Monitoring Lower Back Activity in Daily Life Using Small Unintrusive Sensors and Wearable Electronics in the Context of Rheumatic and Musculoskeletal Diseases
Abstract
:1. Introduction
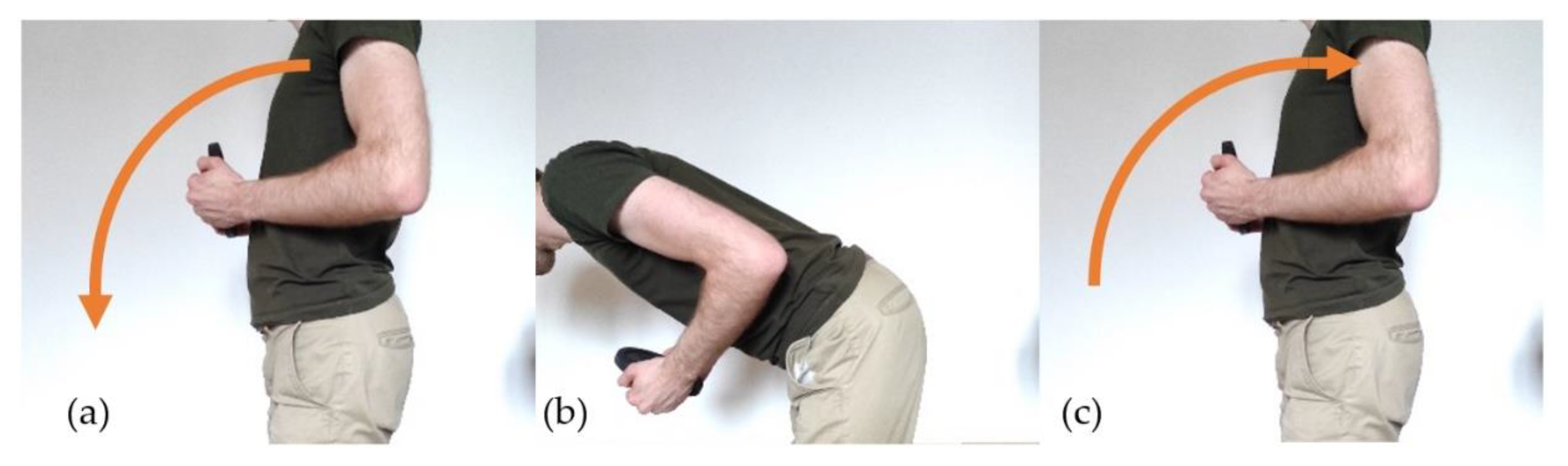
- Diagnosis: Lower back pain can be induced by multiple risk factors. It is complex to measure the posture and movement adopted by the patient during his daily and working life (for example, posture and movement while lifting heavy loads, posture while sitting on a chair, repeated movement in a factory, etc.). It is indeed not trivial for a physician to measure the posture of a patient when the patient is not in a medical facility. Moreover, there could be a need to conduct the measurement over long periods (several days or weeks).
- Therapy: While pain can usually be symptomatically treated by medication, bad posture habits cannot. It usually takes time to turn bad habits into good habits. It is then critical for the therapist to have a mean to help the patient after the diagnosis which can require weeks and months of real-time monitoring and real-time notification to help the patient to learn new good habits about their postures and movements. As demonstrated by [3], home training of a patient is more effective when using sensor-based feedback than using a simple mirror or no feedback.
2. Materials and Methods
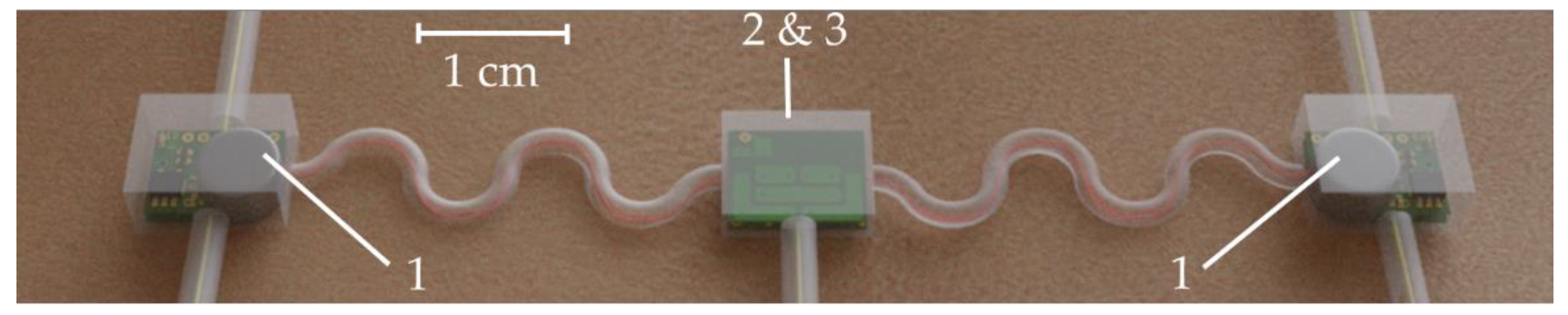
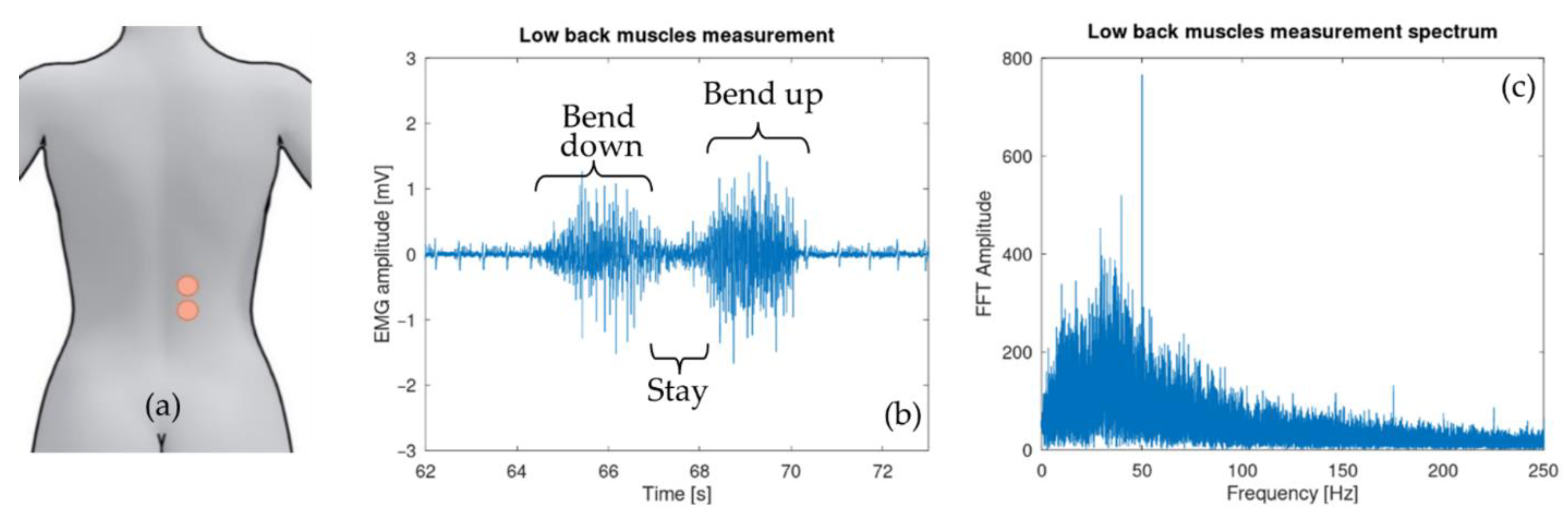
- Unit 1 is intended to acquire the muscles activity via two surface electromyography (sEMG) measurement circuits. One on the left and one on the right side of the body.
