Motorized Treadmill and Optical Recording System for Gait Analysis of Grasshoppers
Abstract
:1. Introduction
2. Materials and Methods
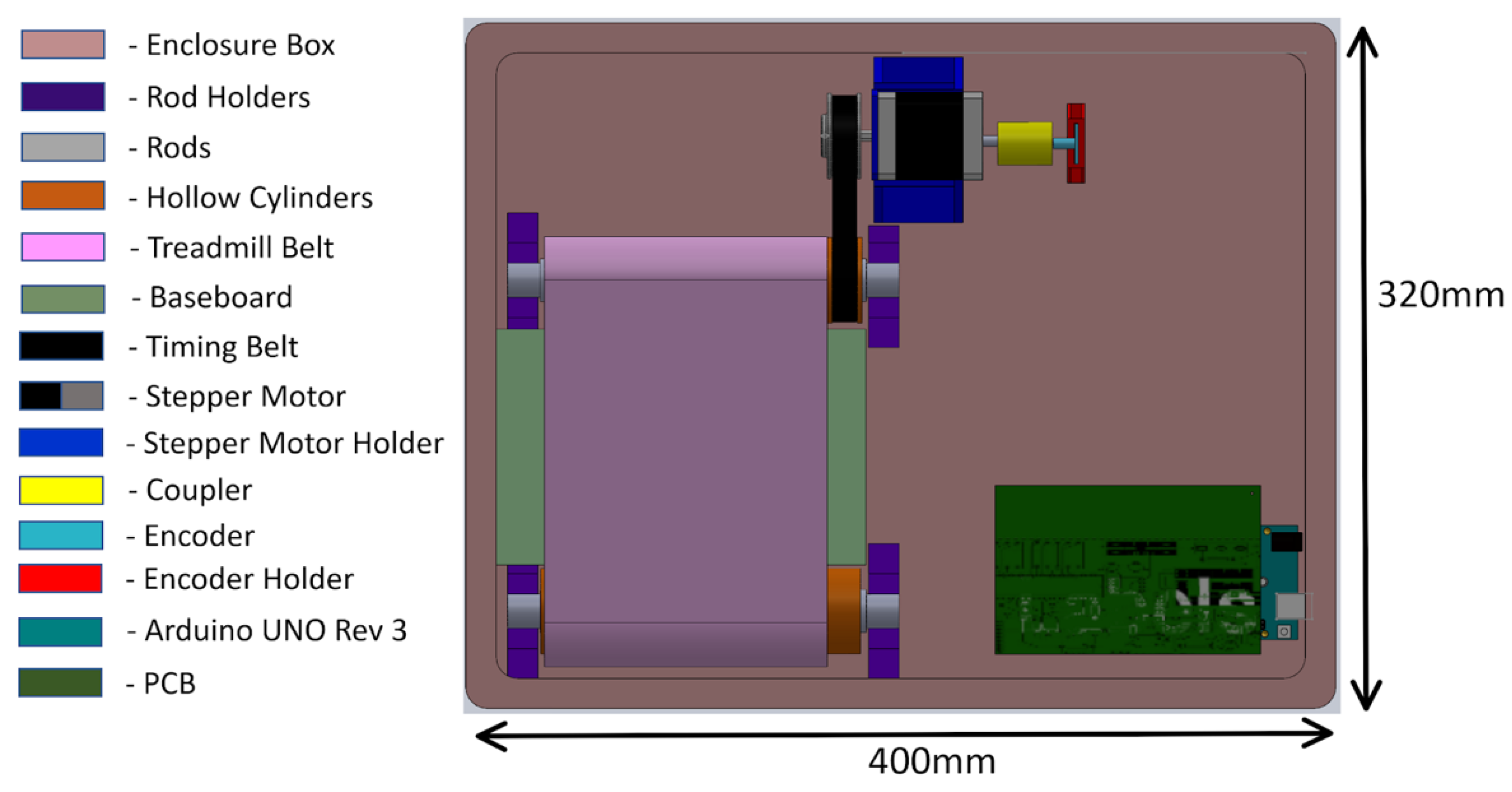
2.1. Development of the Grasshopper Treadmill
2.1.1. Treadmill Electronics
2.1.2. Software
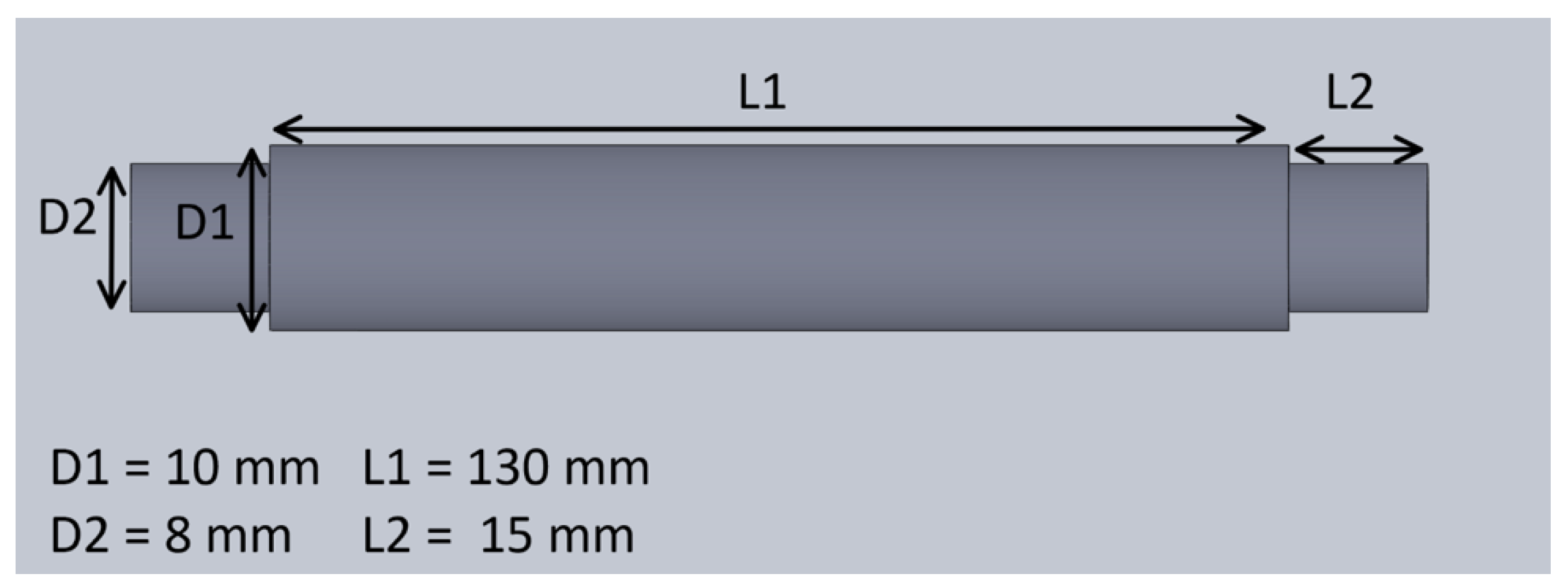
2.1.3. Mechanical System
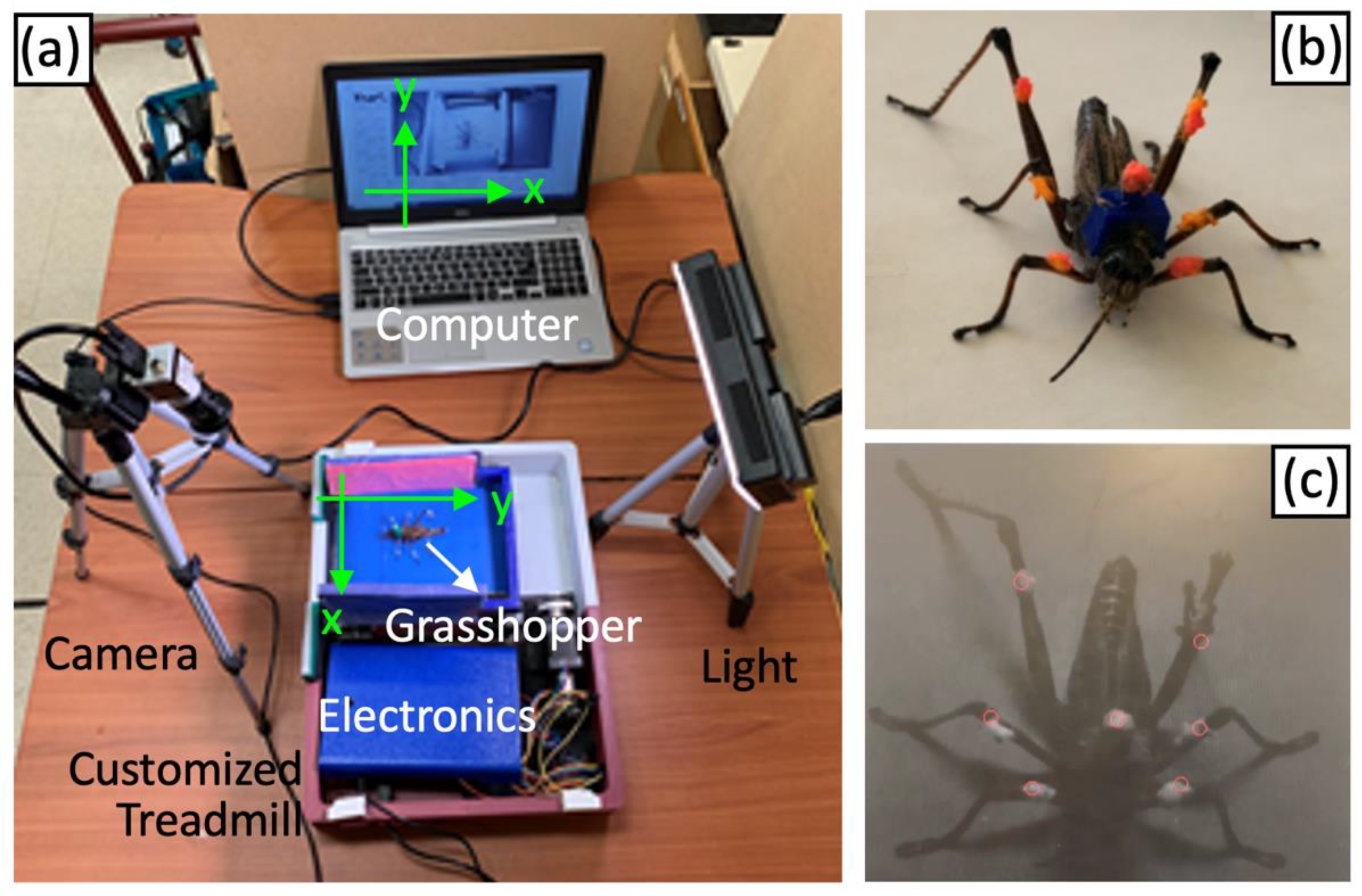
2.2. Optical Recording and Analysis of Grasshopper Gaits
2.3. Calculation of Walking Speed, Stance Duty Factor
2.4. Study Insects
3. Results
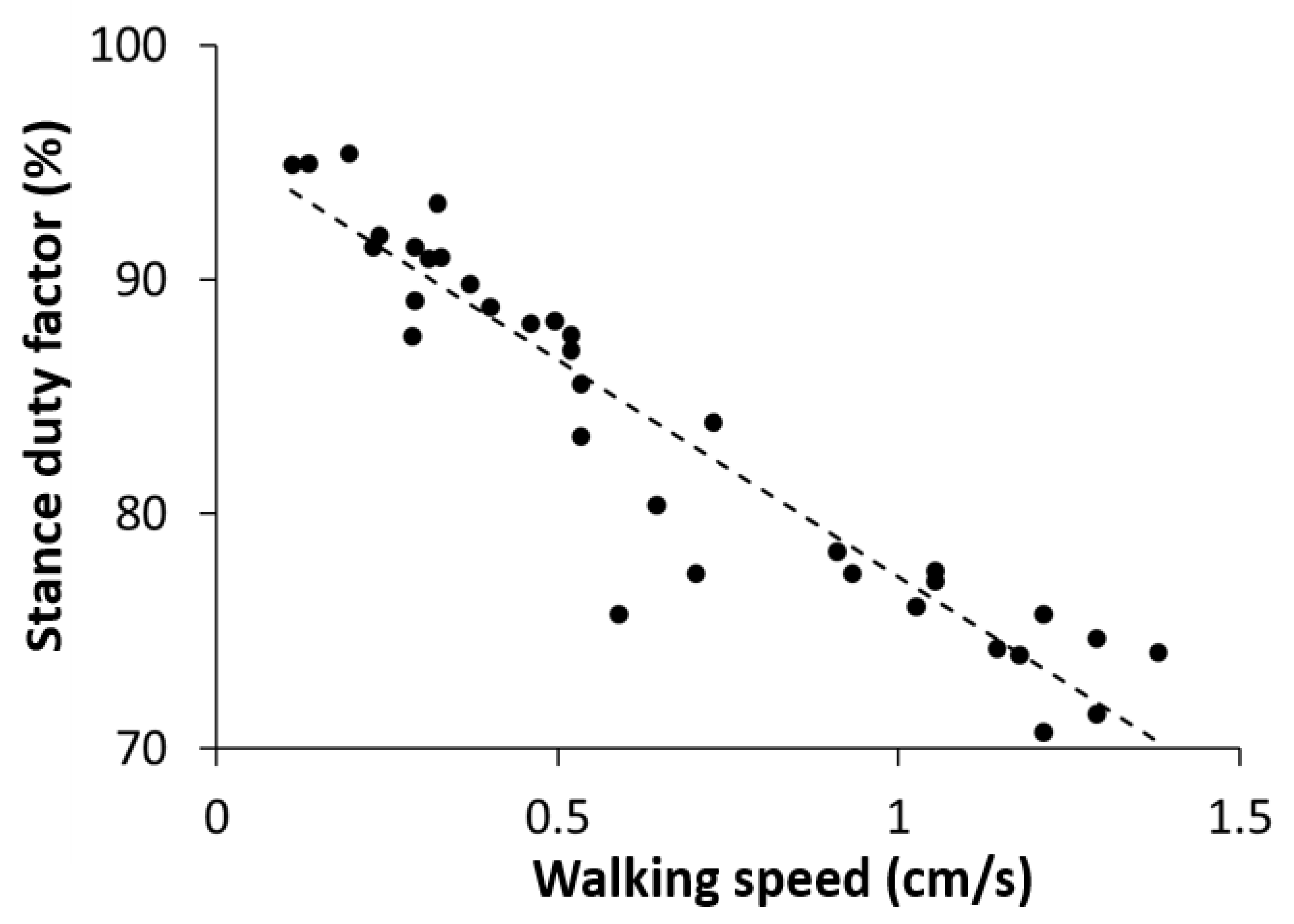
3.1. Relationship between Stance Duty Factor and Walking Speed
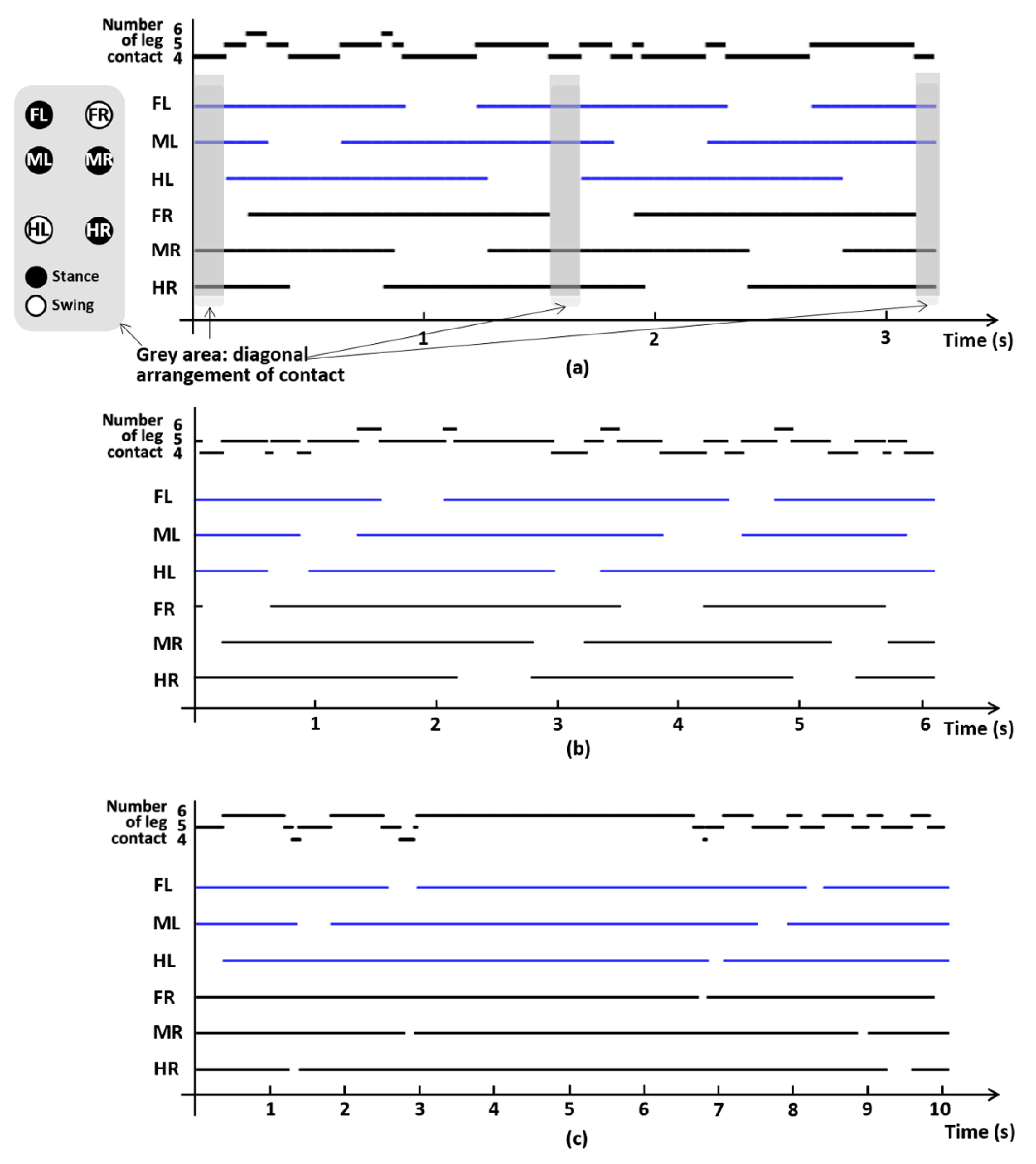
3.2. Gait Phase and Interleg Coordination
3.3. Number of Foot Contacts According to the Walking Speed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chvatal, S.A.; Ting, L.H. Common muscle synergies for balance and walking. Front. Comput. Neurosci. 2013, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prilutsky, B.I.; Sirota, M.G.; Gregor, R.J.; Beloozerova, I.N. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J. Neurophysiol. 2005, 94, 2959–2969. [Google Scholar] [CrossRef] [Green Version]
- Pearson, K.G. Generating the walking gait: Role of sensory feedback. Prog. Brain Res. 2004, 143, 123–129. [Google Scholar] [PubMed]
