Non-Contact Oxygen Saturation Measurement Using YCgCr Color Space with an RGB Camera
Abstract
:1. Introduction
2. Methods
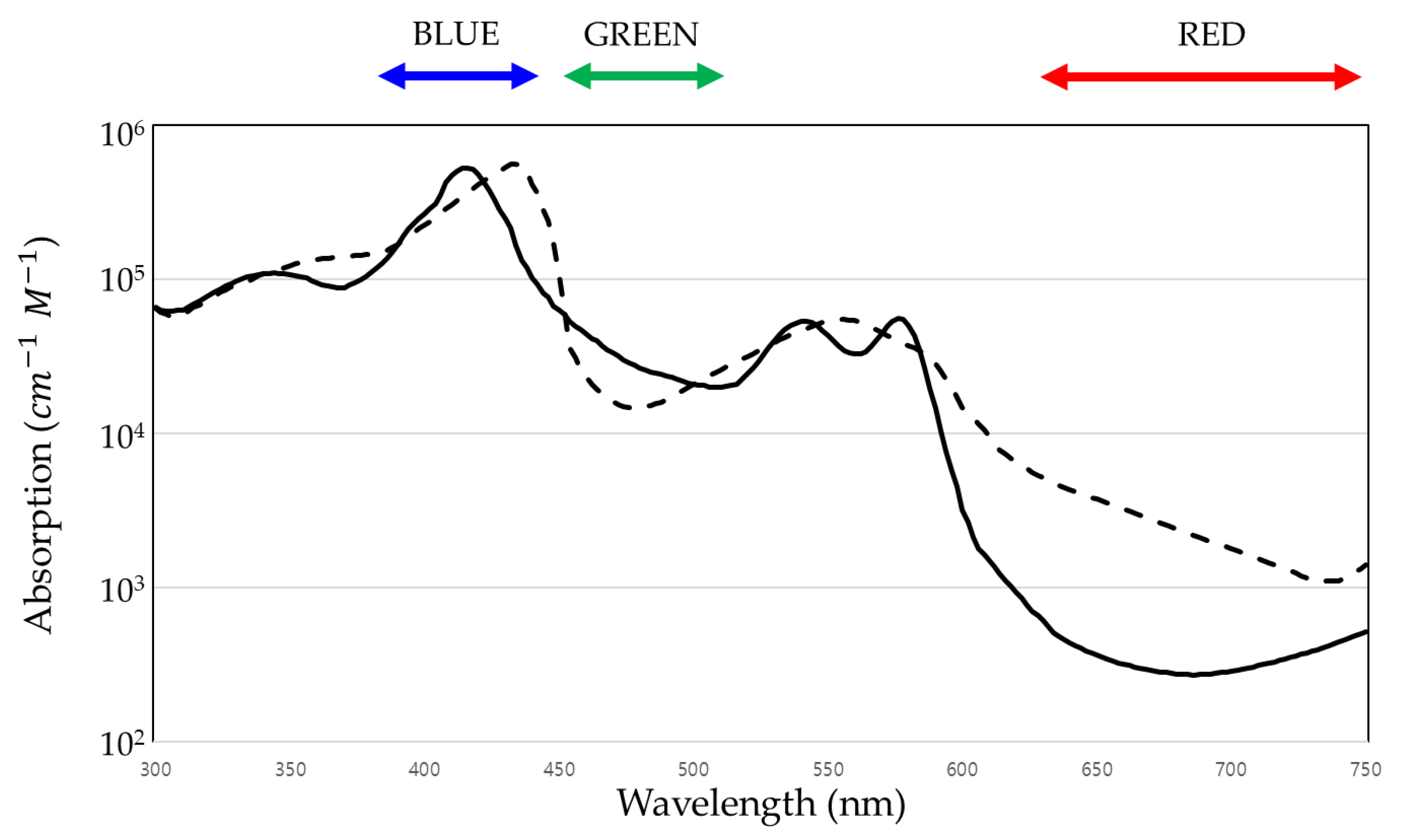
2.1. Oxygen Saturation Measurement Principle
2.2. Non-Contact SPO2 Measurement Method
2.2.1. Remote PPG Signal
2.2.2. SPO2 Signal
SPO2 Measurement Using YCgCr Signal
SPO2 Measurement Using YCbCr Signal
3. Results
3.1. Correlation between Rcgcr Value Obtained Using YCgCr Signal and Reference SPO2
3.2. Correlation between Rcbcr Value Obtained Using YCbCr Signal and Reference SPO2
3.3. Comparison of rSPO2 and Reference SPO2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- West, J.B. Pulmonary Pathophysiology: The Essentials; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Caputo, N.D.; Strayer, R.J.; Levitan, R. Early Self-Proning in Awake, Non-Intubated Patients in the Emergency Department: A Single ED’s Experience During the COVID-19 Pandemic. Acad. Emerg. Med. 2020, 27, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Severinghaus, J.W. Takuo Aoyagi: Discovery of Pulse Oximetry. Anesth. Analg. 2007, 105, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhao, Y.; Dong, L.; Jian, Y.; Jin, X.; Li, B.; Feng, Y.; Liu, M.; Liu, X.; Wu, H. Non-Contact Detection of Oxygen Saturation Based on Visible Light Imaging Device Using Ambient Light. Opt. Express 2013, 21, 17464–17471. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T. Current Progress of Photoplethysmography and SPO2 for Health Monitoring. Biomed. Eng. Lett. 2019, 9, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poh, M.Z.; McDuff, D.J.; Picard, R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express 2010, 18, 10762–10774. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhang, S.; Li, C.; Zhang, Y.; Cheng, J.; Chen, X. Heart rate estimation from facial videos using a spatiotemporal representation with convolutional neural networks. IEEE Trans. Instrum. Meas. 2020, 69, 7411–7421. [Google Scholar] [CrossRef]
- Allado, E.; Poussel, M.; Moussu, A.; Saunier, V.; Bernard, Y.; Albuisson, E.; Chenuel, B. Innovative measurement of routine physiological variables (heart rate, respiratory rate and oxygen saturation) using a remote photoplethysmography imaging system: A prospective comparative trial protocol. BMJ Open 2021, 11, e047896. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabulated Molar Extinction Coefficient for Hemoglobin in Water. Available online: https://omlc.org/spectra/hemoglobin/summary.html (accessed on 10 August 2021).
