Hospitals and Laboratories on Paper-Based Sensors: A Mini Review
Abstract
:1. Introduction
2. Paper-Based Sensors for Physical Factors Sensing
2.1. Humidity Sensing
2.2. Mechanical Sensing
3. Paper-Based Sensor for Biomolecule Sensing
3.1. Nucleic Acid Sensing
3.2. Protein Sensing
3.3. Sugar Sensing
3.4. Antigen/Antibody Sensing
3.5. Vitamin Sensing
3.6. Neurotransmitter Sensing
3.7. Antibiotic Sensing
4. Paper-Based Sensors for Food Safety Testing
5. Paper-Based Sensors for Environmental Quality Inspection
6. Conclusions and Future Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Khorablou, Z.; Shahdost-fard, F.; Razmi, H.; Yola, M.L.; Karimi-Maleh, H. Recent advances in developing optical and elec-trochemical sensors for analysis of methamphetamine: A review. Chemosphere 2021, 278, 130393. [Google Scholar] [CrossRef]
- Meena, K.V.; Sankar, A.R. Biomedical Catheters with Integrated Miniature Piezoresistive Pressure Sensors: A Review. IEEE Sens. J. 2021, 21, 10241–10290. [Google Scholar] [CrossRef]
- Fuh, Y.K.; Wang, B.S. Near field sequentially electrospun three-dimensional piezoelectric fibers arrays for self-powered sensors of human gesture recognition. Nano Energy 2016, 30, 677–683. [Google Scholar] [CrossRef]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Ap-plications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [Green Version]
- White, D.; Keramane, M.; Capretta, A.; Brennan, J.D. A paper-based biosensor for visual detection of glucose-6-phosphate dehydrogenase from whole blood. Analyst 2020, 145, 1817–1824. [Google Scholar] [CrossRef]
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Abas, W.A.B.W.; Pingguan-Murphy, B.; et al. An inte-grated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab Chip 2016, 16, 611–621. [Google Scholar] [CrossRef]
- Alkasir, R.S.J.; Rossner, A.; Andreescu, S. Portable Colorimetric Paper-Based Biosensing Device for the Assessment of Bisphenol A in Indoor Dust. Environ. Sci. Technol. 2015, 49, 9889–9897. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Giordano, G.F.; Soares, C.C.S.P.; de Oliveira, J.F.A.; Mendes, R.K.; Piazzetta, M.H.; Gobbi, A.L.; Cardoso, M.B. Optical paper-based sensor for ascorbic acid quantification using silver nanoparticles. Talanta 2015, 141, 188–194. [Google Scholar] [CrossRef]
- Ming, T.; Luo, J.; Liu, J.; Sun, S.; Xing, Y.; Wang, H.; Xiao, G.; Deng, Y.; Cheng, Y.; Yang, Z.; et al. Paper-based microfluidic aptasensors. Biosens. Bioelectron. 2020, 170, 112649. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-Based Sensors for Gas, Humidity and Strain Detections: A Review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef]
- Wang, T.; Reid, R.C.; Minteer, S.D. A Paper-based Mitochondrial Electrochemical Biosensor for Pesticide Detection. Electroa-nalysis 2016, 28, 854–859. [Google Scholar] [CrossRef]
- Tian, T.; Liu, H.; Li, L.; Yu, J.; Ge, S.; Song, X.; Yan, M. Paper-based biosensor for noninvasive detection of epidermal growth factor receptor mutations in non-small cell lung cancer patients. Sens. Actuators B 2017, 251, 440–445. [Google Scholar] [CrossRef]
