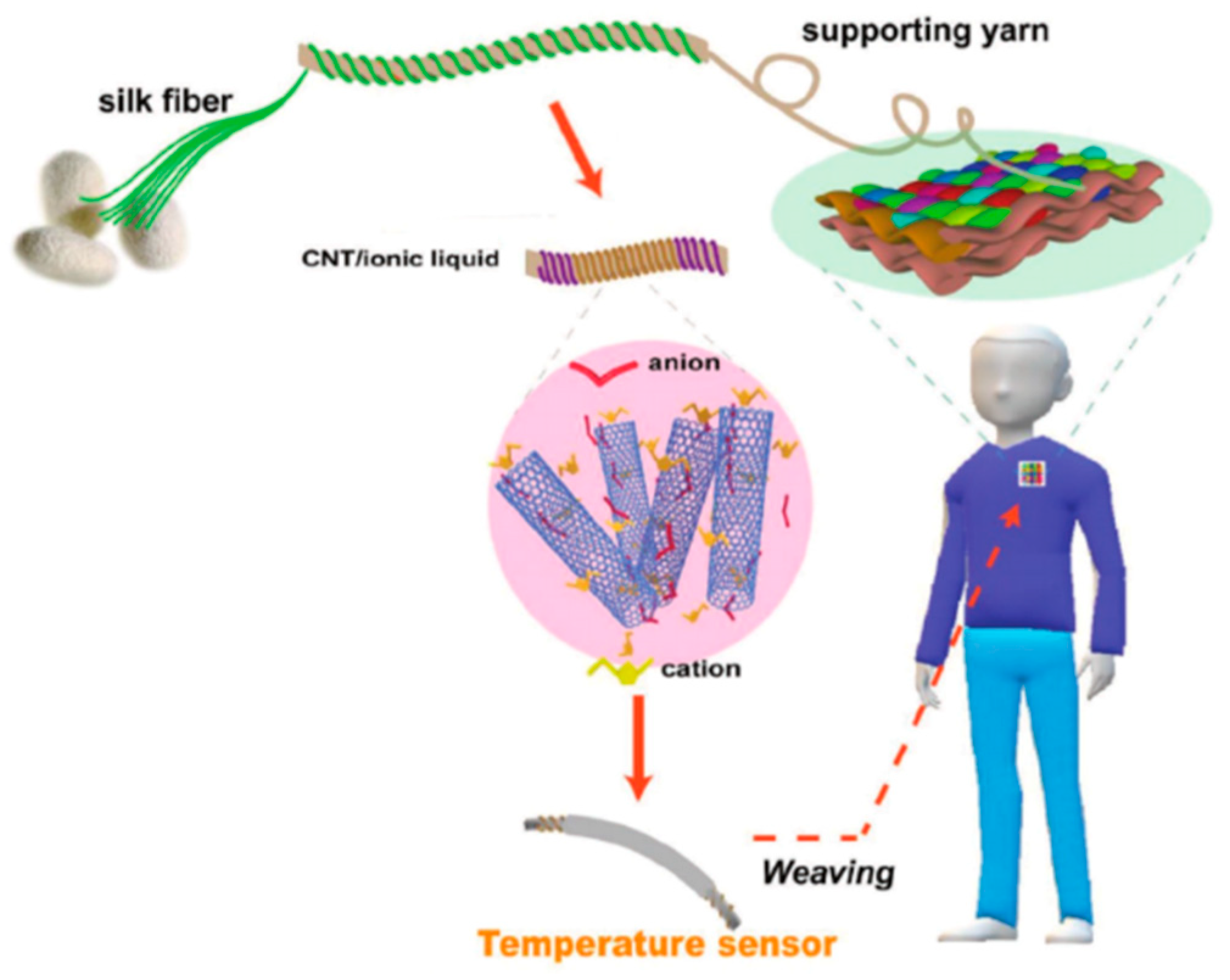
3.5. Temperature
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Monthioux, M.; Kuznetsov, V.L. Who Should Be given the Credit for the Discovery of Carbon Nanotubes? Carbon 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A Review on Carbon Nanotubes and Graphene as Fillers in Reinforced Polymer Nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, G.J. Electrical Conductivity of Carbon Nanotube- and Graphene-Based Nanocomposites. In Micromechanics and Nanomechanics of Composite Solids; Meguid, S.A., Weng, G.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 123–156. ISBN 978-3-319-52794-9. [Google Scholar]
- Arjmand, M.; Chizari, K.; Krause, B.; Pötschke, P.; Sundararaj, U. Effect of Synthesis Catalyst on Structure of Nitrogen-Doped Carbon Nanotubes and Electrical Conductivity and Electromagnetic Interference Shielding of Their Polymeric Nanocomposites. Carbon 2016, 98, 358–372. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Abdi, Y.; Mohajerzadeh, S. Silver Nanoparticles within Vertically Aligned Multi-Wall Carbon Nanotubes with Open Tips for Antibacterial Purposes. J. Mater. Chem. 2011, 21, 387–393. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of Carbon Nanotubes in Drug Delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shahsavar, M. Graphene Nanogrids for Selective and Fast Osteogenic Differentiation of Human Mesenchymal Stem Cells. Carbon 2013, 59, 200–211. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Differentiation of Human Neural Stem Cells into Neural Networks on Graphene Nanogrids. J. Mater. Chem. B 2013, 1, 6291–6301. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal Conductivity of Carbon Nanotube Networks: A Review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef] [Green Version]
- Hone, J.; Llaguno, M.C.; Biercuk, M.J.; Johnson, A.T.; Batlogg, B.; Benes, Z.; Fischer, J.E. Thermal Properties of Carbon Nanotubes and Nanotube-Based Materials. Appl. Phys. A 2002, 74, 339–343. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal Properties of Graphene and Nanostructured Carbon Materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Janas, D. Perfectly Imperfect: A Review of Chemical Tools for Exciton Engineering in Single-Walled Carbon Nanotubes. Mater. Horiz. 2020, 7, 2860–2881. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical Properties of Single-Wall Carbon Nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Muz, İ.; Kurban, M. A Comprehensive Study on Electronic Structure and Optical Properties of Carbon Nanotubes with Doped B, Al, Ga, Si, Ge, N, P and As and Different Diameters. J. Alloy. Compd. 2019, 802, 25–35. [Google Scholar] [CrossRef]
- Vijayan, B.K.; Dimitrijevic, N.M.; Finkelstein-Shapiro, D.; Wu, J.; Gray, K.A. Coupling Titania Nanotubes and Carbon Nanotubes to Create Photocatalytic Nanocomposites. ACS Catal. 2012, 2, 223–229. [Google Scholar] [CrossRef]
- Mayyahi, A.A.; Everhart, B.M.; Shrestha, T.B.; Back, T.C.; Amama, P.B. Enhanced Charge Separation in TiO2/Nanocarbon Hybrid Photocatalysts through Coupling with Short Carbon Nanotubes. RSC Adv. 2021, 11, 11702–11713. [Google Scholar] [CrossRef]
- Yakobson, B.I.; Avouris, P. Mechanical Properties of Carbon Nanotubes. In Carbon Nanotubes: Synthesis, Structure, Properties, and Applications; Dresselhaus, M.S., Dresselhaus, G., Avouris, P., Eds.; Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2001; pp. 287–327. ISBN 978-3-540-39947-6. [Google Scholar]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but Strong: A Review of the Mechanical Properties of Carbon Nanotube–Polymer Composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Buongiorno Nardelli, M.; Fattebert, J.-L.; Orlikowski, D.; Roland, C.; Zhao, Q.; Bernholc, J. Mechanical Properties, Defects and Electronic Behavior of Carbon Nanotubes. Carbon 2000, 38, 1703–1711. [Google Scholar] [CrossRef]
- The Global Carbon Nanotubes (CNT) Market (2018–2023) is Projected to Grow at a CAGR of 16.7%—Technological Advancements and Decreasing Production Cost is Driving Growth. Available online: https://www.prnewswire.com/news-releases/the-global-carbon-nanotubes-cnt-market-2018-2023-is-projected-to-grow-at-a-cagr-of-16-7---technological-advancements-and-decreasing-production-cost-is-driving-growth-300752102.html (accessed on 27 July 2021).
- Global Carbon Nanotube Market to Grow to Around 4000 Tonnes by 2023. Available online: https://www.plasticstoday.com/Materials/Global-Carbon-Nanotube-Market-Grow-around-4000-Tonnes-2023 (accessed on 27 July 2021).
