Dry Wearable Textile Electrodes for Portable Electrical Impedance Tomography
Abstract
:1. Introduction
2. Materials and Methods
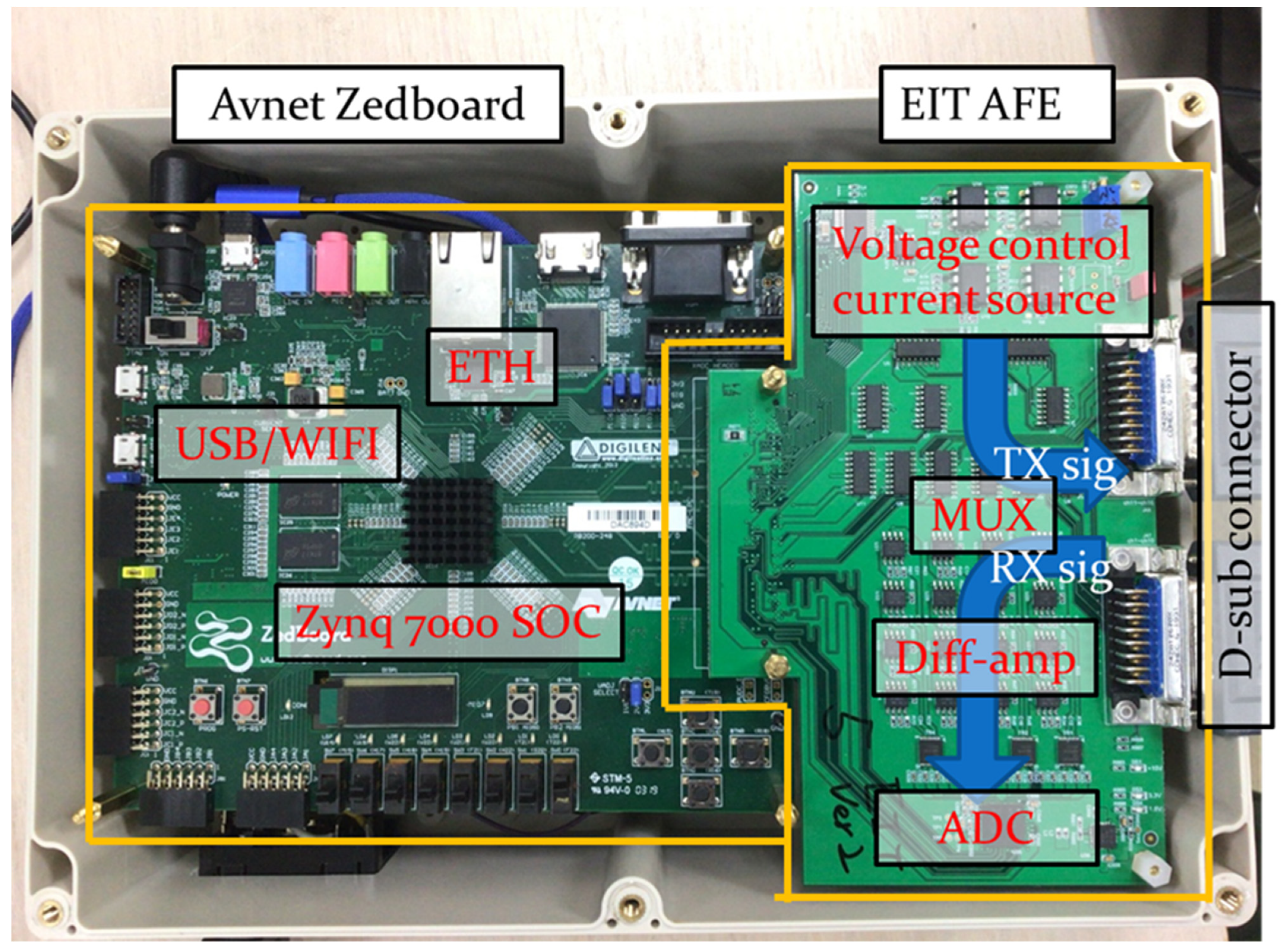
2.1. Portable EIT System
2.2. Image Reconstruction for Difference EIT
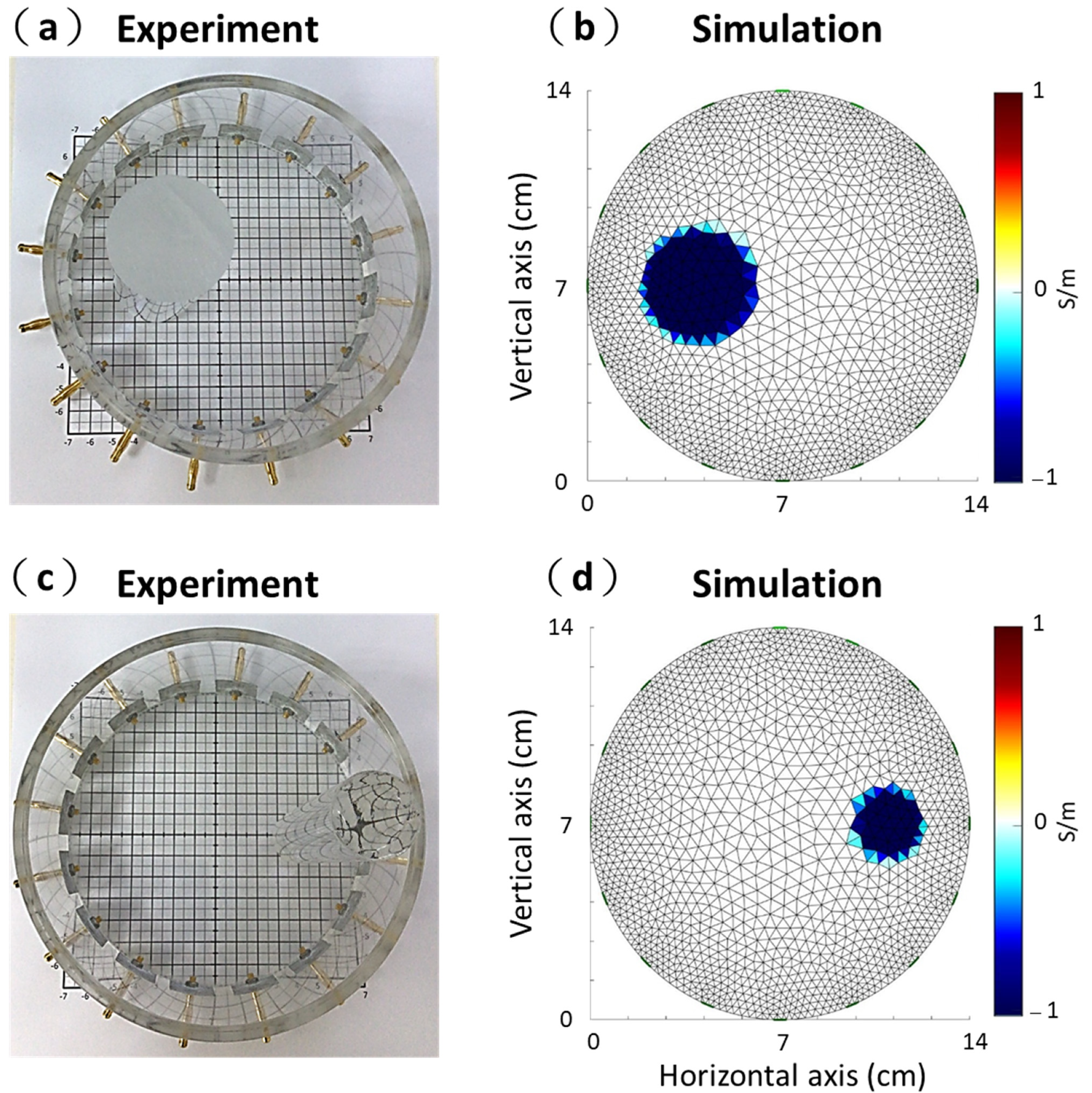
2.3. Experimental Setup and Simulation
2.4. Wearable Textile-Electrode EIT Belt
2.5. In Vivo Lung Imaging during Breathing
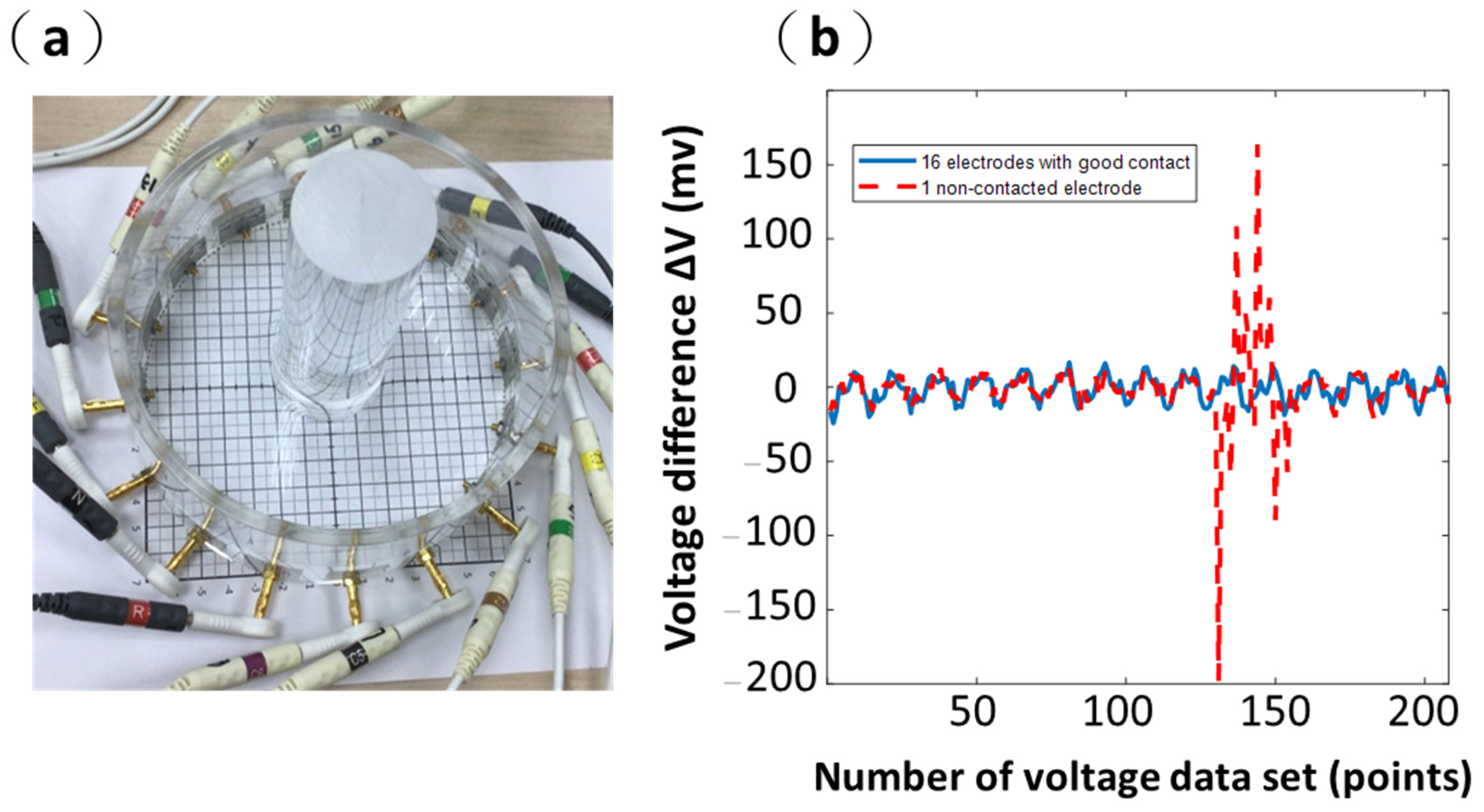
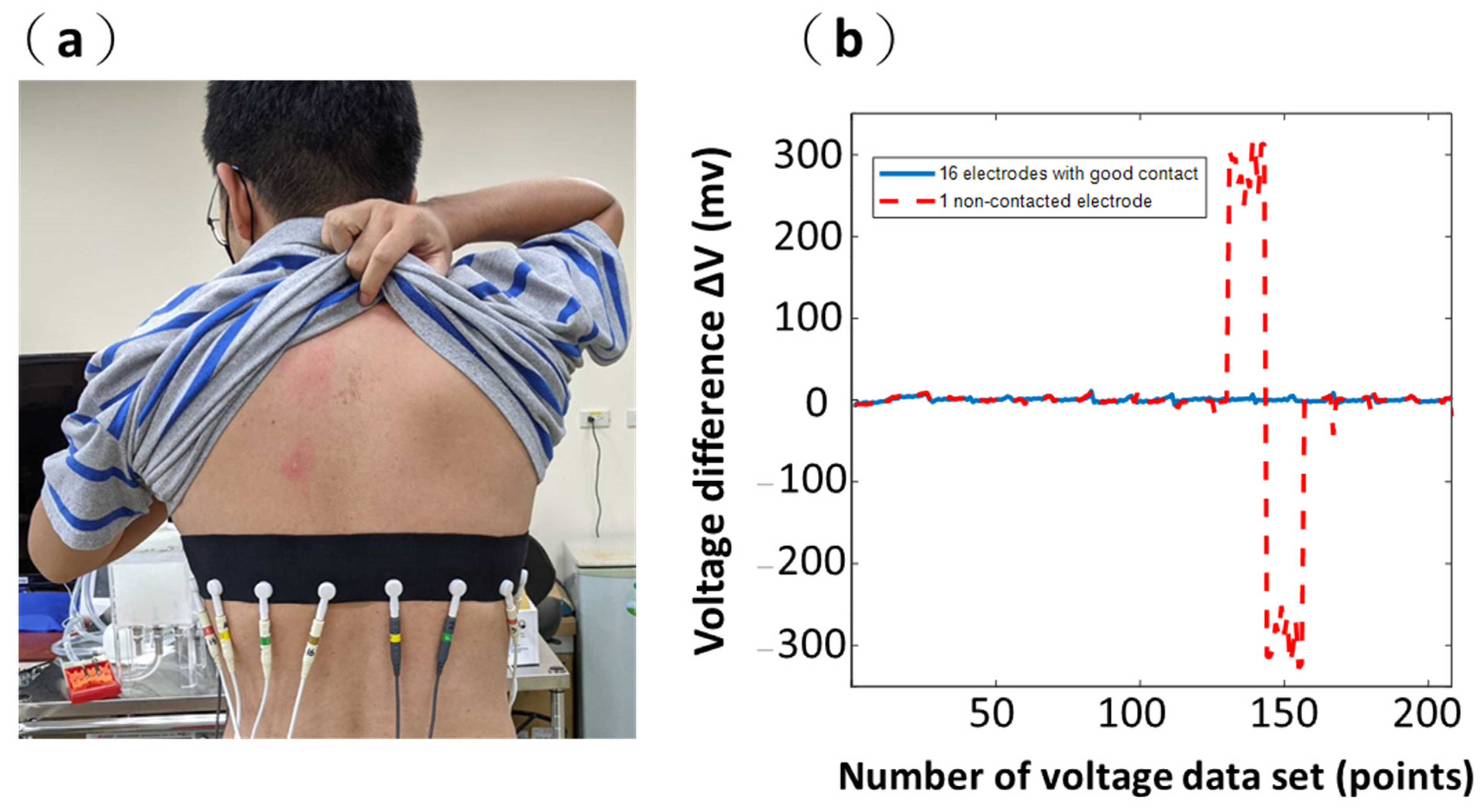
2.6. Contact between Surface and Electrodes
3. Results and Discussion
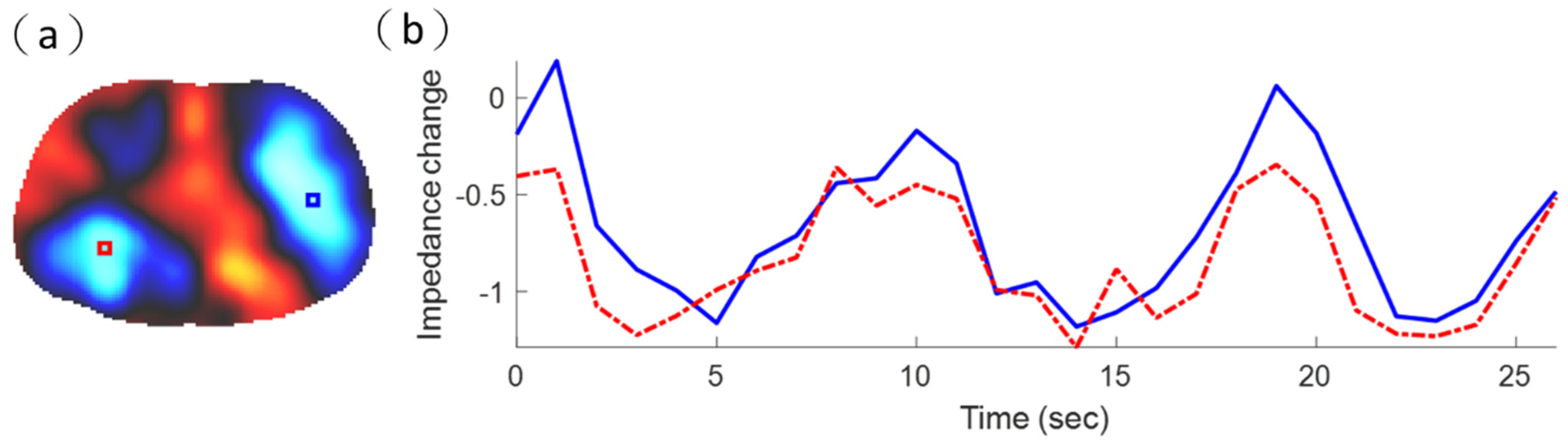
3.1. Experimental and Simulation Results
3.2. Results from Commercial ECG Electrodes (Ag/AgCl Electrodes)
3.3. Results from Wearable Textile-Electrode Belt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowdish, D.M. The aging lung: Is lung health good health for older adults? Chest 2019, 155, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, D.; Bardill, A.; De Gelidi, S.; Bayford, R.; Demosthenous, A. A high frame rate wearable EIT system using ac-tive electrode ASICs for lung respiration and heart rate monitoring. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 65, 3810–3820. [Google Scholar] [CrossRef]
- Brown, B.H.; Barber, D.C.; Seagar, A.D. Clinical physics and physiological measurement applied potential tomography: Pos-sible clinical applications. Clin. Phys. Physiol. Meas. 1985, 6, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.P.; Webster, J.G. An impedance camera for spatially specific measurements of the thorax. IEEE Trans. Biomed. Eng. 1978, 25, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.C.; Brown, B.H.; Freeston, I.L. Imaging spatial distributions of resistivity using applied potential tomog-raphy. Electron. Lett. 1983, 19, 93–95. [Google Scholar] [CrossRef]