- Reisman, D.S.; Wityk, R.; Silver, K.; Bastian, A.J. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 2007, 130, 1861–1872. [Google Scholar] [CrossRef] [Green Version]
- Whittle, M.W. Gait Analysis: An Introduction; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Barbeau, H.; Rossignol, S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987, 412, 84–95. [Google Scholar] [CrossRef]
- Park, H. Gait Optimization with a Real-Time Closed-Loop Artificial Sensory Feedback. Ph.D. Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2017. [Google Scholar]
- McCrea, D.A.; Rybak, I.A. Organization of mammalian locomotor rhythm and pattern generation. Brain Res. Rev. 2008, 57, 134–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybak, I.A.; Dougherty, K.J.; Shevtsova, N.A. Organization of the mammalian locomotor CPG: Review of computational model and circuit architectures based on genetically identified spinal interneurons. eNeuro 2015, 2, 1–20. [Google Scholar] [CrossRef]
- Ebersbach, G.; Sojer, M.; Valldeoriola, F.; Wissel, J.; Müller, J.; Tolosa, E.; Poewe, W. Comparative analysis of gait in Parkinson’s disease, cerebellar ataxia and subcortical arteriosclerotic encephalopathy. Brain 1999, 122, 1349–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, J.J.; Tang, P.-F. Gait training strategies to optimize walking ability in people with stroke: A synthesis of the evidence. Expert Rev. Neurother. 2011, 7, 1417–1436. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.F.; Musselman, K.E. Training to achieve over ground walking after spinal cord injury: A review of who, what, when, and how. J. Spinal Cord Med. 2012, 35, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cham, J.G.; Bailey, S.A.; Clark, J.E.; Full, R.J.; Cutkosky, M.R. Fast and robust: Hexapedal robots via shape deposition manufacturing. Int. J. Rob. Res. 2002, 21, 869–882. [Google Scholar] [CrossRef]
- Belter, D.; Skrzypczyński, P. A biologically inspired approach to feasible gait learning for a hexapod robot. Int. J. Appl. Math. Comput. Sci. 2010, 20, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Skoyles, J.R. Human balance, the evolution of bipedalism and dysequilibrium syndrome. Med. Hypotheses 2006, 66, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.J.; Raichlen, D.A. Lateral sequence walking in infant Papio cynocephalus: Implications for the evolution of diagonal sequence walking in primates. Am. J. Phys. Anthropol. 2005, 126, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, E.; Sato, Y.; Miura, E.; Yamaura, H.; Yuzaki, M.; Yanagihara, D. Characteristics of Gait Ataxia in δ2 Glutamate Receptor Mutant Mice, ho15J. PLoS ONE 2012, 7, e47553. [Google Scholar] [CrossRef]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait analysis using wearable sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef] [PubMed]