- Kaempgen, M.; Duesberg, G.S.; Roth, S. Transparent Carbon Nanotube Coatings. Appl. Surf. Sci. 2005, 252, 425–429. [Google Scholar] [CrossRef]
- Thomas, B.J.C.; Boccaccini, A.R.; Shaffer, M.S.P. Multi-Walled Carbon Nanotube Coatings Using Electrophoretic Deposition (EPD). J. Am. Ceram. Soc. 2005, 88, 980–982. [Google Scholar] [CrossRef]
- Harris, P.J.F. Carbon Nanotube Composites. Int. Mater. Rev. 2004, 49, 31–43. [Google Scholar] [CrossRef]
- Lau, A.K.-T.; Hui, D. The Revolutionary Creation of New Advanced Materials—Carbon Nanotube Composites. Compos. Part. B Eng. 2002, 33, 263–277. [Google Scholar] [CrossRef]
- Biercuk, M.J.; Llaguno, M.C.; Radosavljevic, M.; Hyun, J.K.; Johnson, A.T.; Fischer, J.E. Carbon Nanotube Composites for Thermal Management. Appl. Phys. Lett. 2002, 80, 2767–2769. [Google Scholar] [CrossRef]
- Avouris, P. Carbon Nanotube Electronics. Chem. Phys. 2002, 281, 429–445. [Google Scholar] [CrossRef]
- Peng, L.-M.; Zhang, Z.; Wang, S. Carbon Nanotube Electronics: Recent Advances. Mater. Today 2014, 17, 433–442. [Google Scholar] [CrossRef]
- Chen, K.; Gao, W.; Emaminejad, S.; Kiriya, D.; Ota, H.; Nyein, H.Y.Y.; Takei, K.; Javey, A. Printed Carbon Nanotube Electronics and Sensor Systems. Adv. Mater. 2016, 28, 4397–4414. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Yabuuchi, N.; Gallant, B.M.; Chen, S.; Kim, B.-S.; Hammond, P.T.; Shao-Horn, Y. High-Power Lithium Batteries from Functionalized Carbon-Nanotube Electrodes. Nat. Nanotech. 2010, 5, 531–537. [Google Scholar] [CrossRef]
- Zheng, M.; Chi, Y.; Hu, Q.; Tang, H.; Jiang, X.; Zhang, L.; Zhang, S.; Pang, H.; Xu, Q. Carbon Nanotube-Based Materials for Lithium–Sulfur Batteries. J. Mater. Chem. A 2019, 7, 17204–17241. [Google Scholar] [CrossRef]
- Guo, B.; Wang, X.; Fulvio, P.F.; Chi, M.; Mahurin, S.M.; Sun, X.-G.; Dai, S. Soft-Templated Mesoporous Carbon-Carbon Nanotube Composites for High Performance Lithium-Ion Batteries. Adv. Mater. 2011, 23, 4661–4666. [Google Scholar] [CrossRef]
- Gyamfi, K.S.; Brusey, J.; Gaura, E.; Wilkins, R. Heartbeat Design for Energy-Aware IoT: Are Your Sensors Alive? Expert Syst. Appl. 2019, 128, 124–139. [Google Scholar] [CrossRef]
- Marín-Morales, J.; Higuera-Trujillo, J.L.; Greco, A.; Guixeres, J.; Llinares, C.; Scilingo, E.P.; Alcañiz, M.; Valenza, G. Affective Computing in Virtual Reality: Emotion Recognition from Brain and Heartbeat Dynamics Using Wearable Sensors. Sci. Rep. 2018, 8, 13657. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, G.; Kaur, D. Infant Monitoring System Using Wearable Sensors Based on Blood Oxygen Saturation: A Review. In Proceedings of the Intelligent, Secure, and Dependable Systems in Distributed and Cloud Environments; Traore, I., Woungang, I., Awad, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 162–168. [Google Scholar]
- Davies, H.J.; Williams, I.; Peters, N.S.; Mandic, D.P. In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation. Sensors 2020, 20, 4879. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tang, L. A Wearable Blood Oxygen Saturation Monitoring System Based on Bluetooth Low Energy Technology. Comput. Commun. 2020, 160, 101–110. [Google Scholar] [CrossRef]
- Alfeo, A.L.; Barsocchi, P.; Cimino, M.G.C.A.; La Rosa, D.; Palumbo, F.; Vaglini, G. Sleep Behavior Assessment via Smartwatch and Stigmergic Receptive Fields. Pers Ubiquit Comput. 2018, 22, 227–243. [Google Scholar] [CrossRef]
- Saleem, K.; Bajwa, I.S.; Sarwar, N.; Anwar, W.; Ashraf, A. IoT Healthcare: Design of Smart and Cost-Effective Sleep Quality Monitoring System. J. Sens. 2020, 2020, e8882378. [Google Scholar] [CrossRef]
- Kubley, A.; Chauhan, D.; Kanakaraj, S.N.; Shanov, V.; Xu, C.; Chen, R.; Ng, V.; Bell, G.; Verma, P.; Hou, X.; et al. Chapter 12—Smart Textiles and Wearable Technology Innovation with Carbon Nanotube Technology. In Nanotube Superfiber Materials, 2nd ed.; Schulz, M.J., Shanov, V., Yin, Z., Cahay, M., Eds.; Micro and Nano Technologies; William Andrew Publishing: Amsterdam, The Netherlands, 2019; pp. 263–311. ISBN 978-0-12-812667-7. [Google Scholar]
- Wang, G.; Wang, Y.; Luo, Y.; Luo, S. Carbon Nanomaterials Based Smart Fabrics with Selectable Characteristics for In-Line Monitoring of High-Performance Composites. Materials 2018, 11, 1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart Electronic Yarns and Wearable Fabrics for Human Biomonitoring Made by Carbon Nanotube Coating with Polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Liu, M.; Zhu, D.; Gan, L.; Chen, T. Nanocarbon-Based Materials for Flexible All-Solid-State Supercapacitors. Adv. Mater. 2018, 30, 1705489. [Google Scholar] [CrossRef]