- Unit 2 comprises a 6-DoF IMU measuring linear accelerations and rotation speeds in three axes each to estimate the position and orientation of the subject of interest.
- Unit 3 is the main electronic unit composed of processing, communication and power management.
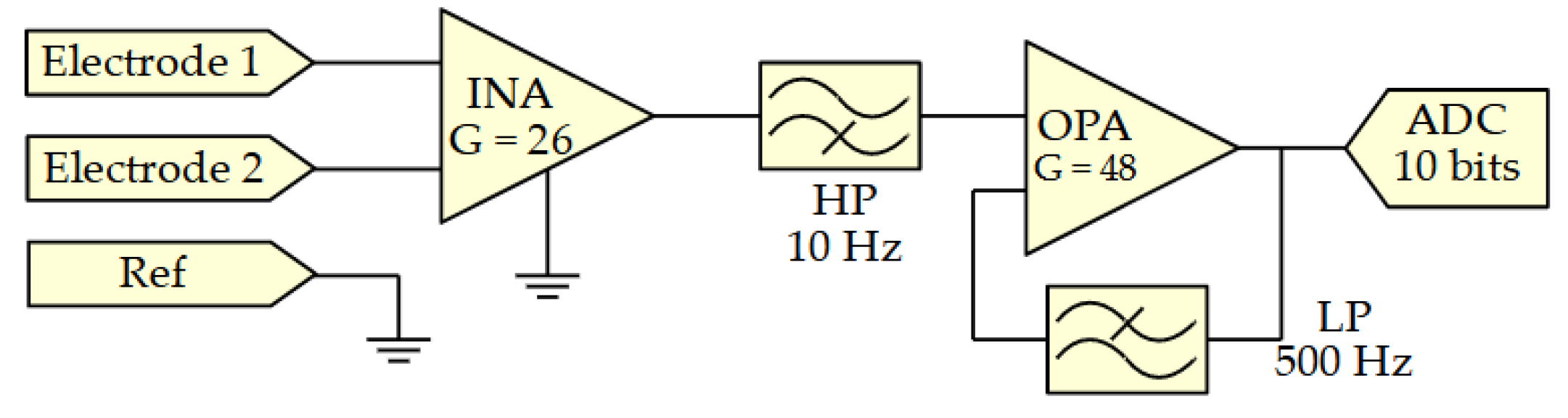
2.1. Surface Electromyography Sensor
2.2. Inertial Sensor
2.3. Main Unit
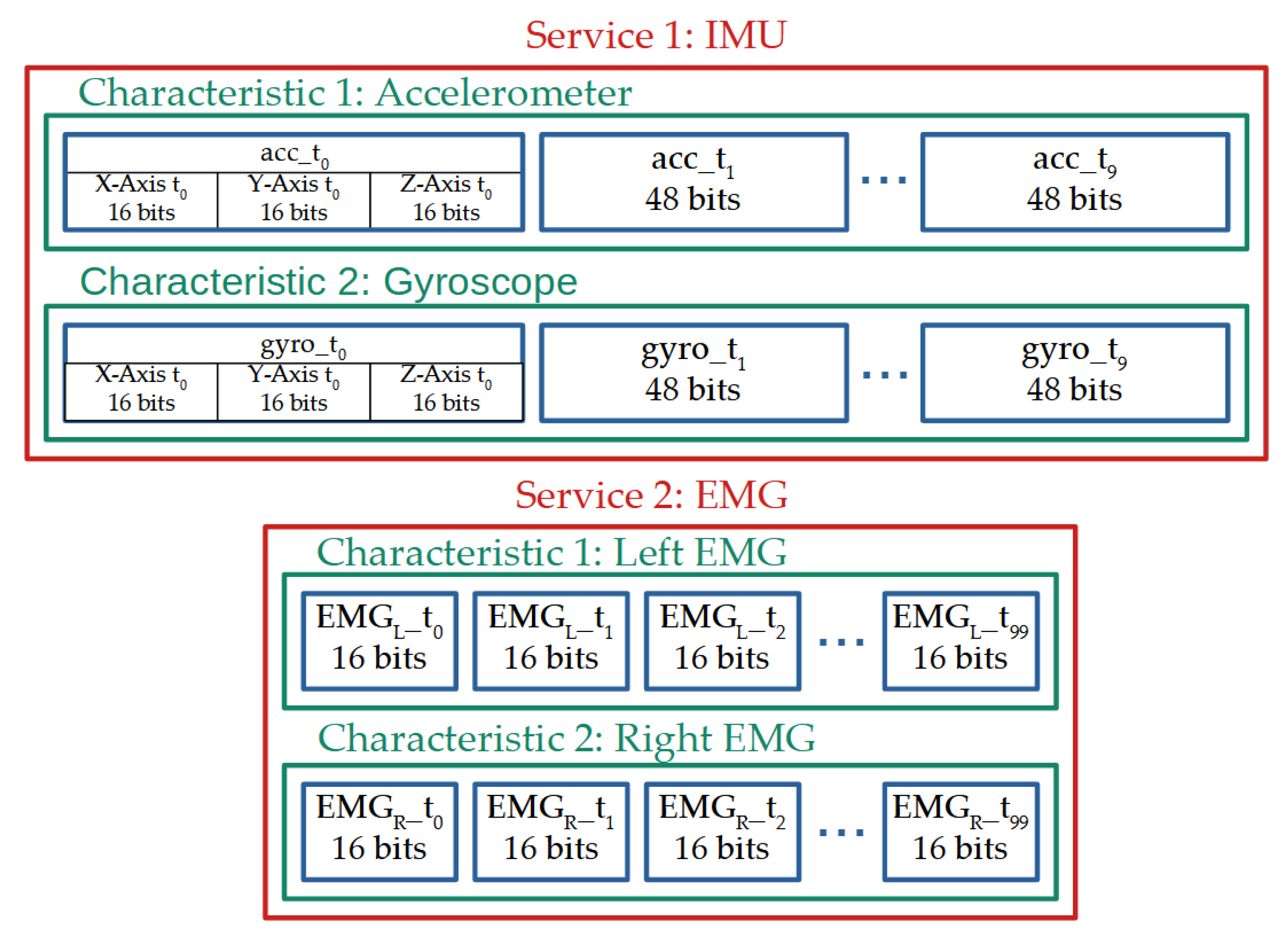
2.4. Wireless Communication
2.5. Sensor System Fabrication
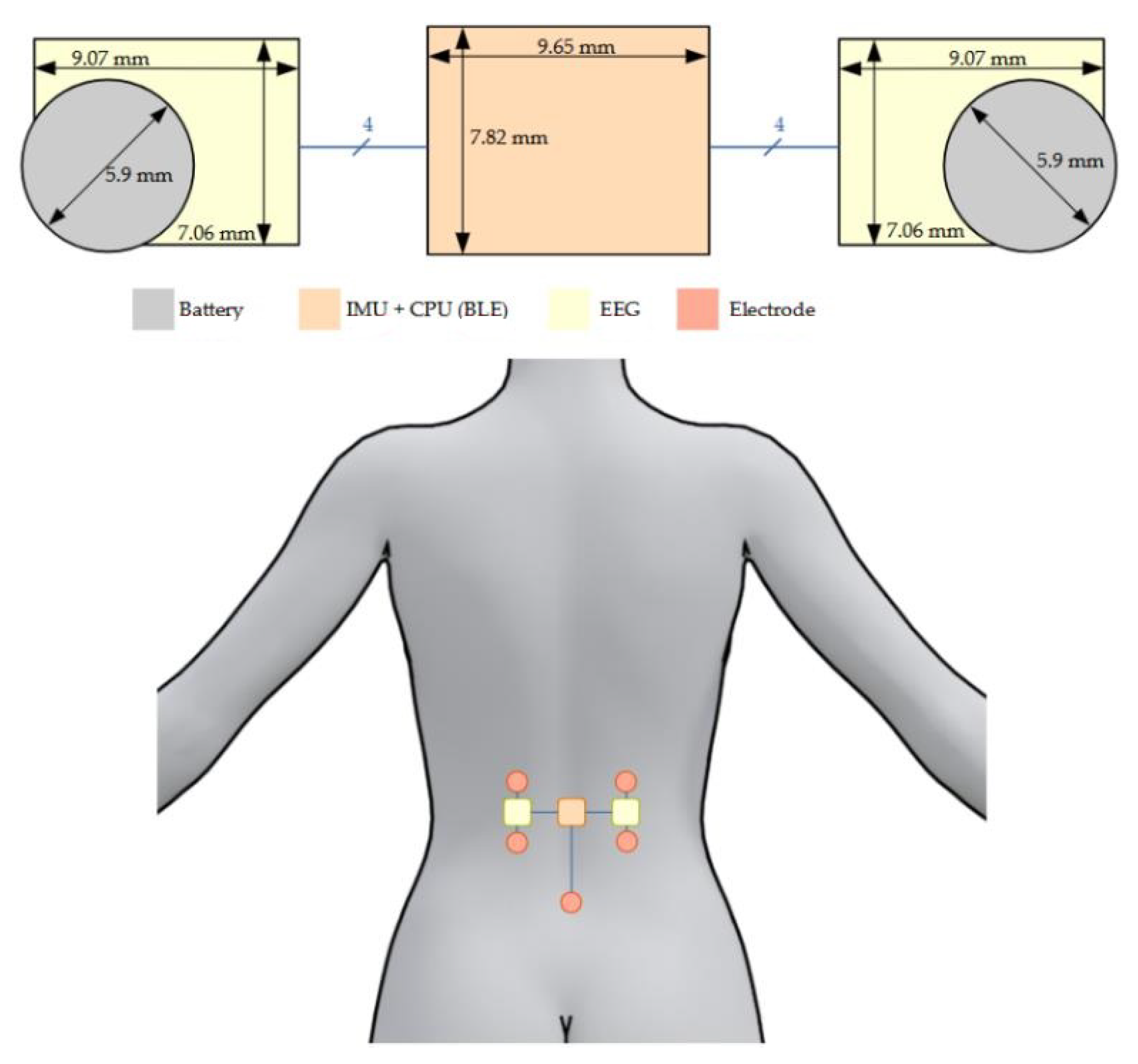
- The central area, placed on the spine, was designed to be as thin as possible while maintaining a reasonable surface. It contains the CPU, the IMU, a crystal, and few passive components with the following respective thicknesses: 400 µm, 910 µm, 650 µm, and 350 µm. This brings a final theoretical PCB thickness of 996 µm with a surface of 9.65 mm × 7.82 mm (without antenna). Despite the fact that we used a zeroth-order resonator (ZOR) antenna measuring only 8.97 mm × 6.27 mm, connecting the antenna next to the PCB would have almost doubled its size. As a tradeoff between size, thickness, and emission performance, it was decided to stack the antenna onto the PCB providing a final sensor measuring 9.65 mm × 7.82 mm × 1.09 mm. Figure 6 shows photos of the PCB, the antenna, and the result when the two PCBs are stacked. The left part of the PCB containing the 4-pin headers is meant to be cut once the CPU is programmed and the prototype is ready to be used.
- The two decentralized parts, placed a few centimeters from the center of the back and on the side, were designed to be as compact as possible. They were designed symmetric from each other. Each part contained an INA, an OPA, and few passive components having the following respective thicknesses: 750 µm, 950 µm, and 250 µm. Since the battery was the biggest element measuring 3.6 mm in thickness, it was decided to split the sEMG acquisition circuit into two PCBs and stack them to reduce its footprint. This resulted in two stacked circuits with a footprint of 25 mm2 instead of 43 mm2 and a thickness of 1.872 mm instead of 0.996 mm. The final area including the battery measured 9.45 mm × 7.27 mm × 3.6 mm. Figure 7 shows pictures of the two layers and the combined parts with the battery.
