Improved Photoresponse Characteristics of a ZnO-Based UV Photodetector by the Formation of an Amorphous SnO2 Shell Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of ZnO/a-SnO2 Core–Shell Nanorods-Based Photodetectors
2.3. Characterization
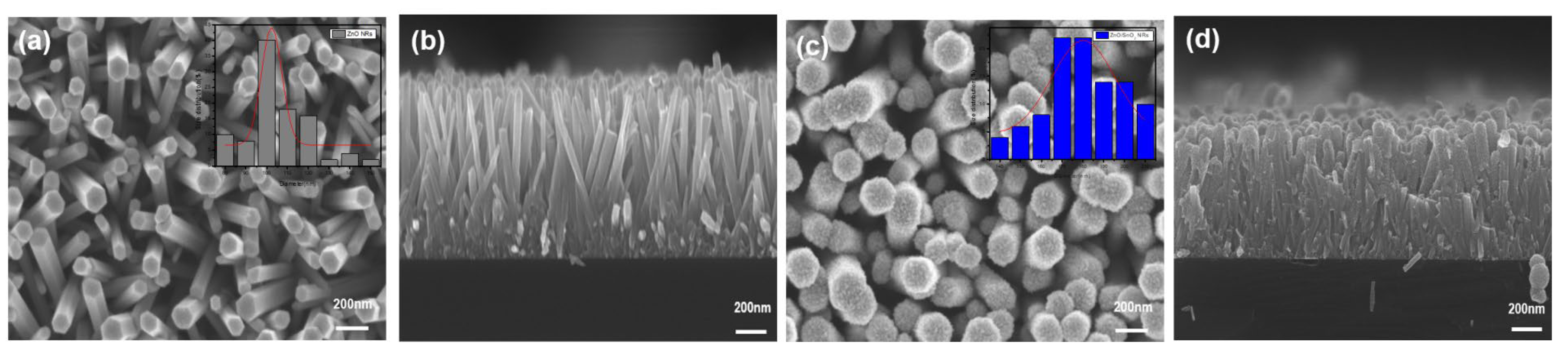
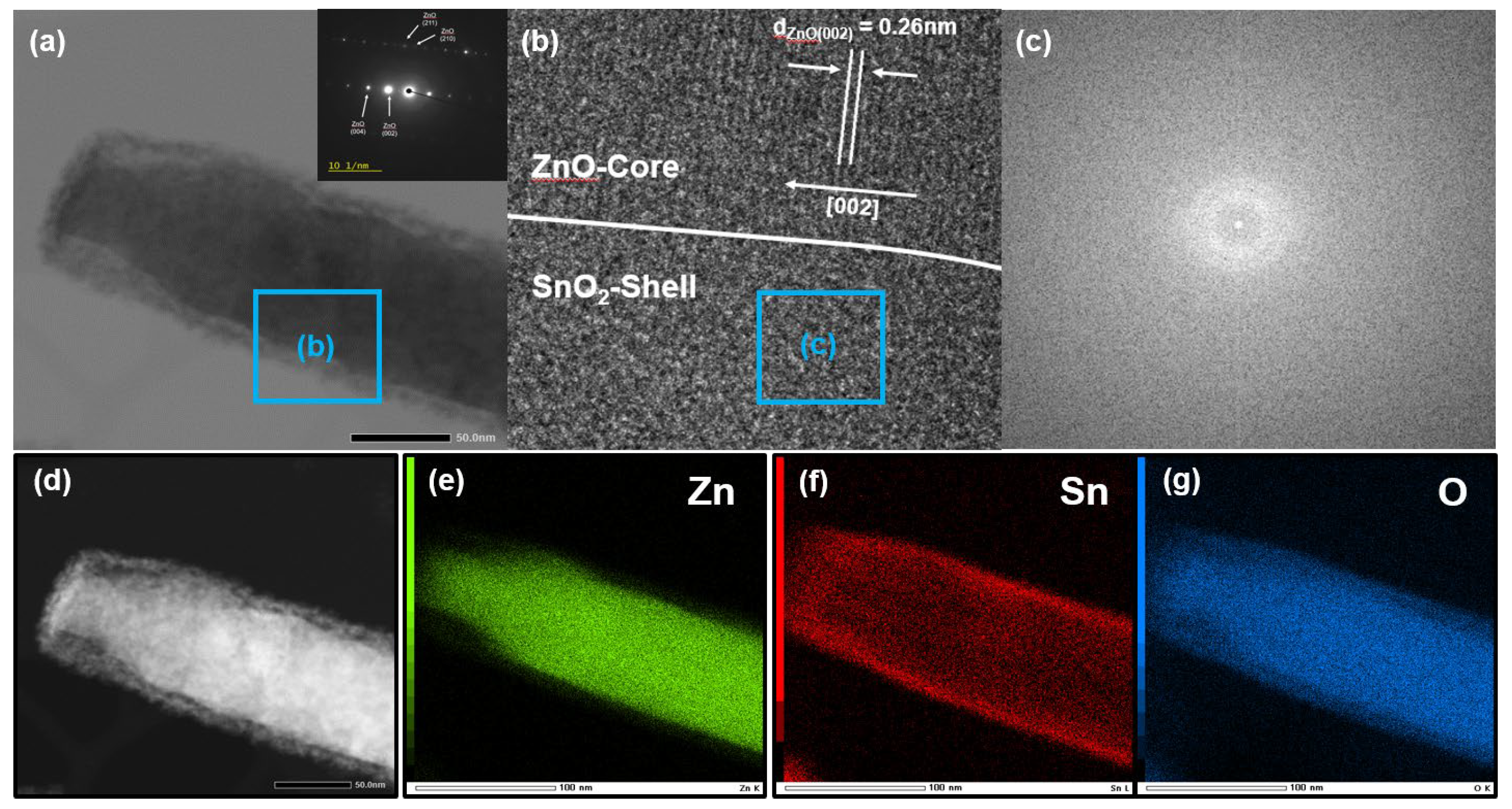
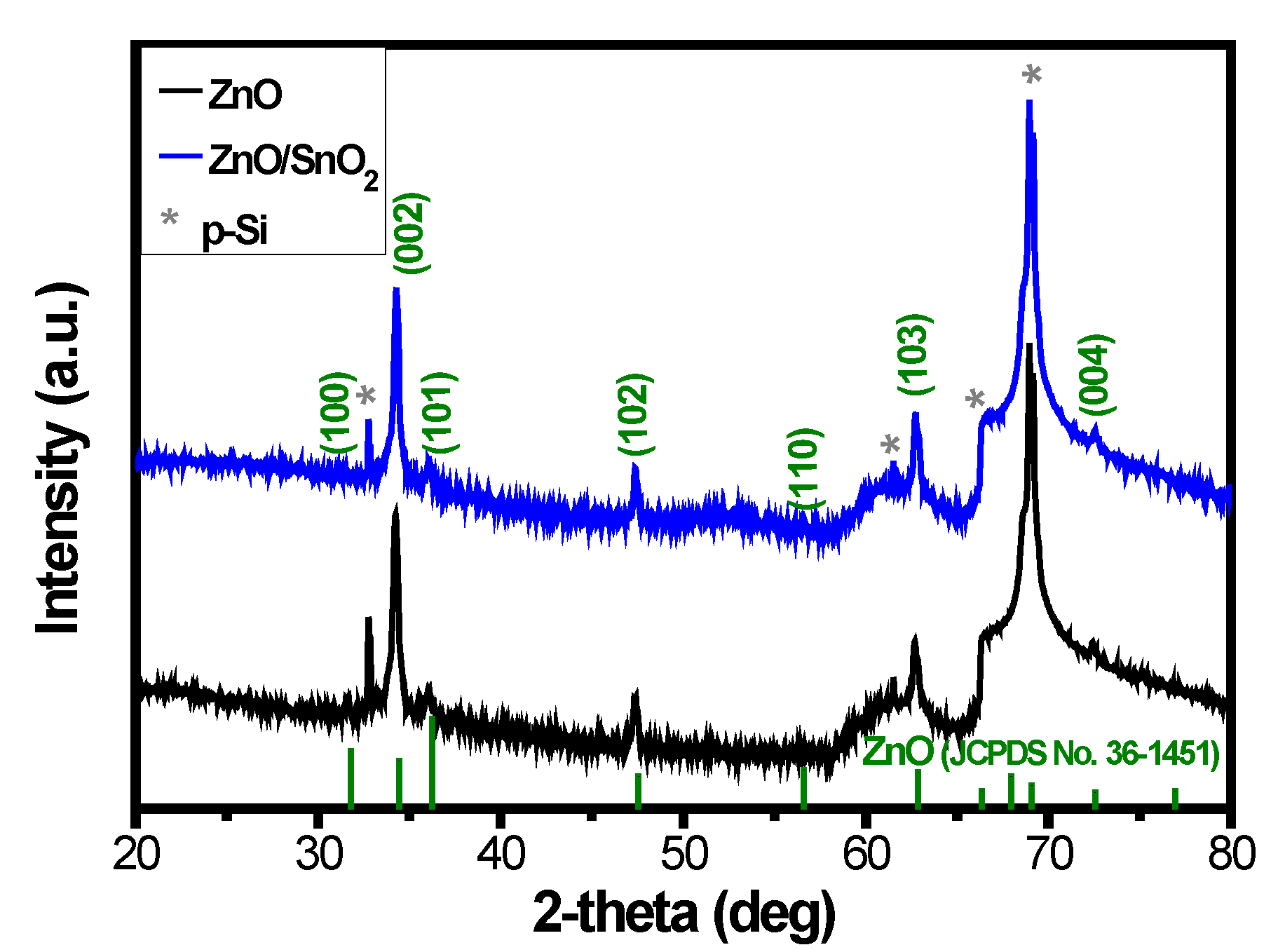
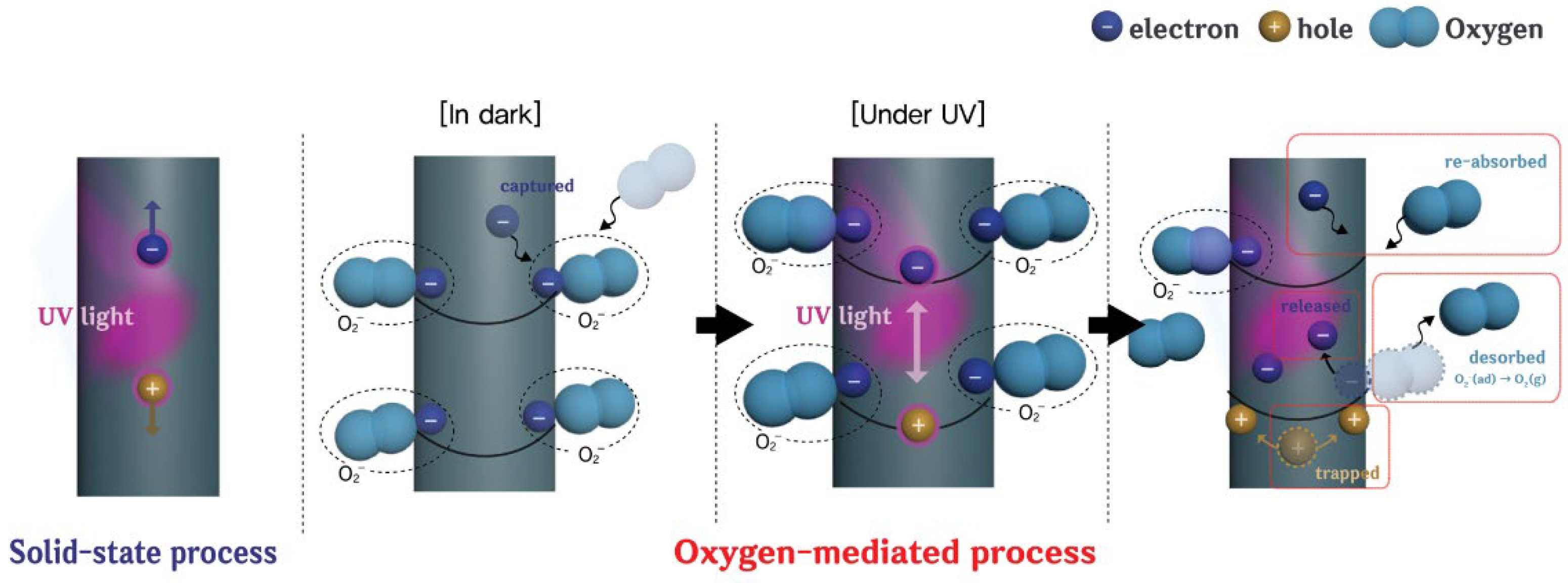
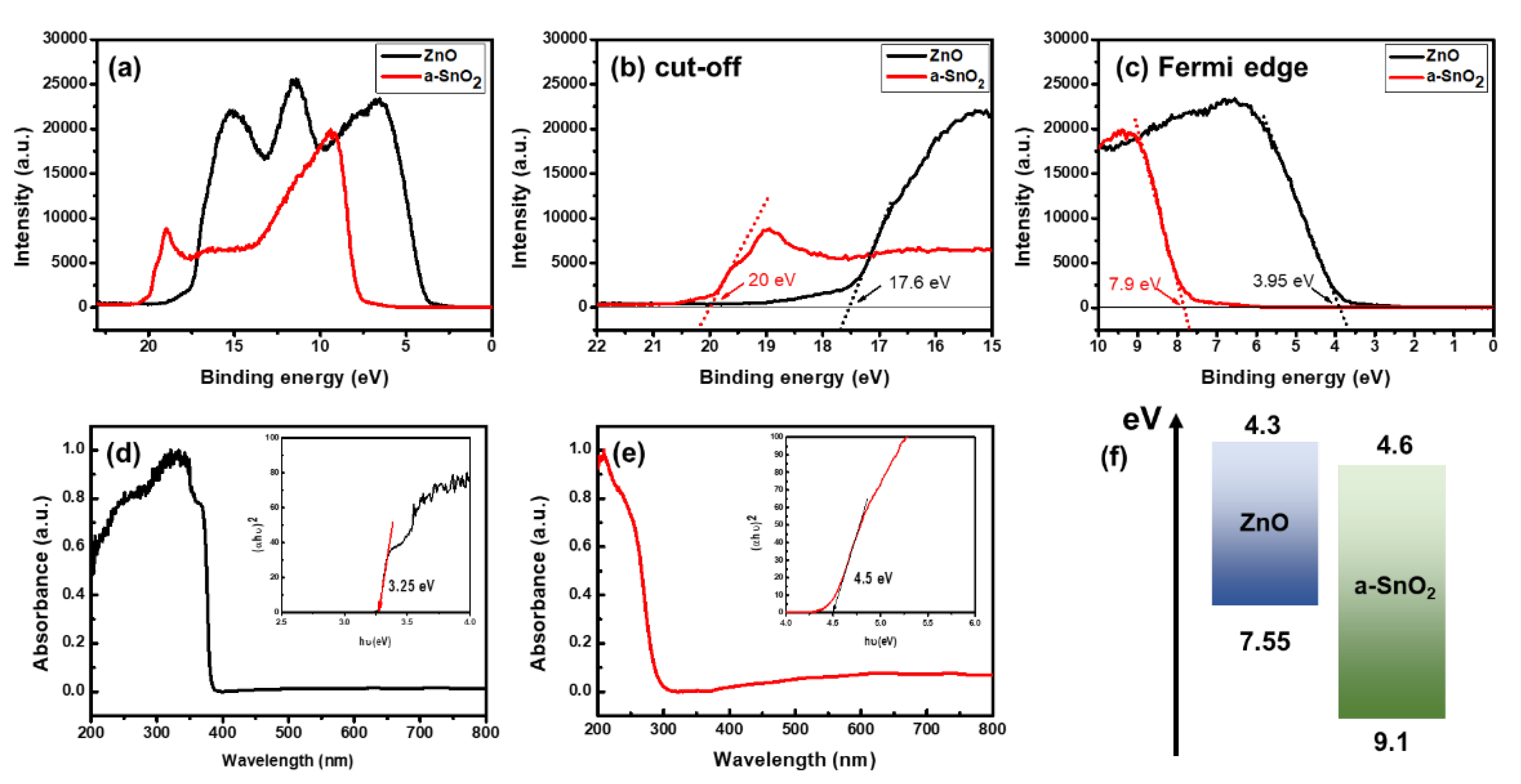
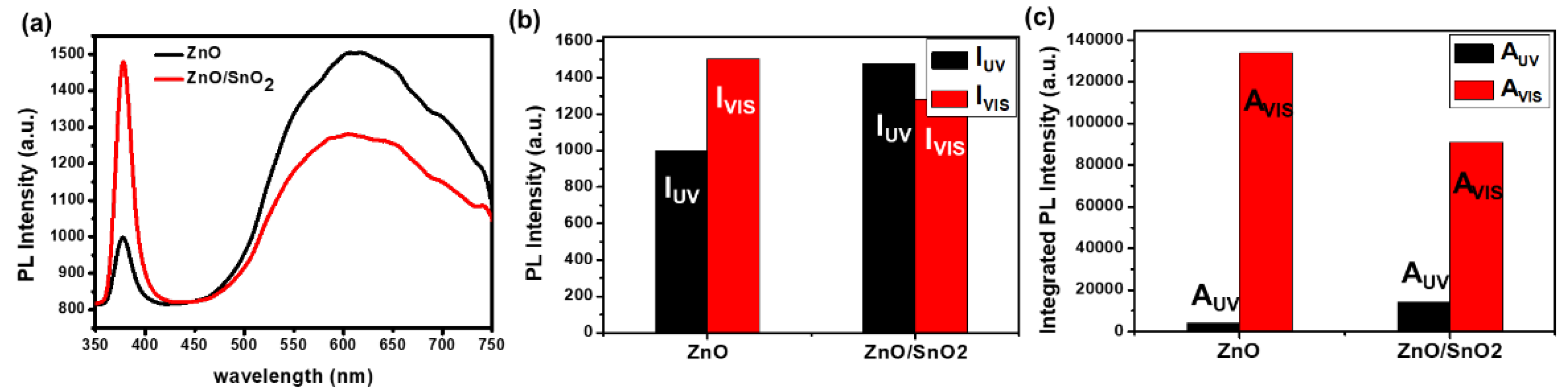
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monroy, E.; Omnès, F.; Calle, F. Wide-bandgap semiconductor ultraviolet photodetectors. Semicond. Sci. Technol. 2003, 18, R33. [Google Scholar] [CrossRef]
- Omnès, F.; Monroy, E.; Muñoz, E.; Reverchon, J.-L. In Wide bandgap UV photodetectors: A short review of devices and applications. Proc. SPIE 2007, 6473. [Google Scholar] [CrossRef]
- Sang, L.; Liao, M.; Sumiya, M. A comprehensive review of semiconductor ultraviolet photodetectors: From thin film to one-dimensional nanostructures. Sensors 2013, 13, 10482–10518. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Sheng, H.; Liu, Y.; Huo, Z.; Lu, Y.; Shen, H. ZnO Schottky ultraviolet photodetectors. J. Cryst. Growth 2001, 225, 110–113. [Google Scholar] [CrossRef]