- Bao, W.; Pickel, A.D.; Zhang, Q.; Chen, Y.; Yao, Y.; Wan, J.; Fu, K.; Wang, Y.; Dai, J.; Zhu, H.; et al. Flexible, High Temperature, Planar Lighting with Large Scale Printable Nanocarbon Paper. Adv. Mater. 2016, 28, 4684–4691. [Google Scholar] [CrossRef]
- Cui, X.; Tian, J.; Zhang, C.; Cai, R.; Ma, J.; Yang, Z.; Meng, Q. Comparative Study of Nanocarbon-Based Flexible Multifunctional Composite Electrodes. ACS Omega 2021, 6, 2526–2541. [Google Scholar] [CrossRef]
- Gao, Y.; Shen Zhou, Y.; Qian, M.; Ming Li, H.; Redepenning, J.; Sha Fan, L.; Nan He, X.; Xiong, W.; Huang, X.; Majhouri-Samani, M.; et al. High-Performance Flexible Solid-State Supercapacitors Based on MnO 2 -Decorated Nanocarbon Electrodes. RSC Adv. 2013, 3, 20613–20618. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Song, Q.; Li, K.; Yin, X.; Sun, J.; Li, H.; Zhang, F.; Ren, X.; Wang, X. Hierarchical, Seamless, Edge-Rich Nanocarbon Hybrid Foams for Highly Efficient Electromagnetic-Interference Shielding. J. Mater. Sci. Technol. 2021, 72, 154–161. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Li, Y.; Chu, B.T.T.; Kuo, I.-T.; Yip, M.; Tai, N. Porous Composites Coated with Hybrid Nano Carbon Materials Perform Excellent Electromagnetic Interference Shielding. Compos. Part. B Eng. 2015, 70, 231–237. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical Conductivity of Individual Carbon Nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon Nanotube Sensors for Gas and Organic Vapor Detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene -Based Gas Sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- He, Q.; Wu, S.; Yin, Z.; Zhang, H. Graphene -Based Electronic Sensors. Chem. Sci. 2012, 3, 1764–1772. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dong, X.; Chen, P. Biological and Chemical Sensors Based on Graphene Materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef]
- Ejehi, F.; Mohammadpour, R.; Asadian, E.; Sasanpour, P.; Fardindoost, S.; Akhavan, O. Graphene Oxide Papers in Nanogenerators for Self-Powered Humidity Sensing by Finger Tapping. Sci. Rep. 2020, 10, 7312. [Google Scholar] [CrossRef]
- Besteman, K.; Lee, J.-O.; Wiertz, F.G.M.; Heering, H.A.; Dekker, C. Enzyme-Coated Carbon Nanotubes as Single-Molecule Biosensors. Nano Lett. 2003, 3, 727–730. [Google Scholar] [CrossRef]
- Guo, X. Single-Molecule Electrical Biosensors Based on Single-Walled Carbon Nanotubes. Adv. Mater. 2013, 25, 3397–3408. [Google Scholar] [CrossRef] [PubMed]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon Nanotube Biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nißler, R.; Bader, O.; Dohmen, M.; Walter, S.G.; Noll, C.; Selvaggio, G.; Groß, U.; Kruss, S. Remote near Infrared Identification of Pathogens with Multiplexed Nanosensors. Nat. Commun. 2020, 11, 5995. [Google Scholar] [CrossRef]
- Mann, F.A.; Herrmann, N.; Opazo, F.; Kruss, S. Quantum Defects as a Toolbox for the Covalent Functionalization of Carbon Nanotubes with Peptides and Proteins. Angew. Chem. Int. Ed. 2020, 59, 17732–17738. [Google Scholar] [CrossRef]
- Ling, Y.; An, T.; Yap, L.W.; Zhu, B.; Gong, S.; Cheng, W. Disruptive, Soft, Wearable Sensors. Adv. Mater. 2020, 32, 1904664. [Google Scholar] [CrossRef]
- Singh, K.; Sharma, S.; Shriwastava, S.; Singla, P.; Gupta, M.; Tripathi, C.C. Significance of Nano-Materials, Designs Consideration and Fabrication Techniques on Performances of Strain Sensors—A Review. Mater. Sci. Semicond. Process. 2021, 123, 105581. [Google Scholar] [CrossRef]
- Ma, C.; Ma, M.-G.; Si, C.; Ji, X.-X.; Wan, P. Flexible MXene-Based Composites for Wearable Devices. Adv. Funct. Mater. 2021, 31, 2009524. [Google Scholar] [CrossRef]
- Pang, Y.; Yang, Z.; Yang, Y.; Ren, T.-L. Wearable Electronics Based on 2D Materials for Human Physiological Information Detection. Small 2020, 16, 1901124. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K. A Review of Production Methods of Carbon Nanotube and Graphene Thin Films for Electrothermal Applications. Nanoscale 2014, 6, 3037–3045. [Google Scholar] [CrossRef]
- Priya, B.R.; Byrne, H.J. Investigation of Sodium Dodecyl Benzene Sulfonate Assisted Dispersion and Debundling of Single-Wall Carbon Nanotubes. J. Phys. Chem. C 2008, 112, 332–337. [Google Scholar] [CrossRef]
- Arnold, M.S.; Green, A.A.; Hulvat, J.F.; Stupp, S.I.; Hersam, M.C. Sorting Carbon Nanotubes by Electronic Structure Using Density Differentiation. Nat. Nanotechnol. 2006, 1, 60–65. [Google Scholar] [CrossRef]
- Poorgholami-Bejarpasi, N.; Sohrabi, B. Self-Assembly of Cationic Surfactants on the Carbon Nanotube Surface: Insights from Molecular Dynamics Simulations. J. Mol. Model. 2013, 19, 4319–4335. [Google Scholar] [CrossRef] [PubMed]