- Brown, B.H. Tissue Impedance Methods, Imaging with Non-Ionising Radiation; Jackson, D.F., Ed.; Surrey University Press: Guilford, UK, 1983. [Google Scholar]
- Garg, D.; Goel, V. Design and development of Electrical Impedance Tomography (EIT) based System. Int. J. Comput. Appl. 2013, 74, 33–36. [Google Scholar] [CrossRef]
- Boverman, G.; Isaacson, D.; Newell, J.C.; Saulnier, G.J.; Kao, T.-J.; Amm, B.C.; Wang, X.; Davenport, D.M.; Chong, D.H.; Sahni, R.; et al. Efficient simultaneous reconstruction of time-varying images and electrode contact impedances in electrical impedance tomography. IEEE Trans. Biomed. Eng. 2016, 64, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.A.; Ling, S.H. Review on electrical impedance tomography: Artificial intelligence methods and its applications. Algorithms 2019, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Woo, E.; Hua, P.; Webster, J.; Tompkins, W.J. Measuring lung resistivity using electrical impedance tomography. IEEE Trans. Biomed. Eng. 1992, 39, 756–760. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Yoo, H.J. Wearable lung-health monitoring system with electrical impedance tomography. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 1707–1710. [Google Scholar]
- Costa, E.L.V.; Chaves, C.N.; Gomes, S.; Beraldo, M.A.; Volpe, M.S.; Tucci, M.R.; Schettino, I.A.L.; Bohm, S.H.; Carvalho, C.R.R.; Tanaka, H.; et al. Real-time detection of pneumothorax using electrical impedance tomography. Crit. Care Med. 2008, 36, 1230–1238. [Google Scholar] [CrossRef]