- Yang, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.J. Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 2002, 12, 323–331. [Google Scholar] [CrossRef]
- Yang, T.; Park, S.-J.; Kim, T.G.; Shin, D.S.; Park, J. Ultraviolet photodetector using pn junction formed by transferrable hollow n-TiO2 nano-spheres monolayer. Opt. Express 2017, 25, 30843–30850. [Google Scholar] [CrossRef]
- Hu, L.; Yan, J.; Liao, M.; Wu, L.; Fang, X. Ultrahigh external quantum efficiency from thin SnO2 nanowire ultraviolet photodetectors. Small 2011, 7, 1012–1017. [Google Scholar] [CrossRef]
- Wu, J.-M.; Kuo, C.-H. Ultraviolet photodetectors made from SnO2 nanowires. Thin Solid Film. 2009, 517, 3870–3873. [Google Scholar] [CrossRef]
- Zhai, T.Y.; Li, L.; Wang, X.; Fang, X.S.; Bando, Y.; Golberg, D. Recent developments in one-dimensional inorganic nanostructures for photodetectors. Adv. Funct. Mater. 2010, 20, 4233–4248. [Google Scholar] [CrossRef]
- Shen, G.; Chen, D. One-dimensional nanostructures for photodetectors. Recent Pat. Nanotechnol. 