- Fakhri, N.; Abarghoei, S.; Dadmehr, M.; Hosseini, M.; Sabahi, H.; Ganjali, M.R. Paper based colorimetric detection of miR-NA-21 using Ag/Pt nanoclusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117529. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Srivastava, S.; Yadav, B.K.; Lee, S.H.; Sharma, J.G.; Doval, D.C.; Malhotra, B.D. Reduced graphene oxide modified smart conducting paper for cancer biosensor. Biosens. Bioelectron. 2015, 73, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Lee, M.; Kim, D. Detection of early stage prostate cancer by using a simple carbon nanotube@paper biosensor. Biosens. Bioelectron. 2018, 102, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.A.; Halweg, S.; Joyce, M.; Lieberman, M.; Goodson, H.V. Incorporating yeast biosensors into paper-based analytical tools for pharmaceutical analysis. Anal. Bioanal. Chem. 2015, 407, 615–619. [Google Scholar] [CrossRef]
- Chaiyo, S.; Mehmeti, E.; Siangproh, W.; Hoang, T.L.; Nguyen, H.P.; Chailapakul, O.; Kalcher, K. Non-enzymatic electro-chemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine–ionic liquid–graphene composite. Biosens. Bioelectron. 2018, 102, 113–120. [Google Scholar] [CrossRef]
- Zhu, P.; Kuang, Y.; Wei, Y.; Li, F.; Ou, H.; Jiang, F.; Chen, G. Electrostatic self-assembly enabled flexible paper-based humidity sensor with high sensitivity and superior durability. Chem. Eng. J. 2020, 404, 127105. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, K.; Tian, H.; Liu, Y.; Wang, D.; Chen, Y.; Yang, Y.; Ren, T. Graphene-Paper Pressure Sensor for Detecting Human Motions. ACS Nano 2017, 11, 8790–8795. [Google Scholar] [CrossRef]
- Long, Y.; He, P.; Xu, R.; Hayasaka, T.; Shao, Z.; Zhong, J.; Lin, L. Molybdenum-carbide-graphene composites for paper-based strain and acoustic pressure sensors. Carbon 2020, 157, e594–e601. [Google Scholar] [CrossRef]
- Wang, C.; Hou, X.; Cui, M.; Yu, J.; Fan, X.; Qian, J.; He, J.; Geng, W.; Mu, J.; Chou, X. An ultra-sensitive and wide measuring range pressure sensor with paper-based CNT film/interdigitated structure. Sci. China Mater. 2019, 63, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Jiao, Z.; Zhang, J.; Wang, Y.; Zhang, C.; Meng, X.; Jiang, X.; Niu, S.; Han, Z.; Ren, L. Bioinspired, Superhydrophobic, and Paper-Based Strain Sensors for Wearable and Underwater Applications. ACS Appl. Mater. Interfaces 2021, 13, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pan, N.; Zhu, Z.; Li, R.; Li, B.; Chu, J.; Li, G.; Chang, Y.; Pan, T. All-in-One Iontronic Sensing Paper. Adv. Funct. Mater. 2019, 29, 1807343. [Google Scholar] [CrossRef]
- Tian, R.; Li, Y.; Bai, J. Hierarchical assembled nanomaterial paper based analytical devices for simultaneously electrochemical detection of microRNAs. Anal. Chim. Acta 2019, 1058, e89–e96. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jian, Y.; Kong, Q.; Liu, H.; Lan, F.; Liang, L.; Ge, S.; Yu, J. Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks. Sens. Actuators B 2018, 257, 561–569. [Google Scholar] [CrossRef]
- Lv, S.; Tang, Y.; Zhang, K.; Tang, D. Wet NH3-Triggered NH2-MIL-125(Ti) structural switch for visible fluorescence immunoassay impregnated on paper. Anal. Chem. 2018, 90, 14121–14125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabria, D.; Caliceti, C.; Zangheri, M.; Mirasoli, M.; Simoni, P.; Roda, A. Smartphone–based enzymatic biosensor for oral fluid L-lactate detection in one minute using confined multilayer paper reflectometry. Biosens. Bioelectron. 