- Clayton, H.M.; Schamhardt, H.C. Measurement techniques for gait analysis. Equine Locomot. 2013, 2, 31–60. [Google Scholar]
- Dierick, F.; Penta, M.; Renaut, D.; Detrembleur, C. A force measuring treadmill in clinical gait analysis. Gait Posture 2004, 20, 299–303. [Google Scholar] [CrossRef]
- Prakash, C.; Gupta, K.; Mittal, A.; Kumar, R.; Laxmi, V. Passive marker based optical system for gait kinematics for lower extremity. Procedia Comput. Sci. 2015, 45, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Carse, B.; Meadows, B.; Bowers, R.; Rowe, P. Affordable clinical gait analysis: An assessment of the marker tracking accuracy of a new low-cost optical 3D motion analysis system. Physiotherapy 2013, 99, 347–351. [Google Scholar] [CrossRef]
- Tranberg, R.; Saari, T.; Zügner, R.; Kärrholm, J. Simultaneous measurements of knee motion using an optical tracking system and radiostereometric analysis (RSA). Acta Orthop. 2011, 82, 171–176. [Google Scholar] [CrossRef]
- Zügner, R.; Tranberg, R.; Timperley, J.; Hodgins, D.; Mohaddes, M.; Kärrholm, J. Validation of inertial measurement units with optical tracking system in patients operated with Total hip arthroplasty. BMC Musculoskelet. Disord. 2019, 20, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Looper, J.; Ulrich, B.D.; Ulrich, D.A.; Angulo-barroso, R.M. Exploring effects of different treadmill interventions on walking onset and gait patterns in infants with Down syndrome. Dev. Med. Child Neurol. 2007, 49, 839–945. [Google Scholar] [CrossRef] [Green Version]