2010, 4, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Lien, D.H.; Retamal, J.R.D.; Ke, J.J.; Kang, C.F.; He, J.H. Surface effects in metal oxide-based nanodevices. Nanoscale 2015, 7, 19874–19884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.; Zhu, Y.K.; Gao, J.R.; Gong, F.; Li, Q.; Xie, R.Z.; Wu, F.; Wang, D.; Yang, J.H.; et al. High performance charge-transfer induced homojunction photodetector based on ultrathin ZnO nanosheet. Appl. Phys. Lett. 2019, 114, 011103. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Ning, Y.; Fang, X.S. From nanofibers to ordered ZnO/NiO heterojunction arrays for self-powered and transparent UV photodetectors. J. Mater. Chem. C 2019, 7, 223–229. [Google Scholar] [CrossRef]
- Liu, S.; Li, M.Y.; Zhang, J.; Su, D.; Huang, Z.; Kunwar, S.; Lee, J. Self-assembled Al nanostructure/ZnO quantum dot heterostructures for high responsivity and fast UV photodetector. Nanomicro Lett. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- You, D.; Xu, C.; Zhang, W.; Zhao, J.; Qin, F.; Shi, Z. Photovoltaic-pyroelectric effect coupled broadband photodetector in self-powered ZnO/ZnTe core/shell nanorod arrays. Nano Energy 2019, 62, 310–318. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wu, H.; Hou, M.; Wang, J.; Zhang, L.; Liao, C.; Liu, S.; Wang, Y. A ZnO nanowire-based microfiber coupler for all-optical photodetection applications. Nanoscale 2019, 11, 8319–8326. [Google Scholar] [CrossRef]
