Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Capture Particle
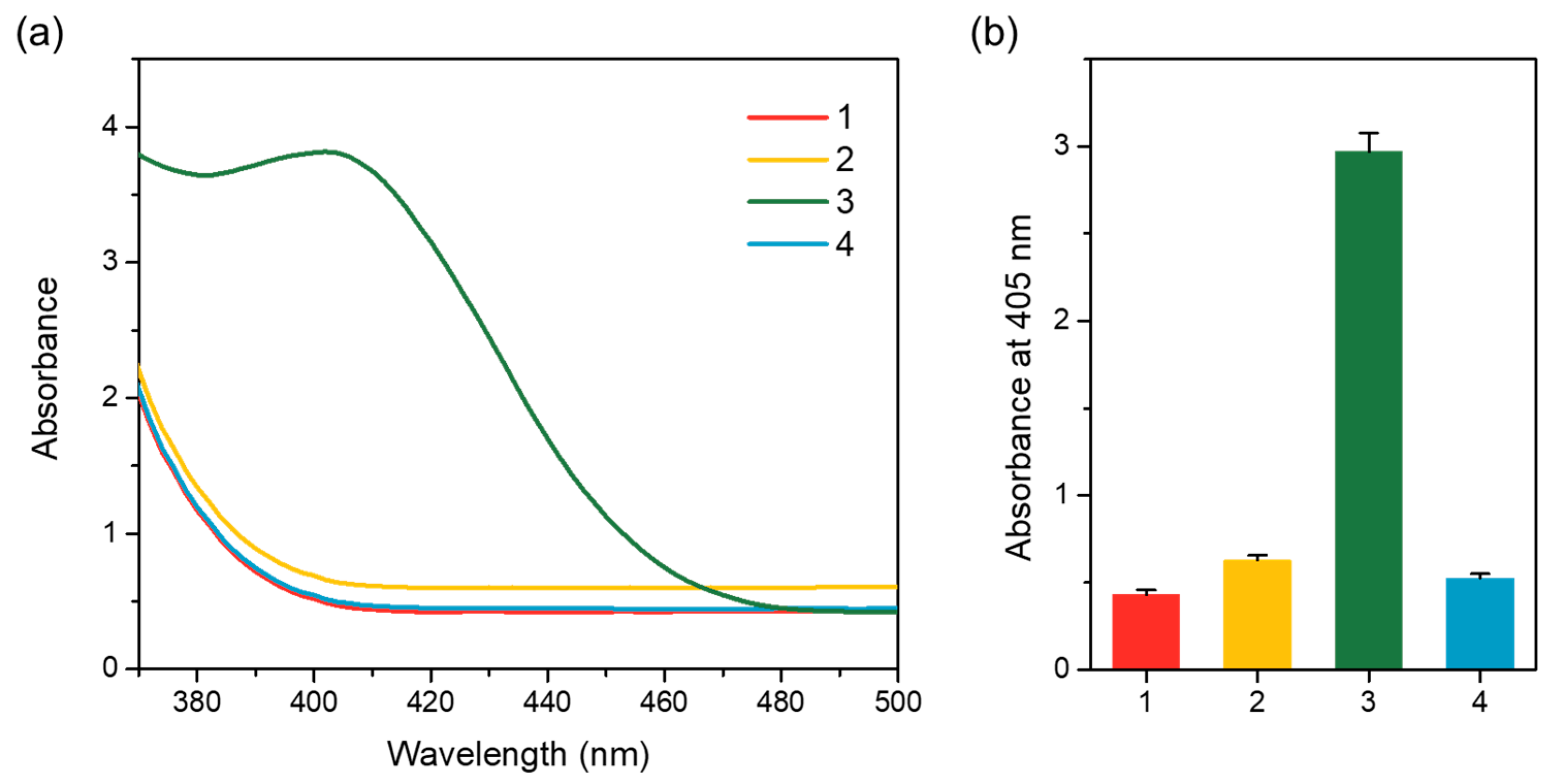
2.3. Characterization of Capture Particle
2.4. Formation of a Magnetic Bead-Based Sandwich Complex
2.5. Human IgE Assay Based on CER Utilizing PGM
2.6. Human IgE Assay Based on Colorimetric p-NPP
2.7. Commercialized ELISA Kit
2.8. Zeta Potential Analysis of Capture Particles and Proteins
2.9. Recovery Test
3. Results and Discussion
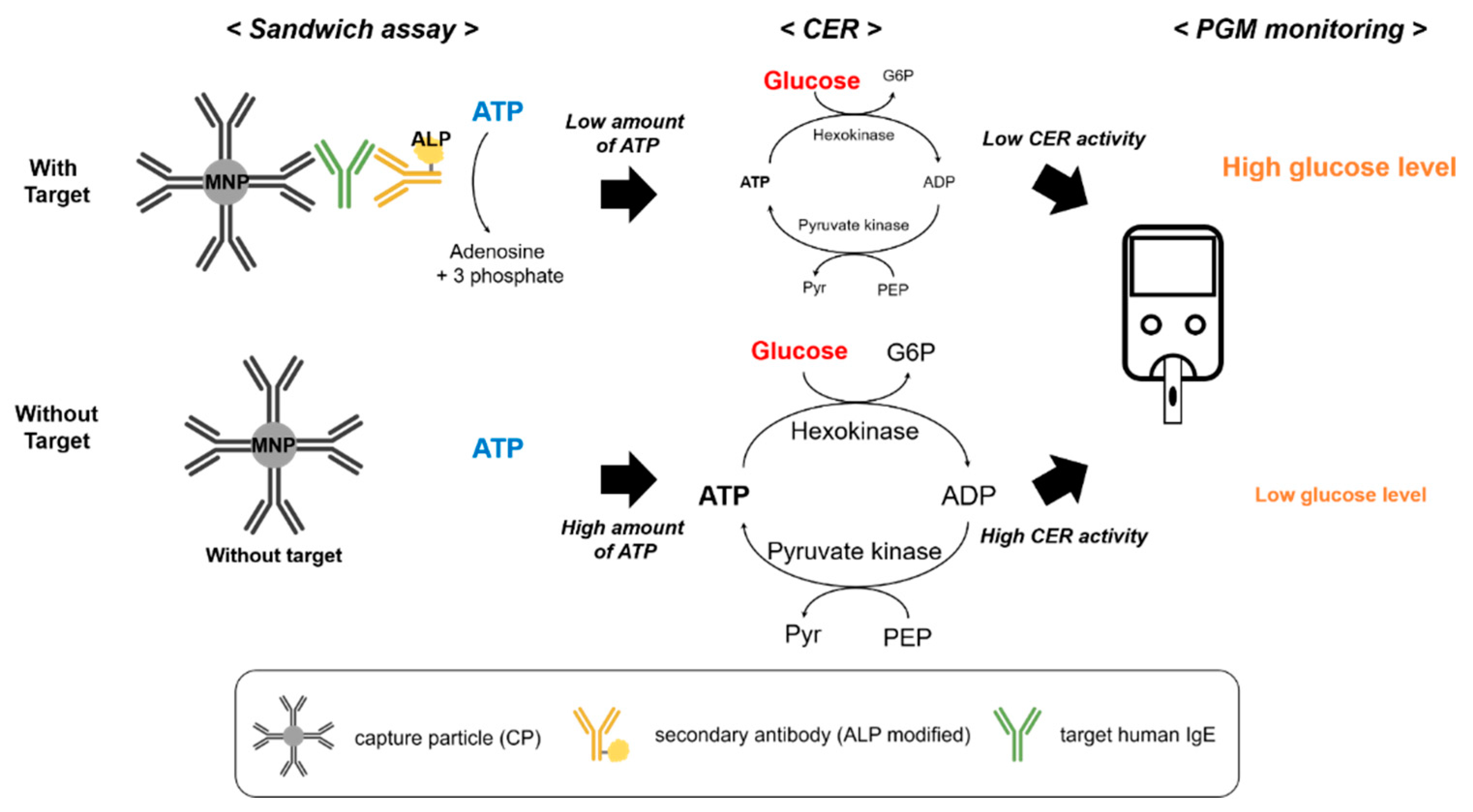
3.1. Detection Principle of the PGM-Based Human IgE Assay
3.2. Characterization of Capture Particle
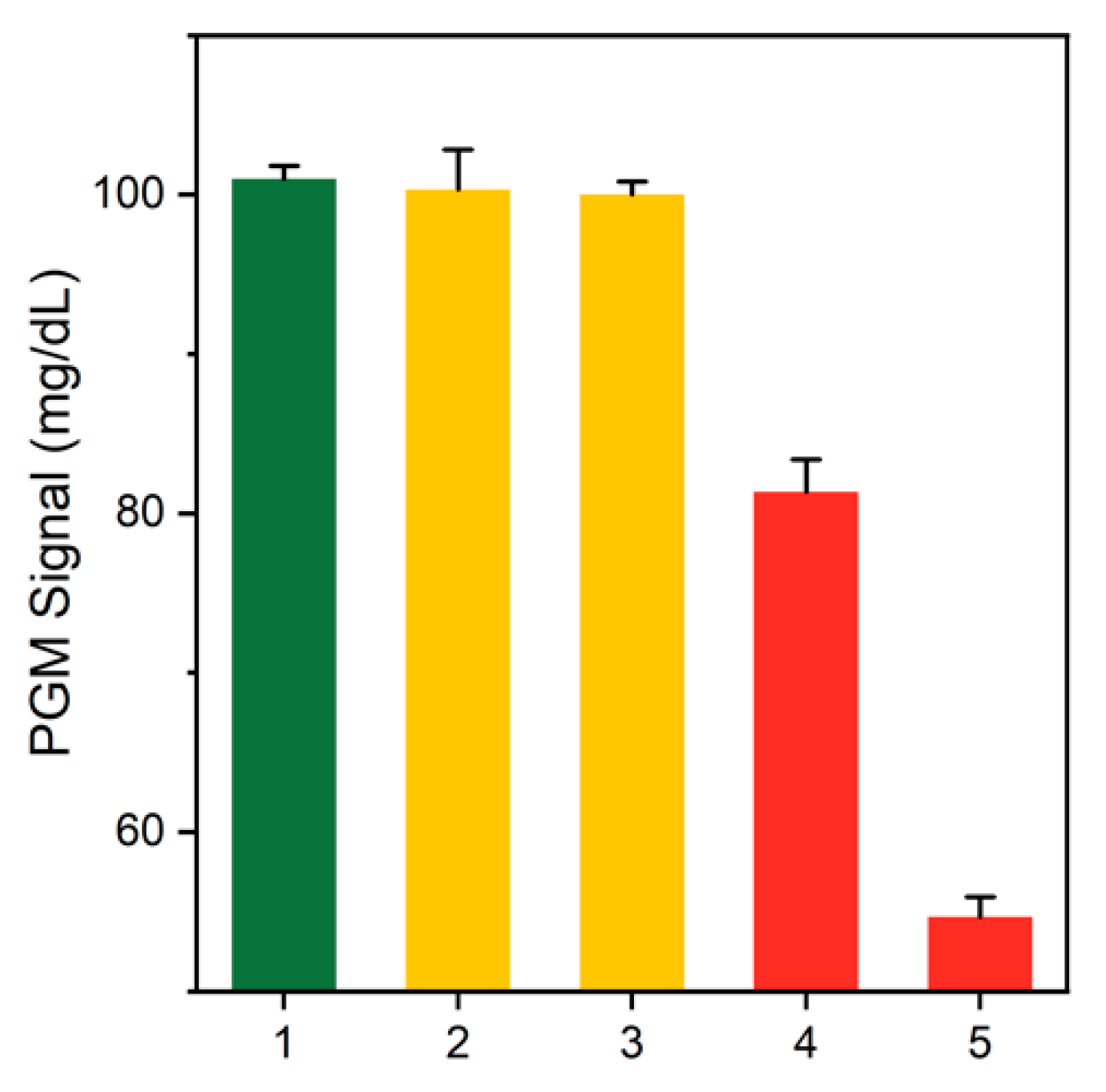
3.3. Detection Feasibility of the PGM-Based Human IgE Assay
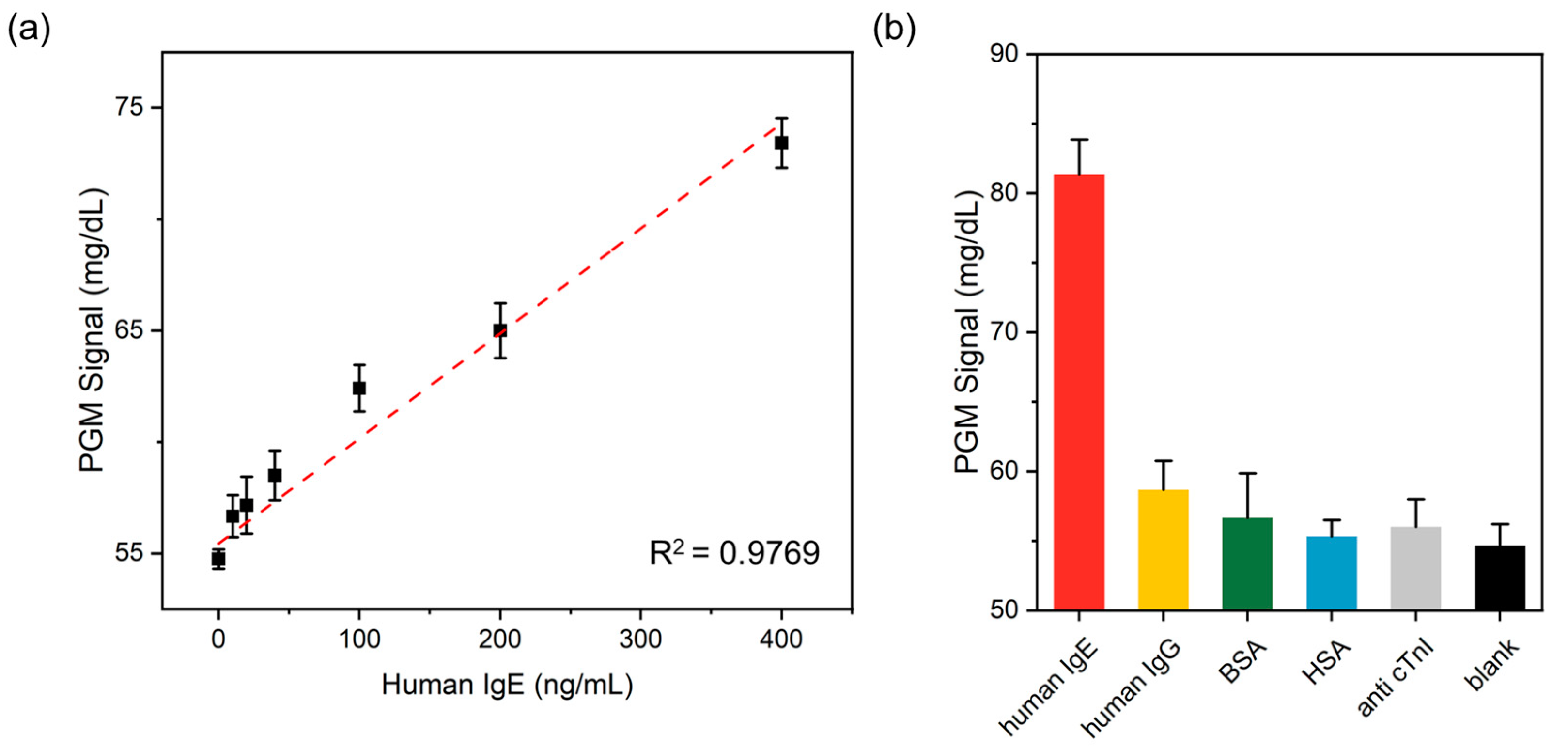
3.4. Sensitivity of the PGM-Based Human IgE Assay
3.5. Selectivity of the PGM-Based Human IgE Assay
3.6. Practical Applicability of the PGM-Based Human IgE Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Morais, S.; Tortajada-Genaro, L.A.; Maquieira, Á.; Martinez, M.Á.G. Biosensors for food allergy detection according to specific IgE levels in serum. Trends Anal. Chem. 2020, 127, 115904. [Google Scholar] [CrossRef]
- Holgate, S.T.; Broide, D. New targets for allergic rhinitis—A disease of civilization. Nat. Rev. Drug Discov. 2003, 2, 903–915. [Google Scholar] [CrossRef]
- Winter, W.E.; Hardt, N.S.; Fuhrman, S. Immunoglobulin E: Importance in parasitic infections and hypersensitivity responses. Arch. Pathol. Lab. Med. 2000, 124, 1382–1385. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.P.; Han, S.J.; Sim, S.J. Aptamer biosensor for lable-free detection of human immunoglobulin E based on surface plasmon resonance. Sens. Actuators B Chem. 2009, 139, 471–475. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, M.; Jiao, M.; Luo, X. Antifouling and ultrasensitive biosensing interface based on self-assembled peptide and aptamer on macroporous gold for electrochemical detection of immunoglobulin E in serum. Anal. Bioanal. Chem. 2018, 410, 5871–5878. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Busnel, J.M.; Peltre, G.; Zhang, X.X.; Girault, H.H. Magnetic beads based immunoaffinity capillary electrophoresis of total serum IgE with laser-induced fluorescence detection. Anal. Chem. 2008, 80, 9583–9588. [Google Scholar] [PubMed] [Green Version]
- Proczek, G.; Gassner, A.L.; Busnel, J.M.; Girault, H.H. Total serum IgE quantification by microfluidic ELISA using magnetic beads. Anal. Bioanal. Chem. 2012, 402, 2645–2653. [Google Scholar]
- King, C.L.; Poindexter, R.W.; Ragunathan, J.; Fleisher, T.A.; Ottesen, E.A.; Nutman, T.B. Frequency analysis of IgE-secreting B lymphocytes in persons with normal or elevated serum IgE levels. J. Immunol. 1991, 146, 1478–1483. [Google Scholar] [PubMed]
- Peavy, R.D.; Metcalfe, D.D. Understanding the mechanisms of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 310. [Google Scholar] [PubMed]
- Oh, S.J.; Ahn, J.K.; Park, H.; Song, Y.; Kwon, S.J.; Shin, H.B. An electrochemical immunosensing system on patterned electrodes for immunoglobulin E detection. Anal. Methods 2019, 11, 4410–4415. [Google Scholar] [CrossRef]
- Ebo, D.G.; Hagendorens, M.M.; Bridts, C.H.; Schuerwegh, A.J.; De Clerck, L.S.; Stevens, W.J. In vitro allergy diagnosis: Should we follow the flow? Clin. Exp. Allergy 2004, 34, 332–339. [Google Scholar] [CrossRef]