2.6. Packaging and Connection
- An acrylonitrile butadiene styrene (ABS) mold with the desired shape was created using 3D printing technology (Figure 8a). ABS was selected over polylactic acid (PLA) for its higher glass transition temperature which is around 105 °C despite its higher complexity to 3D print;
- A first layer of PDMS was deposited onto the bottom of the mold. This prevented a contact between sensors and mold and thus an incomplete sealing (Figure 8b);
- Once the first layer was cured, the sensor system was placed and maintained in position while a second layer of PDMS was added. This layer was used to fix the sensors in its final position and to prevent them from moving during the final step (Figure 8c,d). Special care was taken to prevent the sensors from touching the mold and create a failure in the sealing;
- Once the second layer was cured and the PCB fixed in the correct position, the mold was fully filled with PDMS (Figure 8e);
- The packaged sensors were removed from the mold (Figure 8f).
3. Results
3.1. Surface Electromyography Sensor
3.2. Inertial Sensors
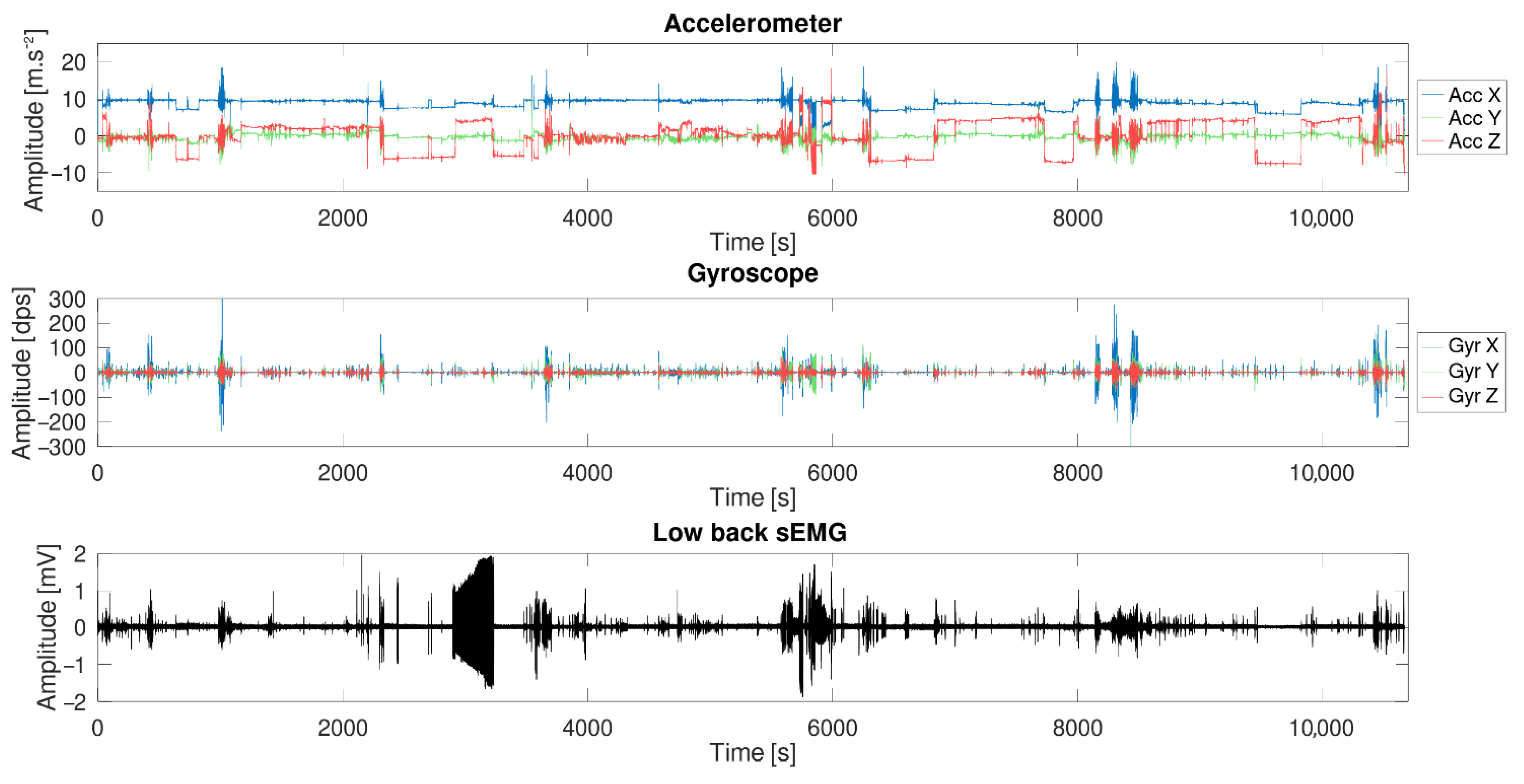
3.3. Long-Term Measurement
3.4. Packaging and Connection
3.5. Communication
4. Discussion
4.1. Surface Electromyography Sensor
4.2. Inertial Sensors
4.3. Long-Term Measurement
4.4. Packaging and Connection
4.5. Communication
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dieleman, J.L.; Baral, R.; Birger, M.; Bui, A.L.; Bulchis, A.; Chapin, A.; Hamavid, H.; Horst, C.; Johnson, E.K.; Joseph, J.; et al. US Spending on Personal Health Care and Public Health, 1996–2013. JAMA 2016, 316, 2627. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, W.; Wirtz, V.J.; Mantel-Teeuwisse, A.; Stolk, P.; Duthey, B.; Laing, R. Priority Medicines for Europe and the World 2013 Update; WHO: Geneva, Switzerland, 2013; pp. 165–168. [Google Scholar]
- Matheve, T.; Brumagne, S.; Demoulin, C.; Timmermans, A. Sensor-Based Postural Feedback Is More Effective than Conventional Feedback to Improve Lumbopelvic Movement Control in Patients with Chronic Low Back Pain: A Randomised Controlled Trial. J. Neuroeng. Rehabil. 2018, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- ShimmerSensing Shimmer ECG & EMG Unit—Specification Sheet. Available online: http://www.shimmersensing.com/images/uploads/docs/Shimmer3_ECG_EMG_Specification_Sheet_Revision_1.7.pdf (accessed on 5 July 2021).