- Zueva, O.S.; Kusova, A.M.; Makarova, A.O.; Turanov, A.; Iskhakova, A.; Salnikov, V.V.; Zuev, Y.F. Reciprocal Effects of Multi-Walled Carbon Nanotubes and Oppositely Charged Surfactants in Bulk Water and at Interfaces. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125296. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, M.Y.; Li, J.; Shi, X.M.; Kim, J.K. Effects of Surfactant Treatment on Mechanical and Electrical Properties of CNT/Epoxy Nanocomposites. Compos. Part. A Appl. Sci. Manuf. 2008, 39, 1876–1883. [Google Scholar] [CrossRef]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually Suspended Single-Walled Carbon Nanotubes in Various Surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Figueiredo, D.T.R.; Correia, A.A.S.; Hunkeler, D.; Rasteiro, M.G.B.V. Surfactants for Dispersion of Carbon Nanotubes Applied in Soil Stabilization. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Borode, A.O.; Ahmed, N.A.; Olubambi, P.A. Surfactant-Aided Dispersion of Carbon Nanomaterials in Aqueous Solution. Phys. Fluids 2019, 31, 071301. [Google Scholar] [CrossRef]
- Lemasson, F.A.; Strunk, T.; Gerstel, P.; Hennrich, F.; Lebedkin, S.; Barner-Kowollik, C.; Wenzel, W.; Kappes, M.M.; Mayor, M. Selective Dispersion of Single-Walled Carbon Nanotubes with Specific Chiral Indices by Poly(N-Decyl-2,7-Carbazole). J. Am. Chem. Soc. 2011, 133, 652–655. [Google Scholar] [CrossRef]
- Akazaki, K.; Toshimitsu, F.; Ozawa, H.; Fujigaya, T.; Nakashima, N. Recognition and One-Pot Extraction of Right- and Left-Handed Semiconducting Single-Walled Carbon Nanotube Enantiomers Using Fluorene-Binaphthol Chiral Copolymers. J. Am. Chem. Soc. 2012, 134, 12700–12707. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Singer, G.; Siedlaczek, P.; Sinn, G.; Rennhofer, H.; Mičušík, M.; Omastová, M.; Unterlass, M.M.; Wendrinsky, J.; Milotti, V.; Fedi, F.; et al. Acid Free Oxidation and Simple Dispersion Method of MWCNT for High-Performance CFRP. Nanomaterials 2018, 8, 912. [Google Scholar] [CrossRef] [Green Version]
- Lassagne, B.; Tarakanov, Y.; Kinaret, J.; Garcia-Sanchez, D.; Bachtold, A. Coupling Mechanics to Charge Transport in Carbon Nanotube Mechanical Resonators. Science 2009, 325, 1107–1110. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, K.; Burghard, M.; Kern, K.; Scolari, M.; Mews, A. Photocurrent Imaging of Charge Transport Barriers in Carbon Nanotube Devices. Nano Lett. 2005, 5, 507–510. [Google Scholar] [CrossRef]
- Hilt, O.; Brom, H.B.; Ahlskog, M. Localized and Delocalized Charge Transport in Single-Wall Carbon-Nanotube Mats. Phys. Rev. B 2000, 61, R5129–R5132. [Google Scholar] [CrossRef] [Green Version]
- Lourie, O.; Wagner, H.D. Evidence of Stress Transfer and Formation of Fracture Clusters in Carbon Nanotube-Based Composites. Compos. Sci. Technol. 1999, 59, 975–977. [Google Scholar] [CrossRef]
- Xiao, K.Q.; Zhang, L.C. The Stress Transfer Efficiency of a Single-Walled Carbon Nanotube in Epoxy Matrix. J. Mater. Sci. 2004, 39, 4481–4486. [Google Scholar] [CrossRef]
- Andrews, R.; Weisenberger, M.C. Carbon Nanotube Polymer Composites. Curr. Opin. Solid State Mater. Sci. 2004, 8, 31–37. [Google Scholar] [CrossRef]
- Rossell, M.D.; Kuebel, C.; Ilari, G.; Rechberger, F.; Heiligtag, F.J.; Niederberger, M.; Koziej, D.; Erni, R. Impact of Sonication Pretreatment on Carbon Nanotubes: A Transmission Electron Microscopy Study. Carbon 2013, 61, 404–411. [Google Scholar] [CrossRef]
- Graf, A.; Zakharko, Y.; Schießl, S.P.; Backes, C.; Pfohl, M.; Flavel, B.S.; Zaumseil, J. Large Scale, Selective Dispersion of Long Single-Walled Carbon Nanotubes with High Photoluminescence Quantum Yield by Shear Force Mixing. Carbon 2016, 105, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Spotnitz, M.E.; Ryan, D.; Stone, H.A. Dip Coating for the Alignment of Carbon Nanotubes on Curved Surfaces. J. Mater. Chem. 2004, 14, 1299–1302. [Google Scholar] [CrossRef]
- Holzinger, M.; Baur, J.; Haddad, R.; Wang, X.; Cosnier, S. Multiple Functionalization of Single-Walled Carbon Nanotubes by Dip Coating. Chem. Commun. 2011, 47, 2450–2452. [Google Scholar] [CrossRef]
- Kang, T.J.; Yoon, J.-W.; Kim, D.-I.; Kum, S.S.; Huh, Y.-H.; Hahn, J.-H.; Moon, S.H.; Lee, H.-Y.; Kim, Y.H. Sandwich-Type Laminated Nanocomposites Developed by Selective Dip-Coating of Carbon Nanotubes. Adv. Mater. 2007, 19, 427–432. [Google Scholar] [CrossRef]
- Sadi, M.S.; Pan, J.; Xu, A.; Cheng, D.; Cai, G.; Wang, X. Direct Dip-Coating of Carbon Nanotubes onto Polydopamine-Templated Cotton Fabrics for Wearable Applications. Cellulose 2019, 26, 7569–7579. [Google Scholar] [CrossRef]