2017, 94, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Leonor, G.; Novell, M.; Blondeau, P.; Andrade, F.J. A disposable, simple, fast and low-cost paper-based biosensor and its ap-plication to the determination of glucose in commercial orange juices. Food Chem. 2018, 265, 64–69. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Y.; Ding, L.; Yu, G.; Leng, Y.; Lai, W.; Xiong, Y.; Chen, X. Supramolecular Recognition-Mediated Lay-er-by-Layer Self-Assembled Gold Nanoparticles for Customized Sensitivity in Paper-Based Strip Nanobiosensors. Small 2019, 15, 1903861. [Google Scholar] [CrossRef]
- Nantaphol, S.; Channon, R.B.; Kondo, T.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Boron Doped Diamond Paste Electrodes for Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2017, 89, 4100–4107. [Google Scholar] [CrossRef]
- Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A paper-based nanomodified electrochemical biosensor for ethanol detection in beers. Anal. Chim. Acta 2017, 960, 123–130. [Google Scholar] [CrossRef]
- Mahato, K.; Chandra, P. Paper-based miniaturized immunosensor for naked eye ALP detection based on digital image col-orimetry integrated with smartphone. Biosens. Bioelectron. 2019, 128, 9–16. [Google Scholar] [CrossRef]
- Wang, H.; Vagin, S.I.; Rieger, B.; Meldrum, A. An Ultrasensitive Fluorescent Paper-Based CO2 Sensor. ACS Appl. Mater. In-terfaces 2020, 12, 20507–20513. [Google Scholar] [CrossRef] [PubMed]
- Noori, R.; Perwez, M.; Mazumder, J.A.; Sardar, M. Development of low-cost paper-based biosensor of polyphenol oxidase for detection of phenolic contaminants in water and clinical samples. Environ. Sci. Pollut. Res. 2020, 27, 30081–30092. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, O.; Fu, H.; Fan, Y.; Xu, L.; Meng, Q.; Zhang, L.; Lan, W.; Wu, C.; Tang, S.; et al. Paper-based sensor for visual detection of Ag+ based on a “turn-off-on” fluorescent design. Microchem. J. 2020, 157, 104887. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Wu, X.; Chen, X.; Qin, G.; Zhang, M.; Wu, H.; Fang, Y.; Lu, H.; Yu, L.; Li, L.; et al. Two-component ratiometric sensor for Cu2+ detection on paper-based device. Anal. Bioanal. Chem. 2019, 411, 6165–6172. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, Flexible, Cost-Saving, and Environment-Friendly Paper-Based Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef]
- Castro, C.; Rosillo, C.; Tsutsui, H. Characterizing effects of humidity and channel size on imbibition in paper-based microfluidic channels. Microfluid Nanofluid 2017, 21, 21. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Xu, Y.; Shen, M.; Duan, C.; Dai, L.; Ni, Y. Green and sustainable cellulose-derived humidity sensors: A review. Carbohydr. Polym. 2021, 270, 118385. [Google Scholar] [CrossRef]
- Shen, L.-L.; Zhang, G.-R.; Etzold, B.J.M. Paper-Based Microfluidics for Electrochemical Applications. ChemElectroChem 2020, 7, 10–30. [Google Scholar] [CrossRef]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Xu, Y.; Fei, Q.; Page, M.; Zhao, G.; Ling, Y.; Stoll, S.B.; Yan, Z. Paper-based wearable electronics. iScience 2021, 24, 102736. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, V.S.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, W.; Cao, K.; Hu, X.; Gao, L.; Lu, Y. Architectured graphene and its composites: Manufacturing and structural applications. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106177. [Google Scholar] [CrossRef]