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse Oximetry: Understanding Its Basic Principles Facilitates Appreciation of Its Limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- CMS50E Fingertip Pulse Oximeter. Available online: https://www.cpap-supply.com/CMS50E-Pulse-Oximeter-p/cms50e.htm (accessed on 10 August 2021).
- Logitech. Available online: https://www.logitech.com/ko-kr/products/webcams/c920-pro-hd-webcam.960-001062.html (accessed on 10 August 2021).
- Suh, K.H.; Lee, E.C. Contactless Physiological Signals Extraction Based on Skin Color Magnification. J. Electron. Imaging 2017, 26, 063003. [Google Scholar] [CrossRef]
- Henriques, J.F.; Caseiro, R.; Martins, P.; Batista, J. High-speed tracking with kernelized correlation filters. IEEE Trans. Pattern Anal. Mach. Intell. 2014, 37, 583–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, G.H. oryong79, 18 May 2020. Real-Time rPPG with Facial Movements [Video File]. Available online: https://www.youtube.com/watch?v=DWW8oS1YRLU (accessed on 10 August 2021).
- De Dios, J.J.; Garcia, N. Face Detection Based on a New Color Space YCgCr. In Proceedings of the International Conference on Image Processing (Cat No. 03CH37429), Barcelona, Spain, 14–17 September 2003; Volume 3. [Google Scholar]
- Ali, A.S.; Radwan, A.G.; Soliman, A.M. Fractional order Butterworth filter: Active and passive realizations. IEEE J. Emerg. Sel. Top. Circuits Syst. 2013, 3, 346–354. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| MAE | RMSE | Cosine Similarity | Intraclass Correlation Coefficient | |||
| Intraclass Correlation | 95% Confidence Interval | Sig | ||||
| 0.537 (±0.436) | 0.692 | 0.999 | 0.922 | 0.905–0.936 | 0.000 | |
| 1.547 (±0.827) | 1.754 | 0.999 | 0.243 | 0.075–0.381 | 0.003 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.H.; Yu, S.-G.; Kim, S.-E.; Lee, E.C. Non-Contact Oxygen Saturation Measurement Using YCgCr Color Space with an RGB Camera. Sensors 2021, 21, 6120. https://doi.org/10.3390/s21186120
Kim NH, Yu S-G, Kim S-E, Lee EC. Non-Contact Oxygen Saturation Measurement Using YCgCr Color Space with an RGB Camera. Sensors. 2021; 21(18):6120. https://doi.org/10.3390/s21186120
Chicago/Turabian StyleKim, Na Hye, Su-Gyeong Yu, So-Eui Kim, and Eui Chul Lee. 2021. "Non-Contact Oxygen Saturation Measurement Using YCgCr Color Space with an RGB Camera" Sensors 21, no. 18: 6120. https://doi.org/10.3390/s21186120
APA StyleKim, N. H., Yu, S.-G., Kim, S.-E., & Lee, E. C. (2021). Non-Contact Oxygen Saturation Measurement Using YCgCr Color Space with an RGB Camera. Sensors, 21(18), 6120. https://doi.org/10.3390/s21186120