- Mirri, F.; Ma, A.W.K.; Hsu, T.T.; Behabtu, N.; Eichmann, S.L.; Young, C.C.; Tsentalovich, D.E.; Pasquali, M. High-Performance Carbon Nanotube Transparent Conductive Films by Scalable Dip Coating. ACS Nano 2012, 6, 9737–9744. [Google Scholar] [CrossRef]
- Jo, J.W.; Jung, J.W.; Lee, J.U.; Jo, W.H. Fabrication of Highly Conductive and Transparent Thin Films from Single-Walled Carbon Nanotubes Using a New Non-Ionic Surfactant via Spin Coating. ACS Nano 2010, 4, 5382–5388. [Google Scholar] [CrossRef]
- Garzon-Roman, A.; Cuate-Gomez, D.H. Graphene Nanoflakes and Carbon Nanotubes on Porous Silicon Layers by Spin Coating, for Possible Applications in Optoelectronics. Sens. Actuators A Phys. 2019, 292, 121–128. [Google Scholar] [CrossRef]
- Maziukiewicz, D.; Maciejewska, B.M.; Litowczenko, J.; Kościński, M.; Warowicka, A.; Wychowaniec, J.K.; Jurga, S. Designing Biocompatible Spin-Coated Multiwall Carbon Nanotubes-Polymer Composite Coatings. Surf. Coat. Technol. 2020, 385, 125199. [Google Scholar] [CrossRef]
- Majumder, M.; Rendall, C.; Li, M.; Behabtu, N.; Eukel, J.A.; Hauge, R.H.; Schmidt, H.K.; Pasquali, M. Insights into the Physics of Spray Coating of SWNT Films. Chem. Eng. Sci. 2010, 65, 2000–2008. [Google Scholar] [CrossRef]
- Komatsu, N.; Nakamura, M.; Ghosh, S.; Kim, D.; Chen, H.; Katagiri, A.; Yomogida, Y.; Gao, W.; Yanagi, K.; Kono, J. Groove-Assisted Global Spontaneous Alignment of Carbon Nanotubes in Vacuum Filtration. Nano Lett. 2020, 20, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- King, B.; Panchapakesan, B. Vacuum Filtration Based Formation of Liquid Crystal Films of Semiconducting Carbon Nanotubes and High Performance Transistor Devices. Nanotechnology 2014, 25, 175201. [Google Scholar] [CrossRef]
- Hu, C.; Ding, Y.; Ji, Y.; Xu, J.; Hu, S. Fabrication of Thin-Film Electrochemical Sensors from Single-Walled Carbon Nanotubes by Vacuum Filtration. Carbon 2010, 48, 1345–1352. [Google Scholar] [CrossRef]
- Dai, L.; Sun, J. Mechanical Properties of Carbon Nanotubes-Polymer Composites; IntechOpen: London, UK, 2016; ISBN 978-953-51-2470-2. [Google Scholar]
- Karakaya, M.; Zhu, J.; Raghavendra, A.J.; Podila, R.; Parler, S.G.; Kaplan, J.P.; Rao, A.M. Roll-to-Roll Production of Spray Coated N-Doped Carbon Nanotube Electrodes for Supercapacitors. Appl. Phys. Lett. 2014, 105, 263103. [Google Scholar] [CrossRef]
- Yamamoto, K.Y.K.; Akita, S.A.S.; Nakayama, Y.N.Y. Orientation of Carbon Nanotubes Using Electrophoresis. Jpn. J. Appl. Phys. 1996, 35, L917. [Google Scholar] [CrossRef]
- Choi, W.B.; Jin, Y.W.; Kim, H.Y.; Lee, S.J.; Yun, M.J.; Kang, J.H.; Choi, Y.S.; Park, N.S.; Lee, N.S.; Kim, J.M. Electrophoresis Deposition of Carbon Nanotubes for Triode-Type Field Emission Display. Appl. Phys. Lett. 2001, 78, 1547–1549. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.J.C.; Jane Minay, E.; Shaffer, M.S.P. Electrophoretic Deposition of Carbon Nanotubes. Carbon 2006, 44, 3149–3160. [Google Scholar] [CrossRef]
- Lekawa-Raus, A.; Patmore, J.; Kurzepa, L.; Bulmer, J.; Koziol, K. Electrical Properties of Carbon Nanotube Based Fibers and Their Future Use in Electrical Wiring. Adv. Funct. Mater. 2014, 24, 3661–3682. [Google Scholar] [CrossRef]
- Wu, A.S.; Chou, T.-W. Carbon Nanotube Fibers for Advanced Composites. Mater. Today 2012, 15, 302–310. [Google Scholar] [CrossRef]
- Kozlov, M.E.; Capps, R.C.; Sampson, W.M.; Ebron, V.H.; Ferraris, J.P.; Baughman, R.H. Spinning Solid and Hollow Polymer-Free Carbon Nanotube Fibers. Adv. Mater. 2005, 17, 614–617. [Google Scholar] [CrossRef]
- Vigolo, B.; Pénicaud, A.; Coulon, C.; Sauder, C.; Pailler, R.; Journet, C.; Bernier, P.; Poulin, P. Macroscopic Fibers and Ribbons of Oriented Carbon Nanotubes. Science 2000, 290, 1331–1334. [Google Scholar] [CrossRef]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; ter Waarbeek, R.F.; de Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional Fibers of Carbon Nanotubes with Ultrahigh Conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Ericson, L.M.; Fan, H.; Peng, H.; Davis, V.A.; Zhou, W.; Sulpizio, J.; Wang, Y.; Booker, R.; Vavro, J.; Guthy, C.; et al. Macroscopic, Neat, Single-Walled Carbon Nanotube Fibers. Science 2004, 305, 1447–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, V.A.; Parra-Vasquez, A.N.G.; Green, M.J.; Rai, P.K.; Behabtu, N.; Prieto, V.; Booker, R.D.; Schmidt, J.; Kesselman, E.; Zhou, W.; et al. True Solutions of Single-Walled Carbon Nanotubes for Assembly into Macroscopic Materials. Nat. Nanotech 2009, 4, 830–834. [Google Scholar] [CrossRef]