- Zheng, M.; Gui, P.; Wang, X.; Zhang, G.; Wan, J.; Zhang, H.; Fang, G.; Wu, H.; Lin, Q.; Liu, C. ZnO ultraviolet photodetectors with an extremely high detectivity and short response time. Appl. Surf. Sci. 2019, 481, 437–442. [Google Scholar] [CrossRef]
- You, D.; Xu, C.; Zhao, J.; Qin, F.; Zhang, W.; Wang, R.; Shi, Z.; Cui, Q. Single-crystal ZnO/AlN core/shell nanowires for ultraviolet emission and dual-color ultraviolet photodetection. Adv. Opt. Mater. 2019, 7, 1801522. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, Y.; Hu, Y.; Mai, W.; Yeh, P.-H.; Bao, G.; Sood, A.K.; Polla, D.L.; Wang, Z.L. Gigantic enhancement in response and reset time of ZnO UV nanosensor by utilizing Schottky contact and surface functionalization. Appl. Phys. Lett. 2009, 94, 191103. [Google Scholar] [CrossRef] [Green Version]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.R.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO nanowire UV photodetectors with high internal gain. Nano Lett. 2007, 4, 1003–1009. [Google Scholar] [CrossRef]
- Liu, Y.; Gorla, C.; Liang, S.; Emanetoglu, N.; Lu, Y.; Shen, H.; Wraback, M. Ultraviolet detectors based on epitaxial ZnO films grown by MOCVD. J. Electron. Mater. 2000, 29, 69–74. [Google Scholar] [CrossRef]