- Mauri, T.; Bellani, G.; Salerno, D.; Mantegazza, F.; Pesenti, A. Regional distribution of air trapping in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1466–1467. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; De, D.R. Advances in textile technologies for unobtrusive monitoring of vital parameters and movements. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 392–395. [Google Scholar]
- Poh, M.Z.; Swenson, N.C.; Picard, R.W. A wearable sensor for unobtrusive, long-term assessment of electrodermal activity. IEEE Trans. Biomed. Eng. 2010, 57, 1243–1252. [Google Scholar] [PubMed] [Green Version]
- Yilmaz, T.; Foster, R.; Hao, Y. Detecting vital signs with wearable wireless sensors. Sensors 2010, 10, 10837–10862. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, M.; Loriga, G.; Taccini, N.; Paradiso, R. Sensing fabrics for monitoring physiological and biomechanical variables: E-textile solutions. In Proceedings of the 2006 3rd IEEE/EMBS International Summer School on Medical Devices and Biosen-sors, Cambridge, MA, USA, 4–6 September 2006; pp. 1–4. [Google Scholar]
- Scilingo, E.P.; Gemignani, A.; Paradiso, R.; Taccini, N.; Ghelarducci, B.; De Rossi, D. Performance evaluation of sensing fabrics for monitoring physiological and biomechanical variables. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 345–352. [Google Scholar] [CrossRef]
- Arquilla, K.; Webb, A.K.; Anderson, A. Textile electrocardiogram (ECG) electrodes for wearable health monitoring. Sensors 2020, 20, 1013. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.J.; Milnes, P.; Waterworth, A.R.; Smallwood, R.; Brown, B.H. Mk3.5: A modular, multi-frequency successor to the Mk3a EIS/EIT system. Physiol. Meas. 2001, 22, 49–54. [Google Scholar] [CrossRef]