- Jestin, S.; Poulin, P. Chapter 6—Wet Spinning of CNT-based Fibers. In Nanotube Superfiber Materials; Schulz, M.J., Shanov, V.N., Yin, Z., Eds.; William Andrew Publishing: Boston, MA, USA, 2014; pp. 167–209. ISBN 978-1-4557-7863-8. [Google Scholar]
- Yeo, L.Y.; Friend, J.R. Electrospinning Carbon Nanotube Polymer Composite Nanofibers. J. Exp. Nanosci. 2006, 1, 177–209. [Google Scholar] [CrossRef] [Green Version]
- Ko, F.; Gogotsi, Y.; Ali, A.; Naguib, N.; Ye, H.; Yang, G.L.; Li, C.; Willis, P. Electrospinning of Continuous Carbon Nanotube-Filled Nanofiber Yarns. Adv. Mater. 2003, 15, 1161–1165. [Google Scholar] [CrossRef]
- Sen, R.; Zhao, B.; Perea, D.; Itkis, M.E.; Hu, H.; Love, J.; Bekyarova, E.; Haddon, R.C. Preparation of Single-Walled Carbon Nanotube Reinforced Polystyrene and Polyurethane Nanofibers and Membranes by Electrospinning. Nano Lett. 2004, 4, 459–464. [Google Scholar] [CrossRef]
- Kimmer, D.; Slobodian, P.; Petráš, D.; Zatloukal, M.; Olejník, R.; Sáha, P. Polyurethane/Multiwalled Carbon Nanotube Nanowebs Prepared by an Electrospinning Process. J. Appl. Polym. Sci. 2009, 111, 2711–2714. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Multiwalled Carbon Nanotube (MWCNT) Reinforced Cellulose Fibers by Electrospinning. ACS Appl. Mater. Interfaces 2010, 2, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Cassell, A.M.; Raymakers, J.A.; Kong, J.; Dai, H. Large Scale CVD Synthesis of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 1999, 103, 6484–6492. [Google Scholar] [CrossRef]
- Magrez, A.; Seo, J.W.; Smajda, R.; Mionić, M.; Forró, L. Catalytic CVD Synthesis of Carbon Nanotubes: Towards High Yield and Low Temperature Growth. Materials 2010, 3, 4871–4891. [Google Scholar] [CrossRef] [Green Version]
- Boncel, S.; Pattinson, S.W.; Geiser, V.; Shaffer, M.S.P.; Koziol, K.K.K. En Route to Controlled Catalytic CVD Synthesis of Densely Packed and Vertically Aligned Nitrogen-Doped Carbon Nanotube Arrays. Beilstein J. Nanotechnol. 2014, 5, 219–233. [Google Scholar] [CrossRef] [Green Version]
- Mayhew, E.; Prakash, V. Thermal Conductivity of High Performance Carbon Nanotube Yarn-like Fibers. J. Appl. Phys. 2014, 115, 174306. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Li, Q.; Fan, S. Spinning Continuous Carbon Nanotube Yarns. Nature 2002, 419, 801. [Google Scholar] [CrossRef]
- Pöhls, J.-H.; Johnson, M.B.; White, M.A.; Malik, R.; Ruff, B.; Jayasinghe, C.; Schulz, M.J.; Shanov, V. Physical Properties of Carbon Nanotube Sheets Drawn from Nanotube Arrays. Carbon 2012, 50, 4175–4183. [Google Scholar] [CrossRef]
- Li, Q.W.; Zhang, X.F.; DePaula, R.F.; Zheng, L.X.; Zhao, Y.H.; Stan, L.; Holesinger, T.G.; Arendt, P.N.; Peterson, D.E.; Zhu, Y.T. Sustained Growth of Ultralong Carbon Nanotube Arrays for Fiber Spinning. Adv. Mater. 2006, 18, 3160–3163. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Tu, Y.; Li, Y.; Coulter, J.Y.; Zheng, L.; Zhao, Y.; Jia, Q.; Peterson, D.E.; Zhu, Y. Strong Carbon-Nanotube Fibers Spun from Long Carbon-Nanotube Arrays. Small 2007, 3, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, B.; Hubert, P. Modeling the Elastic Properties of Carbon Nanotube Array/Polymer Composites. Compos. Sci. Technol. 2006, 66, 387–396. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Y.; Zhou, R.; Zhu, H.; Wang, J.; Liu, L.; Fan, S.; Jiang, K. Carbon Nanotube Yarns with High Tensile Strength Made by a Twisting and Shrinking Method. Nanotechnology 2009, 21, 045708. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, B.; Zhang, M. Optimizing Reaction Condition for Synthesizing Spinnable Carbon Nanotube Arrays by Chemical Vapor Deposition. J. Mater. Sci. 2013, 48, 7749–7756. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, G.; Doorn, S.K.; Htoon, H.; Stan, L.; Hawley, M.E.; Sheehan, C.J.; Zhu, Y.; Jia, Q. Tailoring the Morphology of Carbon Nanotube Arrays: From Spinnable Forests to Undulating Foams. ACS Nano 2009, 3, 2157–2162. [Google Scholar] [CrossRef]
- Li, Y.-L.; Kinloch, I.A.; Windle, A.H. Direct Spinning of Carbon Nanotube Fibers from Chemical Vapor Deposition Synthesis. Science 2004, 304, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Janas, D.; Koziol, K.K. Carbon Nanotube Fibers and Films: Synthesis, Applications and Perspectives of the Direct-Spinning Method. Nanoscale 2016, 8, 19475–19490. [Google Scholar] [CrossRef]
- Yau, R.K.; Strotmeyer, E.S.; Resnick, H.E.; Sellmeyer, D.E.; Feingold, K.R.; Cauley, J.A.; Vittinghoff, E.; Rekeneire, N.D.; Harris, T.B.; Nevitt, M.C.; et al. Diabetes and Risk of Hospitalized Fall Injury Among Older Adults. Diabetes Care 2013, 36, 3985–3991. [Google Scholar] [CrossRef] [Green Version]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1515920 (accessed on 29 July 2021).