- Yang, T.; Mativetsky, J.M. Paper-Based Mechanical Sensors Enabled by Folding and Stacking. ACS Appl. Mater. Interfaces 2019, 11, 26339–26345. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, H.; Zhang, S.; Zhang, D.; Liu, X.; He, Y.; Mi, L.; Zhang, J.; Liu, C.; Shen, C.; et al. Superhydrophobic Electrically Conductive Paper for Ultrasensitive Strain Sensor with Excellent Anticorrosion and Self-Cleaning Property. ACS Appl. Mater. Interfaces 2019, 11, 21904–21914. [Google Scholar] [CrossRef]
- Mao, K.; Min, X.; Zhang, H.; Zhang, K.; Cao, H.; Guo, Y.; Yang, Z. Paper-based microfluidics for rapid diagnostics and drug delivery. J. Control. Release 2020, 322, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Caspersson, T.; Schultz, J. Nucleic Acid Metabolism of the Chromosomes in Relation to Gene Reproduction. Nature 1938, 142, 294–295. [Google Scholar] [CrossRef]
- Hisae, T.-K.; Naoki, S. Roles of non-canonical structures of nucleic acids in cancer and neurodegenerative diseases. Nucleic Acids Res. 2021, 49, 7839–7855. [Google Scholar] [CrossRef]
- Gilboa, T.; Garden, P.M.; Cohen, L. Single-molecule analysis of nucleic acid biomarkers—A review. Anal. Chim. Acta 2020, 1115, 61–85. [Google Scholar] [CrossRef]
- Zhao, Y.; Zuo, X.; Li, Q.; Chen, F.; Chen, Y.-R.; Deng, J.; Han, D.; Hao, C.; Huang, F.; Huang, Y.; et al. Nucleic acids analysis. Sci. China Chem. 2021, 64, 171–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, P.; Zhang, C.; Ma, Z. Epidermal growth factor receptor (EGFR): A rising star in the era of precision medicine of lung cancer. Oncotarget 2017, 8, 50209–50220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Wang, X.; Wu, J.; Li, H.; Li, F. Equipment-Free and Visualized Biosensor for Transcription Factor Rapid Assay Based on Dopamine-Functionalized Cellulose Paper. J. Mater. Chem. B 2019, 7, 5461–5464. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.; Blondeau, P.; Novell, M.; Andrade, F.J.; Rius, F.X.; Riu, J. Paper-based chemiresistor for detection of ultralow concentrations of protein. Biosens. Bioelectron. 2013, 49, 462–465. [Google Scholar] [CrossRef]
- Fischer, C.; Fraiwan, A.; Choi, S. A 3D paper-based enzymatic fuel cell for self-powered, low-cost glucose monitoring. Biosens. Bioelectron. 2016, 79, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Gartia, M.R.; Misra, S.K.; Ye, M.; Schwartz-Duval, A.; Plucinski, L.; Zhou, X.; Kellner, D.; Labriola, L.T.; Pan, D. Point-of-service, quantitative analysis of ascorbic acid in aqueous humor for evaluating anterior globe integrity. Sci. Rep. 2015, 5, 16011. [Google Scholar] [CrossRef]
- Duyen, T.T.M.; Matsuura, H.; Ujiie, K.; Muraoka, M.; Harada, K.; Hirata, K. Paper-based colorimetric biosensor for antibiotics inhibiting bacterial protein synthesis. J. Biosci. Bioeng. 2017, 123, 96–100. [Google Scholar] [CrossRef]
- Wagner, D.R.; Heyward, V.H. Measures of body composition in blacks and whites: A comparative review. Am. J. Clin. Nutr. 2000, 71, 1392–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhelst, X.; Dias, A.M.; Colombel, J.-F.; Vermeire, S.; Vlierberghe, H.V.; Callewaert, N.; Pinho, S.S. Protein Glycosylation as a Diagnostic and Prognostic Marker of Chronic Inflammatory Gastrointestinal and Liver Diseases. Gastroenterology 2020, 158, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Deng, Y.; Yang, X.; Xue, H.; Lang, Y. The Relationship between Protein S-Nitrosylation and Human Diseases: A Review. Neurochem. Res. 2020, 45, 2815–2827. [Google Scholar] [CrossRef]