- Wolf, G.K.; Gómez-Laberge, C.; Rettig, J.S.; Vargas, S.O.; Smallwood, C.D.; Prabhu, S.P.; Arnold, J.H. Mechanical ventila-tion guided by electrical impedance tomography in experimental acute lung injury. Crit. Care Med. 2013, 41, 1296–1304. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Bae, J.; Yoo, H.-J. A 10.4 mW electrical impedance tomography SoC for portable real-time lung ventilation monitoring system. IEEE J. Solid-State Circuits 2015, 50, 2501–2512. [Google Scholar] [CrossRef]
- Kim, M.; Bae, J.; Yoo, H.J. Wearable 3D lung ventilation monitoring system with multi frequency electrical impedance to-mography. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Torino, Italy, 19–21 October 2017; pp. 1–4. [Google Scholar]
- Neruda, M.; Vojtech, L. Verification of surface conductance model of textile materials. J. Appl. Res. Technol. 2012, 10, 578–584. [Google Scholar] [CrossRef]
- Vojtech, L.; Bortel, R.; Neruda, M.; Kozak, M. Wearable textile electrodes for ECG measurement. Adv. Electr. Electron. Eng. 2013, 11, 410–414. [Google Scholar] [CrossRef]
- Lee, Y.-D.; Chung, W.-Y. Wireless sensor network based wearable smart shirt for ubiquitous health and activity monitoring. Sens. Actuators B Chem. 2009, 140, 390–395. [Google Scholar] [CrossRef]
- López, G.; Custodio, V.; Moreno, J.I. LOBIN: E-textile and wireless-sensor-network-based platform for healthcare monitoring in future hospital environments. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 1446–1458. [Google Scholar] [CrossRef]