- Cui, Y.; Zhang, L.; Zhang, M.; Yang, X.; Zhang, L.; Kuang, J.; Zhang, G.; Liu, Q.; Guo, H.; Meng, Q. Prevalence and Causes of Low Vision and Blindness in a Chinese Population with Type 2 Diabetes: The Dongguan Eye Study. Sci. Rep. 2017, 7, 11195. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable Non-Invasive Epidermal Glucose Sensors: A Review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, J.-H.; Chuang, C.-C. Glucose Biosensor Based on Multiwalled Carbon Nanotubes Grown Directly on Si. Carbon 2009, 47, 3106–3112. [Google Scholar] [CrossRef]
- Yan, Y.-M.; Baravik, I.; Yehezkeli, O.; Willner, I. Integrated Electrically Contacted Glucose Oxidase/Carbon Nanotube Electrodes for the Bioelectrocatalyzed Detection of Glucose. J. Phys. Chem. C 2008, 112, 17883–17888. [Google Scholar] [CrossRef]
- Tsai, T.-W.; Heckert, G.; Neves, L.F.; Tan, Y.; Kao, D.-Y.; Harrison, R.G.; Resasco, D.E.; Schmidtke, D.W. Adsorption of Glucose Oxidase onto Single-Walled Carbon Nanotubes and Its Application in Layer-By-Layer Biosensors. Anal. Chem. 2009, 81, 7917–7925. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-C.; Park, B.-S.; Ha, T.-J. Highly Sensitive Wearable Glucose Sensor Systems Based on Functionalized Single-Wall Carbon Nanotubes with Glucose Oxidase-Nafion Composites. Appl. Surf. Sci. 2019, 470, 13–18. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized Helical Fibre Bundles of Carbon Nanotubes as Electrochemical Sensors for Long-Term in Vivo Monitoring of Multiple Disease Biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef]
- Feng, J.; Chen, C.; Sun, X.; Peng, H. Implantable Fiber Biosensors Based on Carbon Nanotubes. Acc. Mater. Res. 2021, 2, 138–146. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A Graphene-Based Electrochemical Device with Thermoresponsive Microneedles for Diabetes Monitoring and Therapy. Nat. Nanotech. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Kolanowska, A.; Herman, A.P.; Jędrysiak, R.G.; Boncel, S. Carbon Nanotube Materials for Electrocardiography. RSC Adv. 2021, 11, 3020–3042. [Google Scholar] [CrossRef]
- Liu, B.; Tang, H.; Luo, Z.; Zhang, W.; Tu, Q.; Jin, X. Wearable Carbon Nanotubes-Based Polymer Electrodes for Ambulatory Electrocardiographic Measurements. Sens. Actuators A Phys. 2017, 265, 79–85. [Google Scholar] [CrossRef]
- Jevon, P. Recording an Accurate ECG. Pract. Nurs. 2010, 21, 404–408. [Google Scholar] [CrossRef]
- Chi, M.; Zhao, J.; Dong, Y.; Wang, X. Flexible Carbon Nanotube-Based Polymer Electrode for Long-Term Electrocardiographic Recording. Materials 2019, 12, 971. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.-C.; Moon, J.-H.; Baek, D.-H.; Lee, J.-H.; Choi, Y.-Y.; Hong, J.-S.; Lee, S.-H. CNT/PDMS Composite Flexible Dry Electrodesfor Long-Term ECG Monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Loh, K.J. Wearable Carbon Nanotube-Based Fabric Sensors for Monitoring Human Physiological Performance. Smart Mater. Struct. 2017, 26, 055018. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Luo, Z.; Zhang, W.; Tu, Q.; Jin, X. Carbon Nanotube-Based Self-Adhesive Polymer Electrodes for Wireless Long-Term Recording of Electrocardiogram Signals. J. Biomater. Sci. Polym. Ed. 2016, 27, 1899–1908. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Y.; Liu, J.; Zhan, Z.; Li, X.; Li, W.J. Single-Wall Carbon Nanotube-Coated Cotton Yarn for Electrocardiography Transmission. Micromachines 2018, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Chu, M.; Naguib, H.E. Soft Flexible Conductive CNT Nanocomposites for ECG Monitoring. Smart Mater. Struct. 2021, 30, 065003. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, H.N.; Seong, M.; Lee, S.-H.; Kang, M.; Yi, H.; Bae, W.G.; Kwak, M.K.; Jeong, H.E. Multifunctional Smart Skin Adhesive Patches for Advanced Health Care. Adv. Healthc. Mater. 2018, 7, 1800275. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hwang, J.-Y.; Hwang, H.R.; Kim, H.S.; Lee, J.H.; Seo, J.-W.; Shin, U.S.; Lee, S.-H. Simple and Cost-Effective Method of Highly Conductive and Elastic Carbon Nanotube/Polydimethylsiloxane Composite for Wearable Electronics. Sci. Rep. 2018, 8, 1375. [Google Scholar] [CrossRef] [Green Version]
- Li, B.M.; Yildiz, O.; Mills, A.C.; Flewwellin, T.J.; Bradford, P.D.; Jur, J.S. Iron-on Carbon Nanotube (CNT) Thin Films for Biosensing E-Textile Applications. Carbon 2020, 168, 673–683. [Google Scholar] [CrossRef]
- Seoane, F.; Soroudi, A.; Lu, K.; Nilsson, D.; Nilsson, M.; Abtahi, F.; Skrifvars, M. Textile-Friendly Interconnection between Wearable Measurement Instrumentation and Sensorized Garments—Initial Performance Evaluation for Electrocardiogram Recordings. Sensors 2019, 19, 4426. [Google Scholar] [CrossRef]
- Sarazan, R.D. The QT Interval of the Electrocardiogram. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 10–15. ISBN 978-0-12-386455-0. [Google Scholar]
- Available online: https://www.cablesysensores.com/pages/12-lead-ecg-placement-guide-with-illustrations (accessed on 18 August 2021).