- Hornby, T.G.; Zemon, D.H.; Campbell, D. Robotic-Assisted, Body-Weight–Supported Treadmill Training in Individuals Following Motor Incomplete Spinal Cord Injury. Phys. Ther. 2005, 85, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Oh, K.; Prilutsky, B.I.; Deweerth, S.P. A real-time closed-loop control system for modulating gait characteristics via electrical stimulation of peripheral nerves. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS 2016), Shanghai, China, 17–19 October 2016; pp. 95–98. [Google Scholar] [CrossRef]
- Park, H.; Latash, E.M.; Molkov, Y.I.; Klishko, A.N.; Frigon, A.; DeWeerth, S.P.; Prilutsky, B.I. Cutaneous sensory feedback from paw pads affects lateral balance control during split-belt locomotion in the cat. J. Exp. Biol. 2019, 222, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mehrholz, J.; Thomas, S.; Elsner, B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2017, 8, CD002840. [Google Scholar] [CrossRef] [PubMed]
- Wittlinger, M.; Wehner, R.; Wolf, H. The desert ant odometer: A stride integrator that accounts for stride length and walking speed. J. Exp. Biol. 2007, 210, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Ramdya, P.; Thandiackal, R.; Cherney, R.; Asselborn, T.; Benton, R.; Ijspeert, A.; Floreano, D. Climbing favours the tripod gait over alternative faster insect gaits. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowska, M.; Godlewska, E.; Schmidt, J.; Daun-Gruhn, S. Research article: Quadrupedal gaits in hexapod animals—Inter-leg coordination in free-walking adult stick insects. J. Exp. Biol. 2012, 215, 4255–4266. [Google Scholar]
- Dahmen, H.; Wahl, V.L.; Pfeffer, S.E.; Mallot, H.A.; Wittlinger, M. Naturalistic path integration of Cataglyphis desert ants on an air-cushioned lightweight spherical treadmill. J. Exp. Biol. 2017, 220, 634–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spirito, B.Y.C.P.; Mushrush, D.L. Interlimb Coordination During Slow Walking in the Cockroach: I. Effects of Substrate Alterations. J. Exp. Biol. 1979, 78, 233–243. [Google Scholar] [CrossRef]
- Oe, M.; Ogawa, H. Neural basis of stimulus-angle-dependent motor control of wind-elicited walking behavior in the cricket Gryllus bimaculatus. PLoS ONE 2013, 8, e80184. [Google Scholar] [CrossRef] [Green Version]
- Parle, E.; Larmon, H.; Taylor, D. Biomechanical factors in the adaptations of insect tibia cuticle. PLoS ONE 2016, 11, e0159262. [Google Scholar] [CrossRef] [Green Version]