- Yoo, J.; Yan, L.; Lee, S.; Kim, H.; Yoo, H.-J. A wearable ECG acquisition system with compact planar-fashionable circuit board-based shirt. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 897–902. [Google Scholar] [CrossRef]
- Merritt, C.R.; Nagle, H.T.; Grant, E. Fabric-based active electrode design and fabrication for health monitoring clothing. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 274–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, J.X.; Bohm, S.H. Belt for Electro Impedance Measurement and Method Using Such Belt. U.S. Patent US10278643B2, 7 May 2019. [Google Scholar]
- Pleyers, T.; Levionnois, O.; Siegenthaler, J.; Spadavecchia, C.; Raillard, M. Investigation of selected respiratory effects of (dex)medetomidine in healthy Beagles. Veter-Anaesth. Analg. 2020, 47, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Sophocleous, L.; Frerichs, I.; Miedema, M.; Kallio, M.; Papadouri, T.; Karaoli, C.; Becher, T.; Tingay, D.G.; van Kaam, A.H.; Bayford, R.; et al. Clinical performance of a novel textile interface for neonatal chest electrical impedance tomography. Physiol. Meas. 2018, 39, 044004. [Google Scholar] [CrossRef] [Green Version]
- Teschner, E.; Imhoff, M.; Leonhardt, S. Electrical Impedance Tomography: The Realisation of Regional Ventilation Monitoring, 2nd ed.; Drager, Technology for Life: Lübeck, Germany, 2015. [Google Scholar]
- Chi, Y.M.; Jung, T.-P.; Cauwenberghs, G. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokus, M.A.; Jur, J. Fabric-based wearable dry electrodes for body surface biopotential recording. IEEE Trans. Biomed. Eng. 2015, 63, 423–430. [Google Scholar] [CrossRef]