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A Review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.-L.; Kuo, P.-C.; Cheng, C.-Y.; Chen, Y.-S. Challenges and Future Perspectives on Electroencephalogram-Based Biometrics in Person Recognition. Front. Neuroinform. 2018, 12, 66. [Google Scholar] [CrossRef] [Green Version]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Van Langenhove, L. The Status of Textile-Based Dry Eeg Electrodes. Autex Res. J. 2021, 21, 63–70. [Google Scholar] [CrossRef]
- Gao, K.-P.; Yang, H.-J.; Wang, X.-L.; Yang, B.; Liu, J.-Q. Soft Pin-Shaped Dry Electrode with Bristles for EEG Signal Measurements. Sens. Actuators A Phys. 2018, 283, 348–361. [Google Scholar] [CrossRef]
- Awara, K.; Kitai, R.; Isozaki, M.; Neishi, H.; Kikuta, K.; Fushisato, N.; Kawamoto, A. Thin-Film Electroencephalographic Electrodes Using Multi-Walled Carbon Nanotubes Are Effective for Neurosurgery. Biomed. Eng. Online 2014, 13, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruffini, G.; Dunne, S.; Fuentemilla, L.; Grau, C.; Farrés, E.; Marco-Pallarés, J.; Watts, P.C.P.; Silva, S.R.P. First Human Trials of a Dry Electrophysiology Sensor Using a Carbon Nanotube Array Interface. Sens. Actuators A Phys. 2008, 144, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.M.; Hossain, M.M. Nanomaterials-Patterned Flexible Electrodes for Wearable Health Monitoring: A Review. J. Mater. Sci. 2021, 56, 14900–14942. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Byeon, H.J.; Lee, J.H.; Baek, D.H.; Lee, K.H.; Hong, J.S.; Lee, S.-H. Self-Adhesive Epidermal Carbon Nanotube Electronics for Tether-Free Long-Term Continuous Recording of Biosignals. Sci. Rep. 2014, 4, 6074. [Google Scholar] [CrossRef]
- Kang, B.-C.; Ha, T.-J. Wearable Carbon Nanotube Based Dry-Electrodes for Electrophysiological Sensors. Jpn. J. Appl. Phys. 2018, 57, 05GD02. [Google Scholar] [CrossRef]
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-Enabled Wearable Sensors for Healthcare. Adv. Healthc. Mater. 2018, 7, 1700889. [Google Scholar] [CrossRef]
- Qi, K.; Zhou, Y.; Ou, K.; Dai, Y.; You, X.; Wang, H.; He, J.; Qin, X.; Wang, R. Weavable and Stretchable Piezoresistive Carbon Nanotubes-Embedded Nanofiber Sensing Yarns for Highly Sensitive and Multimodal Wearable Textile Sensor. Carbon 2020, 170, 464–476. [Google Scholar] [CrossRef]
- Howe, A.S.; Boden, B.P. Heat-Related Illness in Athletes. Am. J. Sports Med. 2007, 35, 1384–1395. [Google Scholar] [CrossRef]
- Donoghue, A.M. Heat Illness in the U.S. Mining Industry. Am. J. Ind. Med. 2004, 45, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Root, W.; Bechtold, T.; Pham, T. Textile-Integrated Thermocouples for Temperature Measurement. Materials 2020, 13, 626. [Google Scholar] [CrossRef] [Green Version]
- Blasdel, N.J.; Monty, C.N. Temperature Sensitive Fabric for Monitoring Dermal Temperature Variations. In Wearable Electronics Sensors: For Safe and Healthy Living; Mukhopadhyay, S.C., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 193–220. ISBN 978-3-319-18191-2. [Google Scholar]
- Rosace, G.; Trovato, V.; Colleoni, C.; Caldara, M.; Re, V.; Brucale, M.; Piperopoulos, E.; Mastronardo, E.; Milone, C.; De Luca, G.; et al. Structural and Morphological Characterizations of MWCNTs Hybrid Coating onto Cotton Fabric as Potential Humidity and Temperature Wearable Sensor. Sens. Actuators B Chem. 2017, 252, 428–439. [Google Scholar] [CrossRef]
- Wu, R.; Ma, L.; Hou, C.; Meng, Z.; Guo, W.; Yu, W.; Yu, R.; Hu, F.; Liu, X.Y. Silk Composite Electronic Textile Sensor for High Space Precision 2D Combo Temperature–Pressure Sensing. Small 2019, 15, 1901558. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rdest, M.; Janas, D. Carbon Nanotube Wearable Sensors for Health Diagnostics. Sensors 2021, 21, 5847. https://doi.org/10.3390/s21175847
Rdest M, Janas D. Carbon Nanotube Wearable Sensors for Health Diagnostics. Sensors. 2021; 21(17):5847. https://doi.org/10.3390/s21175847
Chicago/Turabian StyleRdest, Monika, and Dawid Janas. 2021. "Carbon Nanotube Wearable Sensors for Health Diagnostics" Sensors 21, no. 17: 5847. https://doi.org/10.3390/s21175847
APA StyleRdest, M., & Janas, D. (2021). Carbon Nanotube Wearable Sensors for Health Diagnostics. Sensors, 21(17), 5847. https://doi.org/10.3390/s21175847