- Johansson, S.G. Radioimmunoassay of IgE and IgE antibody and its clinical application. J. Clin. Pathol. 1975, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Grange, R.D.; Thompson, J.P.; Lambert, D.G. Radioimmunoassay, enzyme and non-enzyme-based immunoassays. Br. J. Anaesth. 2014, 112, 213–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilloux, L.; Ricard-Blum, S.; Ville, G.; Motin, J. A new radioimmunoassay using a commercially available solid support for the detection of IgE antibodies against muscle relaxants. J. Allergy Clin. Immunol. 1992, 90, 153–159. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Chen, Y.; Woods, R.; Lee, N.; Yang, H.; Chowdhury, P.S.; Roskos, L.K.; White, W.I. An electrochemiluminescence (ECL)-based assay for the specific detection of anti-drug antibodies of the IgE isotype. J. Pharm. Biomed. Anal. 2013, 86, 73–81. [Google Scholar] [CrossRef]
- Shi, G.F.; Cao, J.T.; Zhang, J.J.; Liu, Y.M.; Chen, Y.H.; Ren, S.W. An electrochemiluminescence aptasensor based on flowerlike CdS–MoS2 composites and DNAzyme for detection of immunoglobulin E. Sens. Actuators B Chem. 2015, 220, 340–346. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Liu, Y. Petal-like CdS nanospheres-based electrochemiluminescence aptasensor for detection of IgE with gold nanoparticles amplification. Spectrochim. Acta A 2015, 151, 274–279. [Google Scholar] [CrossRef]
- Ghosh, S.; Aggarwal, K.; Vinitha, T.U.; Nguyen, T.; Han, J.; Ahn, C.H. A new microchannel capillary flow assay (MCFA) platform with lyophilized chemiluminescence reagents for a smartphone-based POCT detecting malaria. Microsyst. Nanoeng. 2020, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Li, X.; Zhou, J.; Liu, S.; Tian, T.; Song, Y.; Zhu, Z.; Zhou, L.; Ji, T.H.; Yang, C. A fully integrated distance readout ELISA-Chip for point-of-care testing with sample-in-answer-out capability. Biosens. Bioelectron. 