- Park, J.; Shin, D.S.; Kim, D.H. Enhancement of light extraction in GaN-based light-emitting diodes by Al2O3-coated ZnO nanorod arrays. J. Alloy. Compd. 2014, 611, 157–160. [Google Scholar] [CrossRef]
- Park, J.; Ryu, H.; Son, T.; Yeon, S. Epitaxial growth of ZnO/InN core/shell nanostructures for solar cell applications. Appl. Phys. Express 2012, 5, 101201. [Google Scholar] [CrossRef]
- Tian, W.; Zhai, T.; Zhang, C.; Li, S.L.; Wang, X.; Liu, F.; Liu, D.; Cai, X.; Tsukagoshi, K.; Golberg, D. Low-cost fully transparent ultraviolet photodetectors based on electrospun ZnO-SnO2 heterojunction nanofibers. Adv. Mater. 2013, 25, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.Q.; Shing, C.; Sawyer, S.; Dutta, P.S. Enhanced ultraviolet sensitivity of zinc oxide nanoparticle photoconductors by surface passivation. Opt. Mater. 2011, 33, 359–362. [Google Scholar] [CrossRef]
- Cossuet, T.; Resende, J.; Rapenne, L.; Chaix-Pluchery, O.; Jiménez, C.; Appert, E.; Muñoz-Rojas, D.; Consonni, V.; Deschanvres, J.-L. ZnO/CuCrO2 core–shell nanowire heterostructures for self-powered UV photodetectors with fast response. Adv. Funct. Mater. 2018, 28, 1803142. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, B.; Zhang, X.; Cao, S.; Ma, P.; Wang, K.; Fan, Z.; Su, M. Preparation and UV photoelectric properties of aligned ZnO–TiO2 and TiO2–ZnO core–shell structured heterojunction nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 38490–38498. [Google Scholar] [CrossRef]
- Xiong, L.; Guo, Y.; Wen, J.; Liu, H.; Yang, G.; Qin, P.; Fang, G. Review on the application of SnO2 in perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1802757. [Google Scholar] [CrossRef]
- Wali, Q.; Fakharuddin, A.; Jose, R. Tin oxide as a photoanode for dye-sensitised solar cells: Current progress and future challenges. J. Power Sources 2015, 293, 1039–1052. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Shin, D.S.; Babu, B.; Kim, T.G.; Song, K.M.; Park, J. Control of copper nanowire network properties and application to transparent conducting layer in LED. Mater. Des. 2017, 132, 66–71. [Google Scholar] [CrossRef]
- Kwon, D.K.; Lee, S.J.; Myoung, J.M. High-performance flexible ZnO nanorod UV photodetectors with a network-structured Cu nanowire electrode. Nanoscale 2016, 37, 16677–16683. [Google Scholar] [CrossRef]
- Ouyang, W.; Teng, F.; He, J.H.; Fang, X. Enhancing the photoelectric performance of photodetectors based on metal oxide semiconductors by charge-carrier engineering. Adv. Funct. Mater. 2019, 29, 1807672. [Google Scholar] [CrossRef]