- Weihmann, T.; Brun, P.-G.; Pycroft, E. Speed dependent phase shifts and gait changes in cockroaches running on substrates of different slipperiness. Front. Zool. 2017, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Full, R.J.; Tu, M.S. Mechanics of a rapid running insect: Two-, four- and six-legged locomotion. J. Exp. Biol. 1991, 156, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Mantziaris, C.; Bockemühl, T.; Holmes, P.; Borgmann, A.; Daun, S.; Büschges, A. Intra-and intersegmental influences among central pattern generating networks in the walking system of the stick insect. J. Neurophysiol. 2017, 118, 2296–2310. [Google Scholar] [CrossRef] [Green Version]
- Borgmann, A.; Hooper, S.L.; Büschges, A. Sensory feedback induced by front-leg stepping entrains the activity of central pattern generators in caudal segments of the stick insect walking system. J. Neurosci. 2009, 29, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Reches, E.; Knebel, D.; Rillich, J.; Ayali, A.; Barzel, B. The Metastability of the Double-Tripod Gait in Locust Locomotion. iScience 2019, 12, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, K.G.; Franklin, R. Characteristics of Leg Movements and Patterns of Coordination in Locusts Walking on Rough Terrain. Int. J. Robot. Res. 1984, 3, 101–112. [Google Scholar] [CrossRef]
- Vision.PointTracker. Available online: https://www.mathworks.com/help/vision/ref/vision.pointtracker-system-object.html (accessed on 14 August 2021).