- ShimerSensing Shimmer3 200 g IMU Wireless Sensors Platform—Specification Sheet. Available online: http://www.shimmersensing.com/images/uploads/docs/Shimmer3_IMU_200g_Spec_Sheet.pdf (accessed on 5 July 2021).
- Hocoma. Valedo Motion. Available online: https://www.hocoma.com/solutions/valedo-motion/technical-data-sheet/ (accessed on 5 July 2021).
- LP-RESEARCH Inc. LPMS-B2 Series Hardware Manual Ver. 1.0. Available online: https://lp-research.com/wp-content/uploads/2020/03/20200310LpmsB2HardwareManual.pdf (accessed on 5 July 2021).
- Ergotest Innovation. Musclelab. Available online: https://www.ergotest.com/ (accessed on 5 July 2021).
- BTS Bioengineering. FREEMG. Available online: https://www.btsbioengineering.com/products/freeemg-surface-emg-semg/ (accessed on 5 July 2021).
- García Patiño, A.; Khoshnam, M.; Menon, C. Wearable Device to Monitor Back Movements Using an Inductive Textile Sensor. Sensors 2020, 20, 905. [Google Scholar] [CrossRef] [Green Version]
- Valero, E.; Sivanathan, A.; Bosché, F.; Abdel-Wahab, M. Analysis of Construction Trade Worker Body Motions Using a Wearable and Wireless Motion Sensor Network. Autom. Constr. 2017, 83, 48–55. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Zhao, H.; Qiu, S.; Li, J.; Gao, F.; Shi, X. Using Wearable Sensors to Capture Posture of the Human Lumbar Spine in Competitive Swimming. IEEE Trans. Hum.-Mach. Syst. 2019, 49, 194–205. [Google Scholar] [CrossRef]
- Cortell-Tormo, J.M.; Garcia-Jaen, M.; Ruiz-Fernandez, D.; Fuster-Lloret, V. Lumbatex: A Wearable Monitoring System Based on Inertial Sensors to Measure and Control the Lumbar Spine Motion. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Kusaka, T.; Tanaka, T.; Matsuo, Y.; Oda, M.; Sasaki, T.; Kamishima, T.; Yamanaka, M. Calibration Method for Lumbosacral Dimensions in Wearable Sensor System of Lumbar Alignment. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 5 November 2015; pp. 3909–3912. [Google Scholar]
- Brunelli, D.; Farella, E.; Giovanelli, D.; Milosevic, B.; Minakov, I. Design Considerations for Wireless Acquisition of Multichannel SEMG Signals in Prosthetic Hand Control. IEEE Sens. J. 2016, 16, 8338–8347. [Google Scholar] [CrossRef]
- Trigno Wireless Biofeedback System User’s Guide. Available online: https://www.delsys.com/downloads/USERSGUIDE/trigno/wireless-biofeedback-system.pdf (accessed on 5 July 2021).
- Cometa Systems. Available online: https://www.cometasystems.com/ (accessed on 5 July 2021).
- Song, M.-S.; Kang, S.-G.; Lee, K.-T.; Kim, J. Wireless, Skin-Mountable EMG Sensor for Human–Machine Interface Application. Micromachines 2019, 10, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.K.; Han, S.J.; Kim, K.; Kim, Y.H.; Lee, S. Wireless Epidermal Six-Axis Inertial Measurement Units for Real-Time Joint Angle Estimation. Appl. Sci. 2020, 10, 2240. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Kim, K.; Lee, S. Stretchable, Patch-Type, Wireless, 6-Axis Inertial Measurement Unit for Mobile Health Monitoring. Proc. Eng. Technol. Innov. 2020, 14, 16–21. [Google Scholar] [CrossRef]
- Liu, S.-H.; Huang, J.; Huang, Y.-F.; Tan, T.-H.; Huang, T.-S. A Wearable Device for Monitoring Muscle Condition During Exercise. In Proceedings of the Intelligent Information and Database Systems; Sitek, P., Pietranik, M., Krótkiewicz, M., Srinilta, C., Eds.; Springer: Singapore, 2020; pp. 402–410. [Google Scholar]
- Tokarski, T.; Roman-Liu, D. The Effect of the Fatty Tissue on EMG Signal in Young Women. Acta Bioeng Biomech 2010, 12, 87–92. [Google Scholar]
- Nordander, C.; Willner, J.; Hansson, G.-A.; Larsson, B.; Unge, J.; Granquist, L.; Skerfving, S. Influence of the Subcutaneous Fat Layer, as Measure.Pdf. Eur. J. Appl. Physiol. 2003, 89, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Day, S. EMG Introduction. Available online: http://www.bortec.ca/Images/pdf/EMG%20measurement%20and%20recording.pdf (accessed on 9 July 2021).
- Reaz, M.B.I.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG Signal Analysis: Detection, Processing, Classification and Applications. Biol. Proced. Online 2006, 8, 11–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konrad, P. The ABC of EMG—A Practical Introduction to Kinesiological Electromyography; Noraxon Inc.: Scottsdale, AZ, USA, 2014. [Google Scholar]
- Texas Instruments INA333 Micro-Power (50 μA), Zerø-Drift, Rail-to-Rail Out Instrumentation Amplifier. Available online: https://www.ti.com/lit/gpn/ina333 (accessed on 6 July 2021).
- Texas Instruments 1.8 V, 2.9 mA, 90 kHz, Rail-to-Rail I/O OPERATIONAL AMPLIFIERS. Available online: https://www.ti.com/lit/gpn/opa379 (accessed on 6 July 2021).
- TDK InvenSense ICM-42605—Datasheet. Available online: http://invensense.tdk.com/wp-content/uploads/2020/09/DS-000292-ICM-42605-v1.5.pdf (accessed on 6 July 2021).
- TDK InvenSense ICM-20948—Datasheet. Available online: http://invensense.tdk.com/wp-content/uploads/2021/07/DS-000189-ICM-20948-v1.4.pdf (accessed on 6 July 2021).