- Bai, J.; Hashimoto, J.; Suzuki, T.; Nakahara, T.; Kubo, A.; Iwanaga, S.; Ogawa, S. Comparison of image reconstruction algo-rithms in myocardial perfusion scintigraphy. Ann. Nucl. Med. 2001, 15, 79–83. [Google Scholar] [CrossRef]
- Adler, A.; Lionheart, W.R.B. Uses and abuses of EIDORS: An extensible software base for EIT. Physiol. Meas. 2006, 27, 25–42. [Google Scholar] [CrossRef] [Green Version]
- Schöberl, J. NETGEN—An advancing front 2D/3D-mesh generator based on abstract rules. Comput. Vis. Sci. 1997, 1, 41–52. [Google Scholar] [CrossRef]
- Adler, A.; Arnold, J.H.; Bayford, R.; Borsic, A.; Brown, B.; Dixon, P.; Faes, T.J.C.; Frerichs, I.; Gagnon, H.; Gärber, Y.; et al. GREIT: A unified approach to 2D linear EIT reconstruction of lung images. Physiol. Meas. 2009, 30, S35. [Google Scholar] [CrossRef]
- Vauhkonen, M.; Lionheart, W.R.; Heikkinen, L.M.; Vauhkonen, P.J.; Kaipio, J.P. A MATLAB package for the EIDORS project to reconstruct two-dimensional EIT images. Physiol. Meas. 2001, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Nefti-Meziani, S.; Carbonaro, N.; Tognetti, A. A quantitative evaluation of drive pattern selection for optimizing EIT-based stretchable sensors. Sensors 2017, 17, 1999. [Google Scholar] [CrossRef]
- Symeonidou, E.-R.; Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Effects of cable sway, electrode surface area, and electrode mass on electroencephalography signal quality during motion. Sensors 2018, 18, 1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.-L.; Wu, G.-Z.; Chang, C.-C.; Li, M.