- Shalish, I.; Temkin, H.; Narayanamurti, V. Size-dependent surface luminescence in ZnO nanowires. Phys. Rev. B 2004, 69, 245401. [Google Scholar] [CrossRef]
- Guo, L.; Yang, S.H.; Yang, C.L.; Yu, P.; Wang, J.N.; Ge, W.K.; Wong, G.K.L. Highly monodisperse polymer-capped ZnO nanoparticles: Preparation and optical properties. Appl. Phys. Lett. 2000, 76, 2901–2903. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.; Jung, U.; Jung, B.; Shen, W.; Park, J. Improved Photoresponse Characteristics of a ZnO-Based UV Photodetector by the Formation of an Amorphous SnO2 Shell Layer. Sensors 2021, 21, 6124. https://doi.org/10.3390/s21186124
Yoo J, Jung U, Jung B, Shen W, Park J. Improved Photoresponse Characteristics of a ZnO-Based UV Photodetector by the Formation of an Amorphous SnO2 Shell Layer. Sensors. 2021; 21(18):6124. https://doi.org/10.3390/s21186124
Chicago/Turabian StyleYoo, Junhyuk, Uijin Jung, Bomseumin Jung, Wenhu Shen, and Jinsub Park. 2021. "Improved Photoresponse Characteristics of a ZnO-Based UV Photodetector by the Formation of an Amorphous SnO2 Shell Layer" Sensors 21, no. 18: 6124. https://doi.org/10.3390/s21186124