- Ki, H.; Jang, H.; Oh, J.; Han, G.; Lee, H.; Kim, S.; Kim, M. Simultaneous Detection of Serum Glucose and Glycated Albumin on a Paper-Based Sensor for Acute Hyperglycemia and Diabetes Mellitus. Anal. Chem. 2020, 92, 11530–11534. [Google Scholar] [CrossRef]
- Parrilla, M.; Cánovas, R.; Andrade, F.J. Paper-based enzymatic electrode with enhanced potentiometric response for moni-toring glucose in biological fluids. Biosens. Bioelectron. 2017, 90, 110–116. [Google Scholar] [CrossRef]
- Huang, P.; Hong, C.P.; Zhu, J.F.; Chen, T.; Chan, C.; Ko, Y.; Lin, T.; Pan, Z.; Sun, N.; Wang, Y.; et al. Ag@Au nanoprisms-metal organic frameworks-based paper for extending glucose sensing range in human serum and urine. Dalton Trans. 2017, 46, 6985–6993. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Bajo, E.; Fernández-Abedul, M.T. Paper-based platforms with coulometric readout for ascorbic acid determination in fruit juices. Analyst 2020, 145, 3431–3439. [Google Scholar] [CrossRef] [PubMed]
- Sarada, B.V.; Rao, T.N.; Tryk, D.A.; Fujishima, A. Electrochemical Oxidation of Histamine and Serotonin at Highly Bo-ron-Doped Diamond Electrodes. Anal. Chem. 2000, 72, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.Q.; Le, T.P.Q.; Le, N.D.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.-H.; Oeurng, C.; et al. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2021, 764, 142865. [Google Scholar] [CrossRef] [PubMed]
- Magureanu, M.; Bilea, F.; Bradu, C.; Hong, D. A review on non-thermal plasma treatment of water contaminated with anti-biotics. J. Hazard. Mater. 2021, 417, 125481. [Google Scholar] [CrossRef] [PubMed]
- Mentana, A.; Palermo, C.; Nardiello, D.; Quinto, M.; Centonze, D. Simultaneous and accurate real time monitoring of glucose and ethanol in alcoholic drinks, must, and biomass by a dual amperometric biosensor. J. Agric. Food Chem. 2013, 61, 61–68. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Khan, H.; Yang, L. A dual-readout paper-based sensor for onsite detection of penicillinase with a smartphone. Sens. Actuators B Chem. 2021, 335, 129707. [Google Scholar] [CrossRef]
- Hidayat, M.A.; Maharani, D.A.; Purwanto, D.A.; Kuswandi, B.; Yuwono, M. Simple and Sensitive Paper-based Colorimetric Biosensor for Determining Total Polyphenol Content of the Green Tea Beverages. Biotechnol. Bioprocess Eng. 2020, 25, 255–263. [Google Scholar] [CrossRef]
- Zhang, D.; Li, C.; Ji, D.; Wang, Y. Paper-Based Microfluidic Sensors for Onsite Environmental Detection: A Critical Review. Crit. Rev. Anal. Chem. 2021, 1886900. [Google Scholar] [CrossRef]