2017, 96, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 2011, 3, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, Y. An invasive DNA approach toward a general method for portable quantification of metal ions using a personal glucose meter. Chem. Commun. 2013, 49, 585–587. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Lu, Y. Portable and quantitative detection of protein biomarkers and small molecular toxins using antibodies and ubiquitous personal glucose meters. Anal. Chem. 2012, 84, 4174–4178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Y.; Lu, Y. Using commercially available personal glucose meters for portable quantification of DNA. Anal. Chem. 2012, 84, 1975–1980. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Xiang, Y.; Tong, A.; Lu, Y. Simple and efficient method to purify DNA–protein conjugates and its sensing applications. Anal. Chem. 2014, 86, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lan, T.; Shi, H.; Lu, Y. Portable detection of melamine in milk using a personal glucose meter based on an in vitro selected structure-switching aptamer. Anal. Chem. 2015, 87, 7676–7682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Luan, Y.; Xiong, M.; Zhang, J.; Lake, R.; Lu, Y. DNAzyme Amplified Aptasensing Platform for Ochratoxin A Detection Using a Personal Glucose Meter. ACS Appl. Mater. Interfaces 2021, 13, 9472–9481. [Google Scholar] [CrossRef]
- Xu, J.; Qiao, X.; Zhang, J.; Cheng, N.; Sheng, Q.; Zheng, J.; Cao, W.; Yue, T.; Lu, Y. Point-of-care monitoring of intracellular glutathione and serum triglyceride levels using a versatile personal glucose meter. Anal. Methods 2019, 11, 1849–1856. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, Y.; Kang, W.; Wang, B.; Li, J.; Wu, X.; Wang, S.; Liu, F. Quantitative and selective DNA detection with portable personal glucose meter using loop-based DNA competitive hybridization strategy. Sens. Actuators B Chem. 2019, 282, 197–203. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Y.; Cao, Y.; Song, Y.; Xu, L.P.; Zhang, X.; Wang, S. Bioinspired DNA–inorganic hybrid nanoflowers combined with a personal glucose meter for onsite detection of miRNA. ACS Appl. Mater. Interfaces 2018, 10, 42050–42057. [Google Scholar] [CrossRef] [PubMed]
- Lisi, F.; Peterson, J.R.; Gooding, J.J. The application of personal glucose meters as universal point-of-care diagnostic tools. Biosens. Bioelectron. 2020, 148, 111835. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, T.; Xu, L.P.; Zhang, X. Portable detection of Staphylococcus aureus using personal glucose meter based on hybridization chain reaction strategy. Talanta 2021, 226, 122132. [Google Scholar] [CrossRef]
- Ahn, J.K.; Kim, H.Y.; Park, K.S.; Park, H.G. A personal glucose meter for label-free and washing-free biomolecular detection. Anal. Chem. 2018, 90, 11340–11343. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.K.; Kim, H.Y.; Lee, C.Y.; Park, K.S.; Park, H.G. Label-free and washing-free alkaline phosphatase assay using a personal glucose meter. J. Biol. Eng. 2019, 13, 1–5. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ahn, J.K.; Park, K.S.; Park, H.G. Portable glucose meter-based label-free strategy for target DNA detection. Sens. Actuators B Chem. 2020, 310, 127808. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, C.Y.; Kim, H.; Park, K.S.; Park, H.G. Portable glucose meter-utilized label-free and washing-free telomerase assay. Analyst. 2020, 145, 5578–5583. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.R.; Reddington, A.P.; Collins, A.D.; LaBoda, C.; Cretich, M.; Chiari, M.; Little, F.F.; UÜnlü, M.S. Multiplexed method to calibrate and quantitate fluorescence signal for allergen-specific IgE. Anal. Chem. 2011, 83, 9485–9491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papamichael, K.I.; Kreuzer, M.P.; Guilbault, G.G. Viability of allergy (IgE) detection using an alternative aptamer receptor and electrochemical means. Sens. Actuators B Chem. 2007, 121, 178–186. [Google Scholar] [CrossRef]
- Chinnasamy, T.; Segerink, L.I.; Nystrand, M.; Gantelius, J.; Svahn, H.A. A lateral flow paper microarray for rapid allergy point of care diagnostics. Analyst 2014, 139, 2348–2354. [Google Scholar] [CrossRef]
- Reuterswärd, P.; Gantelius, J.; Svahn, H.A. An 8 min colorimetric paper-based reverse phase vertical flow serum microarray for screening of hyper IgE syndrome. Analyst 2015, 140, 7327–7334. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Lee, S.J.; Lee, H.J. Ultra-sensitive detection of IgE using biofunctionalized nanoparticle-enhanced SPR. Talanta 2010, 81, 1755–1759. [Google Scholar] [CrossRef]
- Shyur, S.D.; Jan, R.L.; Webster, J.R.; Chang, P.; Lu, Y.J.; Wang, J.Y. Determination of multiple allergen-specific IgE by microfluidic immunoassay cartridge in clinical settings. Pediatr. Allergy Immunol. 2010, 21, 623–633. [Google Scholar] [CrossRef]
- Tai, L.W.; Tseng, K.Y.; Wang, S.T.; Chiu, C.C.; Kow, C.H.; Chang, P.; Chen, C.; Wang, J.Y.; Webster, J.R. An automated microfluidic-based immunoassay cartridge for allergen screening and other multiplexed assays. Anal. Biochem. 2009, 391, 98–105. [Google Scholar] [CrossRef] [PubMed]