- Ligorio, G.; Bergamini, E.; Truppa, L.; Guaitolini, M.; Raggi, M.; Mannini, A.; Sabatini, A.M.; Vannozzi, G.; Garofalo, P. A Wearable Magnetometer-Free Motion Capture System: Innovative Solutions for Real-World Applications. IEEE Sens. J. 2020, 20, 8844–8857. [Google Scholar] [CrossRef]
- Molnar, M.; Kok, M.; Engel, T.; Kaplick, H.; Mayer, F.; Seel, T. A Method for Lower Back Motion Assessment Using Wearable 6D Inertial Sensors. In Proceedings of the 2018 21st International Conference on Information Fusion (FUSION), Cambridge, UK, 10–13 July 2018; pp. 799–806. [Google Scholar]
- Kang, S.-W.; Choi, H.; Park, H.-I.; Choi, B.-G.; Im, H.; Shin, D.; Jung, Y.-G.; Lee, J.-Y.; Park, H.-W.; Park, S.; et al. The Development of an IMU Integrated Clothes for Postural Monitoring Using Conductive Yarn and Interconnecting Technology. Sensors 2017, 17, 2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barim, M.S.; Lu, M.-L.; Feng, S.; Hughes, G.; Hayden, M.; Werren, D. Accuracy of An Algorithm Using Motion Data Of Five Wearable IMU Sensors For Estimating Lifting Duration And Lifting Risk Factors. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2019, 63, 1105–1111. [Google Scholar] [CrossRef]
- Ultra Low Power Bluetooth 5.1 SoC. Available online: https://www.dialog-semiconductor.com/sites/default/files/2021-03/DA14531_datasheet_3v3_0.pdf (accessed on 4 July 2021).
- Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles. Available online: Http://www.Seniam.Org/ (accessed on 7 July 2021).
- Martin, W. Bluetooth Core Specification Version 5.0 Feature Overview. Available online: https://www.bluetooth.com/bluetooth-resources/bluetooth-5-go-faster-go-further/ (accessed on 5 July 2021).
- Gnu Octave. Available online: https://www.gnu.org/software/octave/ (accessed on 5 July 2021).
- Nordi Semiconductor NRF Connect for Mobile. Available online: https://www.nordicsemi.com/Software-and-tools/Development-Tools/nRF-Connect-for-mobile (accessed on 5 July 2021).
- Willigenburg, N.W.; Daffertshofer, A.; Kingma, I.; van Dieën, J.H. Removing ECG Contamination from EMG Recordings: A Comparison of ICA-Based and Other Filtering Procedures. J. Electromyogr. Kinesiol. 2012, 22, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Billiet, L.; Swinnen, T.; Westhovens, R.; de Vlam, K.; Van Huffel, S. Accelerometry-Based Activity Recognition and Assessment in Rheumatic and Musculoskeletal Diseases. Sensors 2016, 16, 2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karantonis, D.M.; Narayanan, M.R.; Mathie, M.; Lovell, N.H.; Celler, B.G. Implementation of a Real-Time Human Movement Classifier Using a Triaxial Accelerometer for Ambulatory Monitoring. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.; Cufí, C.; Akiba; Davidson, R. Getting Started with Bluetooth Low Energy; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2014; ISBN 978-1-4919-4951-1. [Google Scholar]