- Lin, Y.; Gritsenko, D.; Feng, S.; Teh, Y.C.; Lu, X.; Xu, J. Detection of heavy metal by paper-based microfluidics. Biosens. Bioe-lectron. 2016, 83, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Dabhade, A.; Jayaraman, S.; Paramasivan, B. Colorimetric paper bioassay by horseradish peroxidase for the detection of cat-echol and resorcinol in aqueous samples. Prep. Biochem. Biotechnol. 2020, 50, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Dungchai, W.; Sameenoi, Y.; Chailapakul, O.; Volckens, J.; Henry, C.S. Determination of aerosol oxidative activity using silver nanoparticle aggregation on paper-based analytical devices. Analyst 2013, 138, 6766–6773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liang, F. Progress in Paper-based Colorimetric Sensor Array. Chin. J. Anal. Chem. 2020, 48, 1448–1457. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Tong, J.; Guan, X.; Bian, C.; Xia, S. Paper-Based Sensor Chip for Heavy Metal Ion Detection by SWSV. Mi-cromachines 2018, 9, 150. [Google Scholar] [CrossRef] [Green Version]
- Cinti, S.; Talarico, D.; Palleschi, G.; Moscone, D.; Arduini, F. Novel reagentless paper-based screen-printed electrochemical sensor to detect phosphate. Anal. Chim. Acta 2016, 919, 78–84. [Google Scholar] [CrossRef]
- Yakoh, A.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and selective paper-based colorimetric sensor for determi-nation of chloride ion in environmental samples using label-free silver nanoprisms. Talanta 2018, 178, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.; Lee, K.; Lin, C.; Zhang, S.; Wang, C.; Liu, T. Portable Nanohybrid Paper-Based Chemiresistive Sensor for Free Chlorine Detection. ACS Omega 2020, 5, 25209–25215. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandi, M.; Thiruppathi, D.; Vasimalai, N. One minute synthesis of green fluorescent copper nanocluster: The prep-aration of smartphone aided paper-based kit for onsite monitoring of nanomolar level mercury and sulfide ions in environ-mental samples. J. Hazard. Mater. 2020, 392, 122294. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, C.; Lu, B.; Lin, P.; Ho, M. Determination of nitrite ions in environment analysis with a paper-based microfluidic device. Dalton Trans. 2018, 47, 14799–14807. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.E.E.; Kamel, A.H.; Almehizia, A.A.; Sayed, A.Y.A.; Elsayed, E.A.; Abd-Rabboh, H.S.M. Paper-Based Potentiometric Sensors for Nicotine Determination in Smokers’ Sweat. ACS Omega 2021, 6, 11340–11347. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, D.; Qiu, S.; Ren, L.; Huang, S.; Liu, R.; Wang, L.; Wang, H. Polyphenylene sulfide paper-based sensor modified by Eu-MOF for efficient detection of Fe3+. React. Funct. Polym. 2021, 165, 104954. [Google Scholar] [CrossRef]
- Prabpal, J.; Vilaivan, T.; Praneenararat, T. Paper-Based Heavy Metal Sensors from the Concise Synthesis of an Anionic Por-phyrin: A Practical Application of Organic Synthesis to Environmental Chemistry. J. Chem. Educ. 2017, 94, 1137–1142. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Wang, X.; Zhang, Z.; Wang, Z.; Han, J.; Chen, L. Three-dimensional paper-based microfluidic chip device for multiplexed fluorescence detection of Cu2+ and Hg2+ ions based on ion imprinting technology. Sens. Actuators B Chem. 2017, 251, 224–233. [Google Scholar] [CrossRef]
- Ding, R.; Cheong, Y.H.; Ahamed, A.; Lisak, G. Heavy Metals Detection with Paper-Based Electrochemical Sensors. Anal. Chem. 2021, 93, 1880–1888. [Google Scholar] [CrossRef] [PubMed]



| Output Signal | Principle | Analyte | Limit | Shelf Time | Year | Ref |
|---|---|---|---|---|---|---|
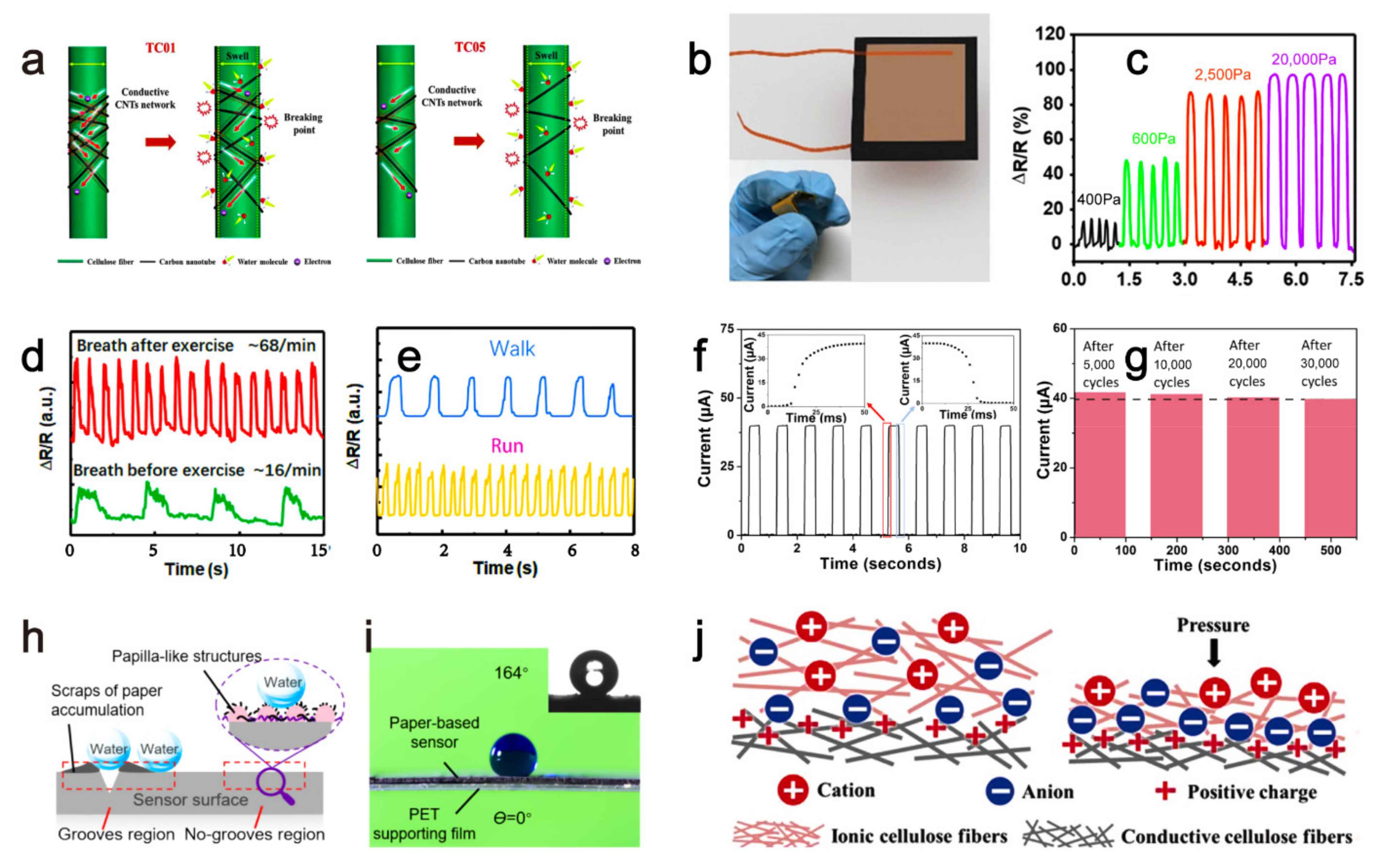