-L. Acoustic-field beamforming for low-power portable ultrasound. Ultrason. Imaging 2021, 43, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, H.; Sigmen, Y.; Hartinger, A.E.; Guardo, R. An active phantom to assess the robustness of EIT systems to electrode contact impedance variations. In Proceedings of the International Conference on Biomedical Applications of Electrical Im-Pedance Tomography (EIT’09), Manchester, UK, 15–19 June 2009. [Google Scholar]
- Boverman, G.; Isaacson, D.; Saulnier, G.J.; Newell, J.C. Methods for compensating for variable electrode contact in EIT. IEEE Trans. Biomed. Eng. 2009, 56, 2762–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frerichs, I.; Amato, M.B.P.; Van Kaam, A.H.; Tingay, D.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2016, 72, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, N.D.; Suggett, A.J.; Barber, D.C.; Brown, B.H. Applications of applied potential tomography (APT) in respiratory medicine. Clin. Phys. Physiol. Meas. 1987, 8, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Riera, J.; Riu, P.; Casan, P.; Masclans, J. Electrical impedance tomography in acute lung injury. Med. Intensiv. 2011, 35, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Tomicic, V.; Cornejo, R. Lung monitoring with electrical impedance tomography: Technical considerations and clinical appli-cations. J. Thorac. Dis. 2019, 11, 3122. [Google Scholar] [CrossRef]
- Grychtol, B.; Adler, A. Uniform background assumption produces misleading lung EIT images. Physiol. Meas. 2013, 34, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Schiffmann, H.; Frerichs, A.; Klockgether-Radke, A.; Frerichs, I. Body and head position effects on regional lung ventilation in infants: An electrical impedance tomography study. Intensiv. Care Med. 2006, 32, 1392–1398. [Google Scholar] [CrossRef]