- Geuens, J.; Geurts, L.; Swinnen, T.W.; Westhovens, R.; Abeele, V.V. Mobile Health Features Supporting Self-Management Behavior in Patients With Chronic Arthritis: Mixed-Methods Approach on Patient Preferences. JMIR mHealth uHealth 2019, 7, e12535. [Google Scholar] [CrossRef]







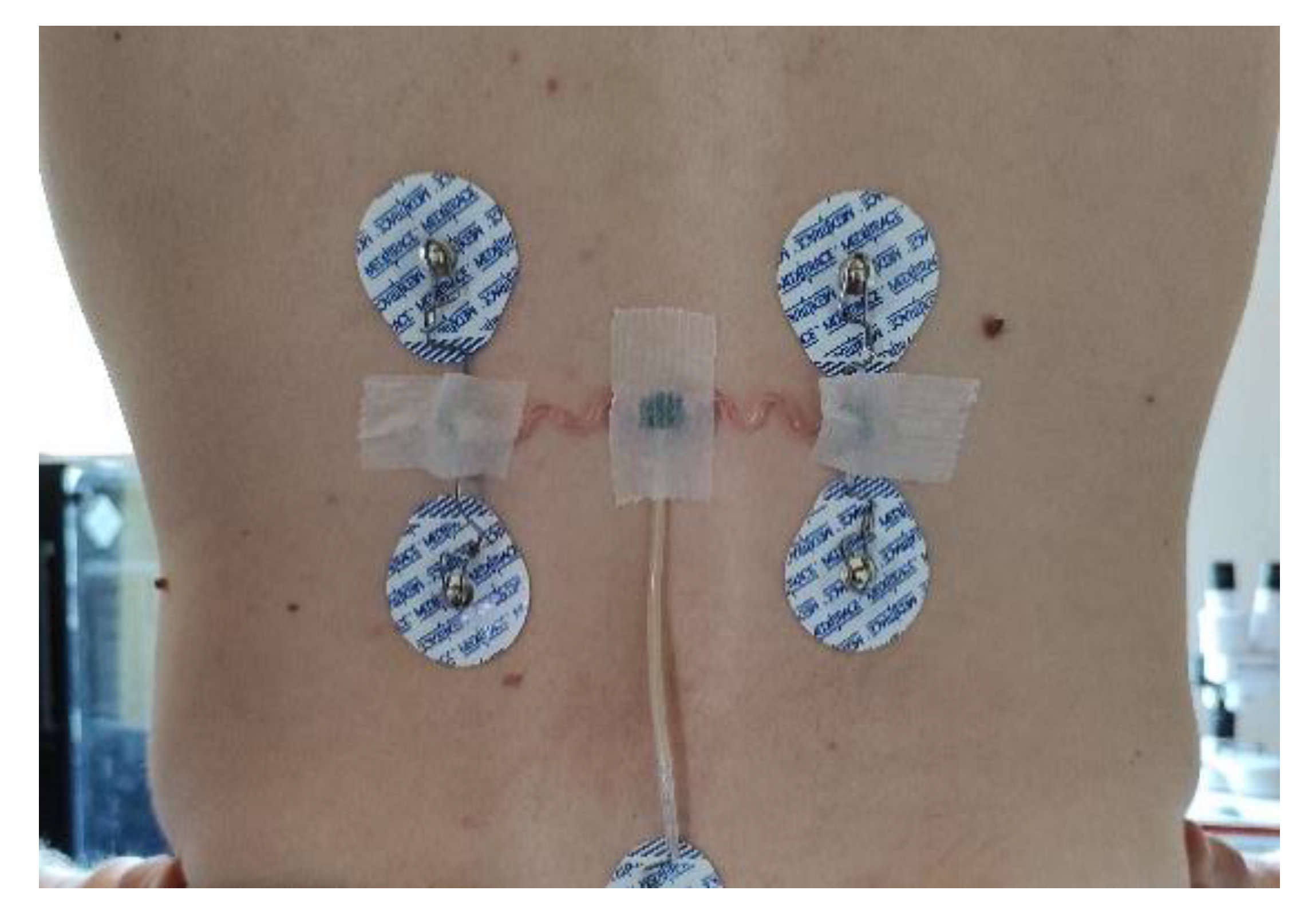

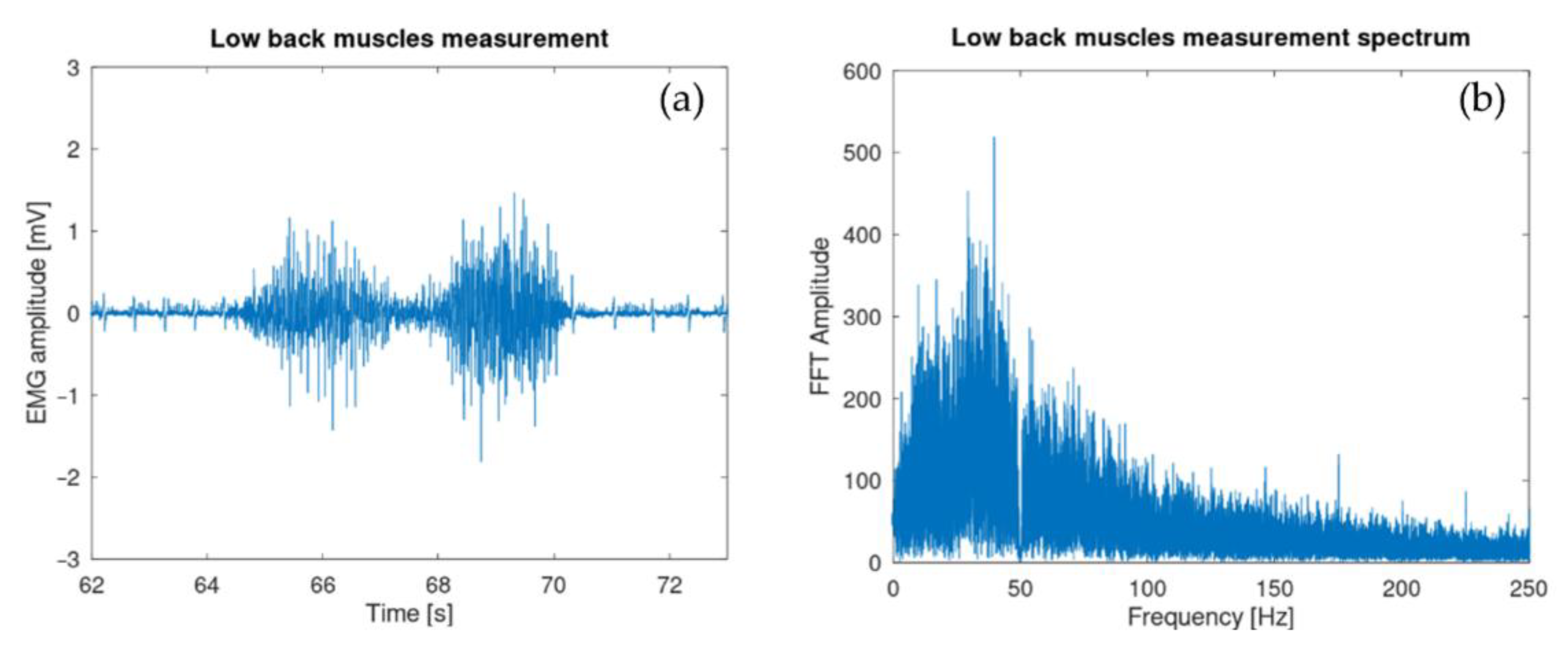

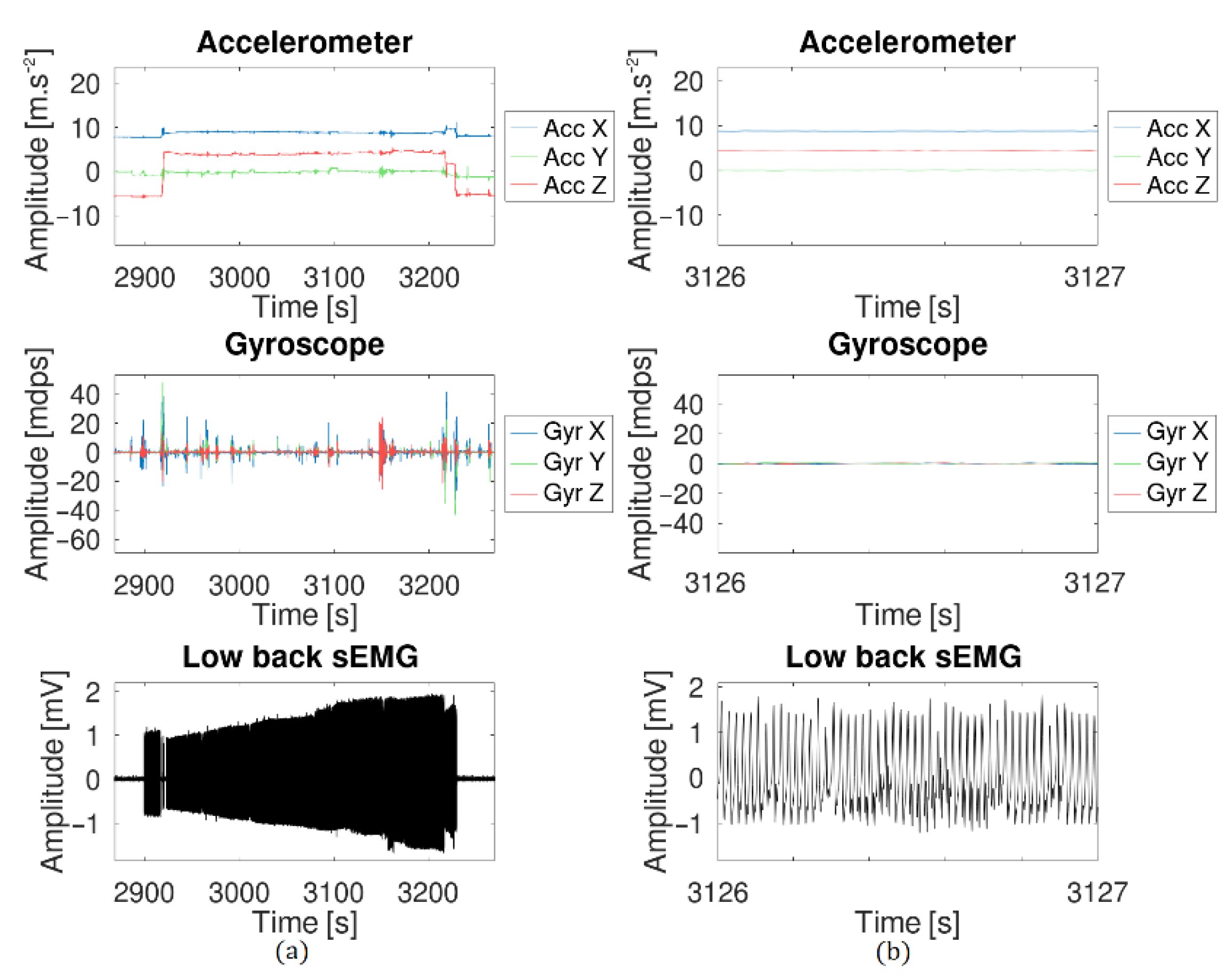
| Properties | Accelerometer | Gyroscope |
|---|---|---|
| Resolution (x, y, z) | 14 bits | 16 bits |
| Bandwidth | 125 Hz | 100 Hz |
| Range | ±16 g | 2000 dps |
| Noise | 70 µg/√Hz | 3.8 mdps/√Hz |
| Packet Size (Bytes) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Update Frequency [Hz] | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 200 | 300 | 400 | 500 | |
| 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 200 | 300 | 400 | 0 | |
| 2 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 40 | 60 | 80 | 100 | 120 | 140 | 160 | 180 | 200 | 400 | 600 | 800 | 0 | |
| 3 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | 600 | 900 | 1200 | 0 | |
| 4 | 4 | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 | 80 | 120 | 160 | 200 | 240 | 280 | 320 | 360 | 400 | 800 | 1200 | 0 | 0 | |
| 5 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 | 1000 | 1500 | 0 | 0 | |
| 6 | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | 120 | 180 | 240 | 300 | 360 | 420 | 480 | 540 | 600 | 1200 | 1800 | 0 | 0 | |
| 7 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 | 63 | 70 | 140 | 210 | 280 | 350 | 420 | 490 | 560 | 630 | 700 | 1400 | 2100 | 0 | 0 | |
| 8 | 8 | 16 | 24 | 32 | 40 | 48 | 56 | 64 | 72 | 80 | 160 | 240 | 320 | 400 | 480 | 560 | 640 | 720 | 800 | 1600 | 0 | 0 | 0 | |
| 9 | 9 | 18 | 27 | 36 | 45 | 54 | 63 | 72 | 81 | 90 | 180 | 270 | 360 | 450 | 540 | 630 | 720 | 810 | 900 | 1800 | 0 | 0 | 0 | |
| 10 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 900 | 1000 | 2000 | 0 | 0 | 0 | |
| 20 | 20 | 40 | 60 | 80 | 100 | 120 | 140 | 160 | 180 | 200 | 400 | 600 | 800 | 1000 | 1200 | 1400 | 1600 | 1800 | 2000 | 0 | 0 | 0 | 0 | |