| current | Swelling of cellulose network after absorbing water causes the separation of the conductive carbon nanotubes attached to cellulose network, increasing sensor’s resistance, then the sensor’s current changed by the humidity. | humidity | - | Three months | 2020 | [18] |
| resistance | Applied pressure reduces the gap between the conductive paper layers carrying the reduced graphene oxide material, then the sensor’s resistance decreases. | pressure | - | - | 2017 | [19] |
| resistance | Conductive molybdenum carbide-graphene layers with porous and coral-shaped microstructure on the paper matrix allow micro-cracks under external compressive or tensile pressures, which eventually causes a resistance increase of the sensor. | pressure | - | - | 2020 | [20] |
| current | Low pressure reduces the gap between the PVP layer containing conductive carbon nanotubes and the interdigital electrodes, resulting in a resistance decrease. While high pressure reduces the gap between the conductive carbon nanotubes inside the PVP layer to reduce the sensor’s resistance, leading to an increased current. | pressure | 8 Pa | - | 2019 | [21] |
| resistance | Vibration caused deformation of the sensor, which increased/decreased the distance between the conductive grooves’ two walls and subsequently increase/decrease the resistance. | vibration | - | - | 2021 | [22] |
| capacitance | External pressure caused an increment of the contact area among the ionic and conductive cellulose fibers, leading to a capacitance rise of the ISP sensor. | pressure | 6.25 Pa | - | 2019 | [23] |
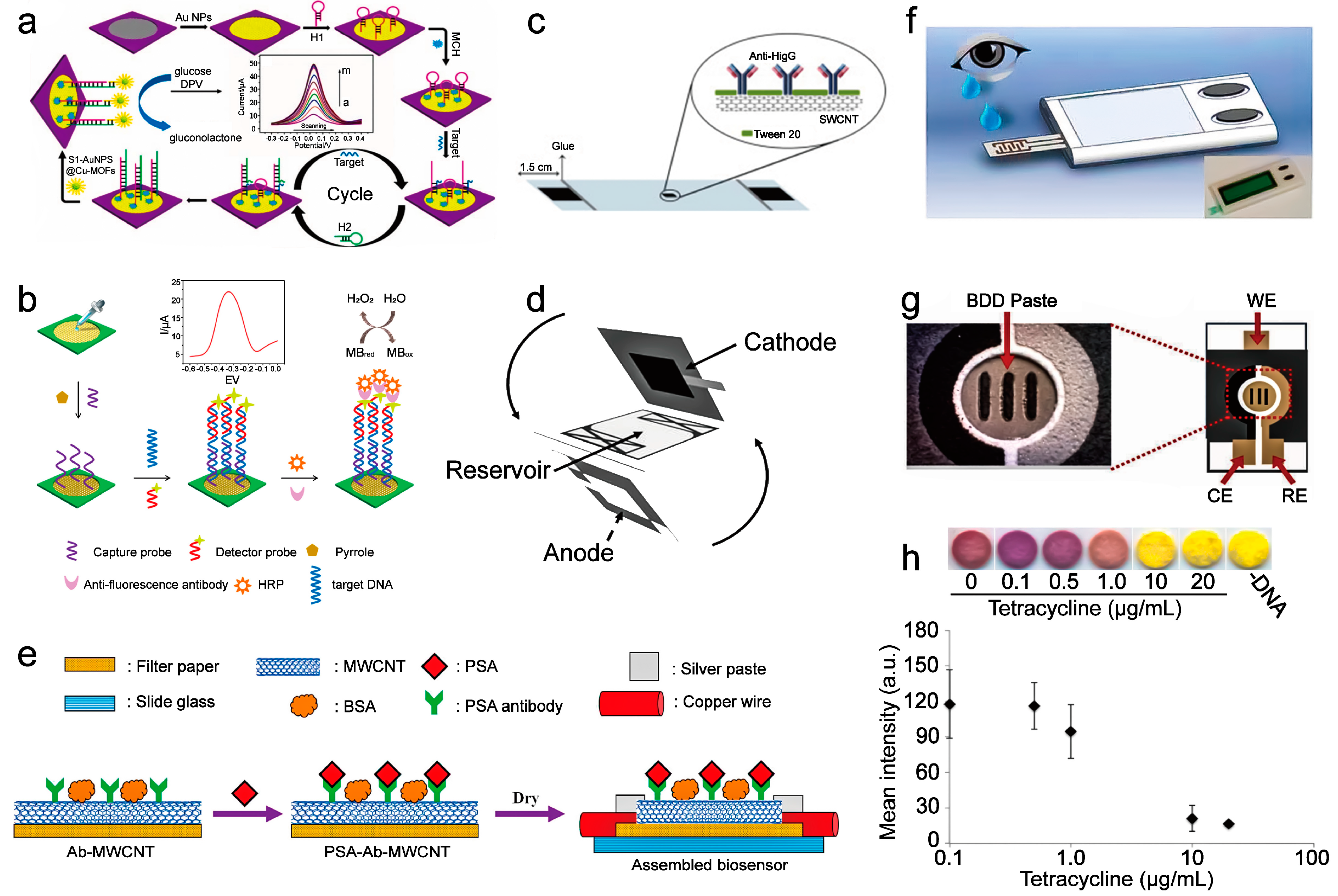
| current | After capturing the target miRNA, PtCuMOFs/ DNA3, and PtCuMOFs/DNA4 reporter probes are hybridized to the captured miRNA on the electrode surface and generates faradaic current with conjugated methylene blue and ferrocene. | miR−141, miR−21 | 0.1 fM/L | - | 2019 | [24] |
| current | The hairpin structure of H1 is opened after the miRNA−155 binding. Then the hairpin structure H2 competes with miRNA−155 from H1 through a stronger combination with H1, then continuously going through this process. Then the amplified H1-H2 duplexes combine with S1-AuNPs@Cu-MOFs to oxidize glucose to generate an electrical signal. | miRNA−155 | 0.35 fM/L | - | 2017 | [25] |
| current | After the mutation-related DNA is captured on the sensor, it then hybridizes with another DNA strand labeled with HRP and catalyzes hydrogen peroxide to generate electrical signals. | EGFR mutation DNA | 0.167 nM/L | Four weeks | 2017 | [12] |
| fluorescence | NH3-triggered Structure change of NH2-MIL-125(Ti) on the sensor is utilized for the visible fluorescence immunoassay of target CEA by coupling with a sandwich-type detection mode in the microplates. | CEA | 0.041 ng/mL | - | 2018 | [26] |
| chemiluminescence | Lactate oxidase on the sensor oxidizes lactate to generate hydrogen peroxide. Subsequently, the HRP fixed on the sensor catalyzes the oxidation of TMB by hydrogen peroxide to realize the lactate-concentration-dependent color delivery. | L-lactate | 0.1 mM/L | - | 2017 | [27] |
| electrochemical potential | The glucose oxidase on the sensor oxidizes glucose to generate hydrogen peroxide, which changes the redox potential of the solution directly. | glucose | 0.02 mM/L | One month | 2018 | [28] |