- González, T.Y.; Leonard, A.; Gaude, V.; Delplanque, A.; Barre, A.; Rougé, P.; Garnier, L.; Bienvenu, F.; Bienvenu, J.; Zelsmann, M.; et al. IgE detection in allergic patient’s serum by absorption analysis of biofunctionalised microparticles. Microelectron. Eng. 2019, 207, 27–32. [Google Scholar] [CrossRef]
- Platt, G.W.; Damin, F.; Swann, M.J.; Metton, I.; Skorski, G.; Cretich, M.; Chiari, M. Allergen immobilisation and signal amplification by quantum dots for use in a biosensor assay of IgE in serum. Biosens. Bioelectron. 2014, 52, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Z.; Niu, Q.; Li, H.; Vuki, M.; Xu, D. Visual microarray detection for human IgE based on silver nanoparticles. Sens. Actuators B Chem. 2017, 239, 45–51. [Google Scholar] [CrossRef]
- Teste, B.; Malloggi, F.; Siaugue, J.M.; Varenne, A.; Kanoufi, F.; Descroix, S. Microchip integrating magnetic nanoparticles for allergy diagnosis. Lab. Chip 2011, 11, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Unal, D.; Gelincik, A.; Elitok, A.; Demir, S.; Olgac, M.; Coskun, R.; Kocaaga, M.; Colakoglu, B.; Buyukozturk, S. Impact of high serum Immunoglobulin E levels on the risk of atherosclerosis in humans. Asia Pac. Allergy 2017, 7, 74–81. [Google Scholar] [CrossRef]
- Ahn, J.K.; Park, K.S.; Won, B.Y.; Park, H.G. A novel electrochemical method to detect theophylline utilizing silver ions captured within abasic site-incorporated duplex DNA. Biosens. Bioelectron. 2015, 67, 590–594. [Google Scholar] [CrossRef]
- Ahn, J.K.; Kim, H.Y.; Baek, S.; Park, H.G. A new s-adenosylhomocysteine hydrolase-linked method for adenosine detection based on DNA-templated fluorescent Cu/Ag nanoclusters. Biosens. Bioelectron. 2017, 93, 330–334. [Google Scholar] [CrossRef]