- Hahn, G.; Sipinkova, I.; Baisch, F.; Hellige, G. Changes in the thoracic impedance distribution under different ventilatory conditions. Physiol. Meas. 1995, 16, A161. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Boyle, A. Electrical impedance tomography: Tissue properties to image measures. IEEE Trans. Biomed. Eng. 2017, 64, 2494–2504. [Google Scholar] [CrossRef] [PubMed]








| Experiment | Simulation | |||
|---|---|---|---|---|
| Diameter of 4 cm | Diameter of 2.5 cm | Diameter of 4 cm | Diameter of 2.5 cm | |
| SD (%) | 16.51 | 19.41 | 13.42 | 15.1 |
| PE (mm) | 1.13 | 1.32 | 1.12 | 1.02 |
| PER (%) | 5.65 | 10.56 | 5.6 | 8.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.-L.; Cheng, I.-C.; Huang, C.-H.; Liao, Y.-T.; Lin, W.-C.; Tsai, K.-J.; Chi, C.-H.; Chen, C.-W.; Wu, C.-H.; Lin, I.-T.; et al. Dry Wearable Textile Electrodes for Portable Electrical Impedance Tomography. Sensors 2021, 21, 6789. https://doi.org/10.3390/s21206789
Hu C-L, Cheng I-C, Huang C-H, Liao Y-T, Lin W-C, Tsai K-J, Chi C-H, Chen C-W, Wu C-H, Lin I-T, et al. Dry Wearable Textile Electrodes for Portable Electrical Impedance Tomography. Sensors. 2021; 21(20):6789. https://doi.org/10.3390/s21206789
Chicago/Turabian StyleHu, Chang-Lin, I-Cheng Cheng, Chih-Hsien Huang, Yu-Te Liao, Wei-Chieh Lin, Kun-Ju Tsai, Chih-Hsien Chi, Chang-Wen Chen, Chia-Hsi Wu, I-Te Lin, and et al. 2021. "Dry Wearable Textile Electrodes for Portable Electrical Impedance Tomography" Sensors 21, no. 20: 6789. https://doi.org/10.3390/s21206789