| resistance | Adsorption of PSA through the specific reaction between PSA and PSA antibody increases the distance between the multi-wall carbon nanotubes on the sensor, forcing the resistance increment. | PSA | 1.18 ng/mL | Two hours | 2018 | [15] |
| fluorescence | In the presence of the analytes, a layer-by-layer self-assembly reaction is allowed due to the recognition between the β-cyclodextrin-coated gold nanoparticles and 1-adamantane acetic acid or tetrakis (4-carboxyphenyl) porphyrin. And then, the accumulation of Au nanoparticles will give an increasing fluorescence. | carcinoembryonic antigen, p24 antigen | dozens of molecules per strip | - | 2019 | [29] |
| fluorescence | Reduced Ag+ in the silver nanoparticles to Ag atoms will aggregate into oligomeric clusters, distributing to the UV–vis absorption band. | AA | 82.8 µM/L | Three weeks | 2015 | [8] |
| current | Adsorption of norepinephrine or serotonin gives an electrode fouling, leading to the decrease of oxidation current and the shift of the positive potential peak. | NE, 5-HT | 2.5 µM/L, 0.5 µM/L | - | 2017 | [30] |
| chemiluminescence | Doxycycline relieves the expression of the reporter of the yeast bacteria, causing a noticeable color change in the sensor. | doxycycline | - | One year | 2015 | [16] |
| current | Ethanol oxidase on the senor oxides ethanol to generate hydrogen peroxide. Then the hydrogen peroxide reacted with the Prussian blue/carbon black nanoparticles to trigger an electric current on the sensor. | ethanol | 0.52 mM/L | Three weeks | 2017 | [31] |
| After the capture of the ALP by the ALP antibody on the sensor, it will catalyze the color-developing substrate to deliver an ALP-concentration-dependent color change. | ALP | 0.87 U/mL | Four weeks | 2019 | [32] | |
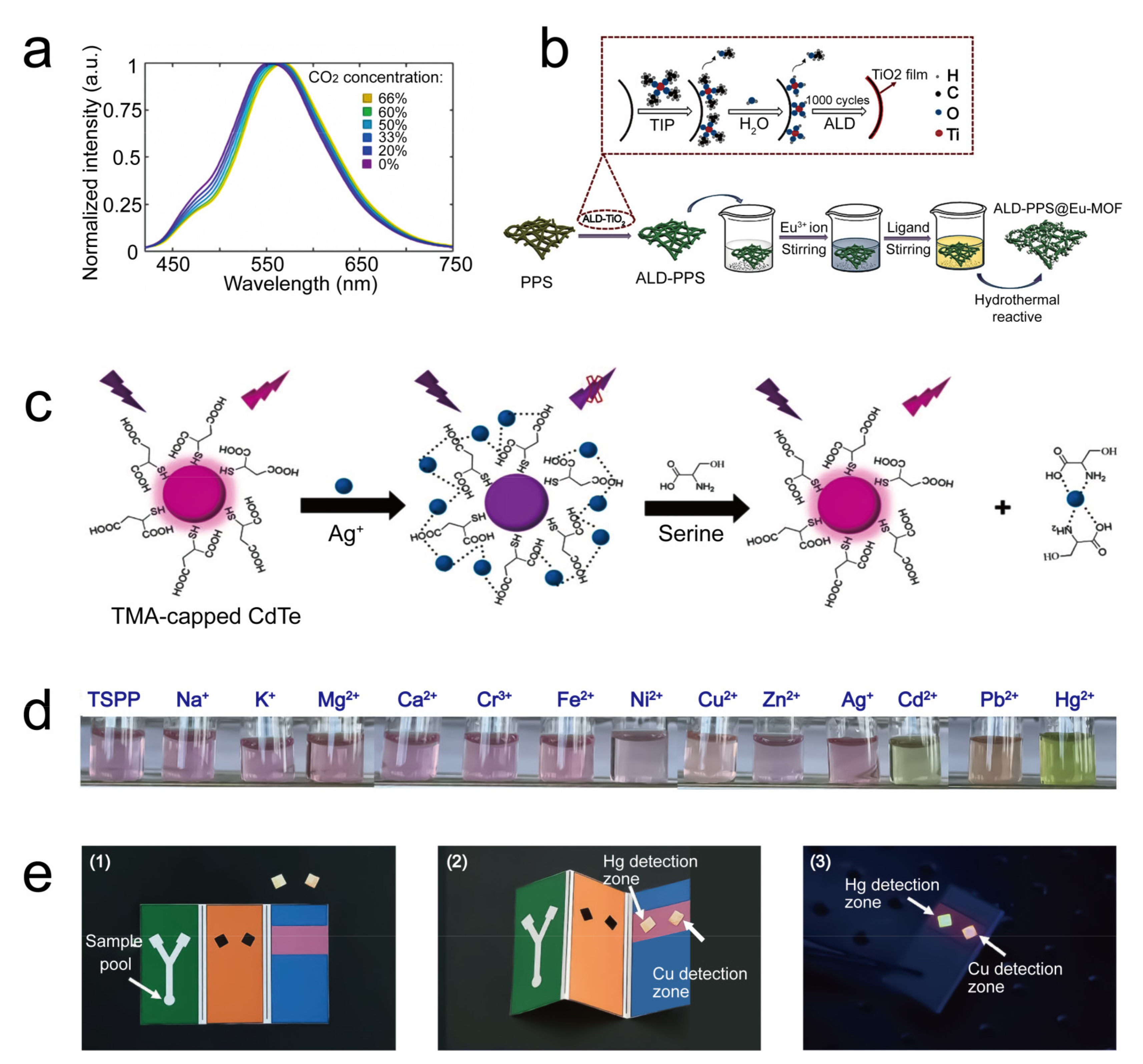
| fluorescence | In the presence of CO2, the fluorescent spectra peak of the P4VB on the sensor shows a CO2-concentration-dependent redshift. | CO2 | 5.7 ppm | - | 2020 | [33] |
| chemiluminescence | After the oxidation of phenol substrates by the polyphenol oxidase, the oxidization product will react with the benzothiazolinone hydrazine to present a color change of the sensor paper. | catechol, phenol, p-cresol, 4-methyl catechol | 0.5 µM/L | One month | 2020 | [34] |
| fluorescence | Ag+ binding quenches the fluorescence of the CdTe quantum dots. | Ag+ | 13.16 nM/L | - | 2020 | [35] |
| fluorescence | In the presence of Cu2+, the fluorescence of the fluorophore P2017 decreased while that of B001 was maintained well, thus giving a ratio-based fluorescence detection technology. | Cu2+ | 2.4 nM/L | - | 2019 | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xia, C.; Feng, G.; Fang, J. Hospitals and Laboratories on Paper-Based Sensors: A Mini Review. Sensors 2021, 21, 5998. https://doi.org/10.3390/s21185998
Zhang H, Xia C, Feng G, Fang J. Hospitals and Laboratories on Paper-Based Sensors: A Mini Review. Sensors. 2021; 21(18):5998. https://doi.org/10.3390/s21185998
Chicago/Turabian StyleZhang, Huaizu, Chengbin Xia, Guangfu Feng, and Jun Fang. 2021. "Hospitals and Laboratories on Paper-Based Sensors: A Mini Review" Sensors 21, no. 18: 5998. https://doi.org/10.3390/s21185998
APA StyleZhang, H., Xia, C., Feng, G., & Fang, J. (2021). Hospitals and Laboratories on Paper-Based Sensors: A Mini Review. Sensors, 21(18), 5998. https://doi.org/10.3390/s21185998