| Method | Key Elements | LOD (ng/mL) | Sample | Ref. |
|---|---|---|---|---|
| Arrays | Indirect assay with bound allergens | 49.3 | Non-diluted serum | [37] |
| Electrochemical | Aptasensor | 300 | Non-diluted serum | [38] |
| Immunochemical | Paper-based assay | 2.4 | Non-diluted serum | [39] |
| Vertical flow assays | 1900 | Diluted serum (10%) | [40] | |
| Label-free | SPR | 190 | Buffer solution | [41] |
| Microfluidics | Miniaturized array | 27 | Non-diluted serum | [42] |
| 2.4 | Non-diluted serum | [43] | ||
| Miniaturized immunodiffusion | 1 | Diluted serum (20%) | [43] | |
| Nanomaterial-based | Magnetic capture | 24 | Diluted serum | [44] |
| Quantum dots | 84 | Diluted serum (2%) | [45] | |
| Silver particle | 20 | Diluted serum (20%) | [46] | |
| PGM | Cascade enzymatic reaction | 29.6 | Non-diluted serum | This work |
| Added IgE (μg/mL) | Measured IgE (μg/mL) | SD | CV (%) | Recovery (%) |
|---|---|---|---|---|
| 1.0 | 1.05 | 0.082 | 7.75 | 105.21 |
| 0.5 | 0.49 | 0.036 | 7.35 | 99.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Park, J.; Ahn, J.K. Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter. Sensors 2021, 21, 6396. https://doi.org/10.3390/s21196396
Han H, Park J, Ahn JK. Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter. Sensors. 2021; 21(19):6396. https://doi.org/10.3390/s21196396
Chicago/Turabian StyleHan, Hyogu, Junhyun Park, and Jun Ki Ahn. 2021. "Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter" Sensors 21, no. 19: 6396. https://doi.org/10.3390/s21196396
APA StyleHan, H., Park, J., & Ahn, J. K. (2021). Immunoglobulin E Detection Method Based on Cascade Enzymatic Reaction Utilizing Portable Personal Glucose Meter. Sensors, 21(19), 6396. https://doi.org/10.3390/s21196396