- Mstafa, R.J.; Elleithy, K.M. A video steganography algorithm based on Kanade-Lucas-Tomasi tracking algorithm and error correcting codes. Multimed. Tools Appl. 2016, 75, 10311–10333. [Google Scholar] [CrossRef]
- Barnouti, N.H.; Al-Mayyahi, M.H.; Al-Dabbagh, S.S. Real-Time Face Tracking and Recognition System Using Kanade-Lucas-Tomasi and Two-Dimensional Principal Component Analysis. In Proceedings of the 2018 International Conference on Advanced Science and Engineering (ICOASE), Duhok, Iraq, 9 October 2018; pp. 24–29. [Google Scholar]
- Ahmed, T.; Singh, D.P.; Raman, B. Potential application of Kanade–Lucas–Tomasi tracker on satellite images for automatic change detection. J. Appl. Remote Sens. 2016, 10, 026018. [Google Scholar] [CrossRef]
- Rogers, S.M.; Cullen, D.; Anstey, M.L.; Burrows, M.; Despland, E.; Dodgson, T.; Matheson, T.; Ott, S.; Stettin, K.; Sword, G.A.; et al. Rapid behavioural gregarization in the desert locust, Schistocerca gregaria entails synchronous changes in both activity and attraction to conspecifics. J. Insect Physiol. 2014, 65, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, J.P.; Büschges, A. Control of stepping velocity in a single insect leg during walking. Philos. Trans. R Soc. A Math. Phys. Eng. Sci. 2007, 365, 251–271. [Google Scholar] [CrossRef]
- Witney, A.G.; Hedwig, B. Kinematics of phonotactic steering in the walking cricket Gryllus bimaculatus (de Geer). J. Exp. Biol. 2011, 214, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, M.P.; Lewis, C.J.; Whitman, D.W. Effects of males on the fecundity and fertility of female Romalea microptera grasshoppers. J. Orthoptera Res. 1999, 277–283. [Google Scholar] [CrossRef]
- Hatle, J.D.; Awan, A.; Nicholas, J.; Koch, R.; Vokrri, J.R.; McCue, M.D.; Williams, C.M.; Davidowitz, G.; Hahn, D.A. Life-extending dietary restriction and ovariectomy each increase leucine oxidation and alter leucine allocation in grasshoppers. Exp. Gerontol. 2017, 96, 155–161. [Google Scholar] [CrossRef]
- Schowalter, T.D. Biology and Management of the Eastern Lubber Grasshopper (Orthoptera: Acrididae). J. Integr. Pest Manag. 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Asboth, L.; Friedli, L.; Beauparlant, J.; Martinez-Gonzalez, C.; Anil, S.; Rey, E.; Baud, L.; Pidpruzhnykova, G.; Anderson, M.A.; Shkorbatova, P.; et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci. 2018, 21, 576–588. [Google Scholar] [CrossRef]
- Tian, Z.Z.; Kyte, M.D.; Messer, C.J. Parallax error in video-image systems. J. Transp. Eng. 2002, 128, 218–223. [Google Scholar] [CrossRef]
- Alluin, O.; Karimi-Abdolrezaee, S.; Delivet-Mongrain, H.; Leblond, H.; Fehlings, M.G.; Rossignol, S. Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J. Neurotrauma 2011, 28, 1963–1981. [Google Scholar] [CrossRef]
- Eftaxiopoulou, T.; Macdonald, W.; Britzman, D.; Bull, A.M.J. Gait compensations in rats after a temporary nerve palsy quantified using temporo-spatial and kinematic parameters. J. Neurosci. Methods 2014, 232, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyan, G.S.; Ball, E.E. The grasshopper, Drosophila and neuronal homology (advantages of the insect nervous system for the neuroscientist). Prog. Neurobiol. 1993, 41, 657–682. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, L.; Shon, A.; Knox, D.; Song, H.; Park, H.; Kim, J. Motorized Treadmill and Optical Recording System for Gait Analysis of Grasshoppers. Sensors 2021, 21, 5953. https://doi.org/10.3390/s21175953
Barreto L, Shon A, Knox D, Song H, Park H, Kim J. Motorized Treadmill and Optical Recording System for Gait Analysis of Grasshoppers. Sensors. 2021; 21(17):5953. https://doi.org/10.3390/s21175953
Chicago/Turabian StyleBarreto, Leslie, Ahnsei Shon, Derrick Knox, Hojun Song, Hangue Park, and Jeonghee Kim. 2021. "Motorized Treadmill and Optical Recording System for Gait Analysis of Grasshoppers" Sensors 21, no. 17: 5953. https://doi.org/10.3390/s21175953