| 30 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | 600 | 900 | 1200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 40 | 40 | 80 | 120 | 160 | 200 | 240 | 280 | 320 | 360 | 400 | 800 | 1200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 50 | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 | 1000 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 60 | 60 | 120 | 180 | 240 | 300 | 360 | 420 | 480 | 540 | 600 | 1200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 70 | 70 | 140 | 210 | 280 | 350 | 420 | 490 | 560 | 630 | 700 | 1400 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 80 | 80 | 160 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| This Work (IMU) | Shimmer [4] | FreeMG [9] | Brunelli et al. [15] | Trigno [16] | |
|---|---|---|---|---|---|
| Length [mm] | 11.65 | 65 | 41.5 | 27 | 27 |
| Height [mm] | 9.27 | 32 | 24.8 | 18 | 37 |
| Thickness [mm] | 5.6 | 12 | 14 | 9.2 | 13 |
| Number of channels | 1 | 2 | 1 | 32 | 1 |
| Resolution [bit] | 10 | NA | 16 | 12 | 16 |
| Max sample rate [Hz] | 1000 | 8400 | 1000 | 1000 | 4370 |
| Weight [g] | 0.5 | 31 | 13 | NA | 7 |
| Packaging | Soft PDMS | Rigid plastic | Rigid plastic | NA | Rigid plastic |
| This Work (EMG) | LPMS-B2 [7] | Valero et al. [11] | Trigno [16] | Lee et al. [19] | |
|---|---|---|---|---|---|
| Length [mm] | 11.45 | 39 | 60 | 27 | 40 |
| Height [mm] | 9.82 | 39 | 40 | 37 | 37 |
| Thickness [mm] | 3.1 | 8 | 15 | 13 | NA |
| DOF | 6 | 9 | NA | 6 | 6 |
| Resolution [bit] | 10 | NA | NA | 16 | 16 |
| Max sample rate [Hz] | 100 | 400 | 50 | 963/741 | 100 |
| Weight [g] | 0.9 (each) | 12 | NA | 10 | NA |
| Packaging | Soft PDMS | Rigid plastic | Rigid plastic | Rigid plastic | Adhesive film |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baijot, M.; Puers, R.; Kraft, M. Monitoring Lower Back Activity in Daily Life Using Small Unintrusive Sensors and Wearable Electronics in the Context of Rheumatic and Musculoskeletal Diseases. Sensors 2021, 21, 6362. https://doi.org/10.3390/s21196362
Baijot M, Puers R, Kraft M. Monitoring Lower Back Activity in Daily Life Using Small Unintrusive Sensors and Wearable Electronics in the Context of Rheumatic and Musculoskeletal Diseases. Sensors. 2021; 21(19):6362. https://doi.org/10.3390/s21196362
Chicago/Turabian StyleBaijot, Mathieu, Robert Puers, and Michael Kraft. 2021. "Monitoring Lower Back Activity in Daily Life Using Small Unintrusive Sensors and Wearable Electronics in the Context of Rheumatic and Musculoskeletal Diseases" Sensors 21, no. 19: 6362. https://doi.org/10.3390/s21196362
APA StyleBaijot, M., Puers, R., & Kraft, M. (2021). Monitoring Lower Back Activity in Daily Life Using Small Unintrusive Sensors and Wearable Electronics in the Context of Rheumatic and Musculoskeletal Diseases. Sensors, 21(19), 6362. https://doi.org/10.3390/s21196362







