New Stimulation Device to Drive Multiple Transverse Intrafascicular Electrodes and Achieve Highly Selective and Rich Neural Responses
Abstract
:1. Introduction
2. Materials and Methods
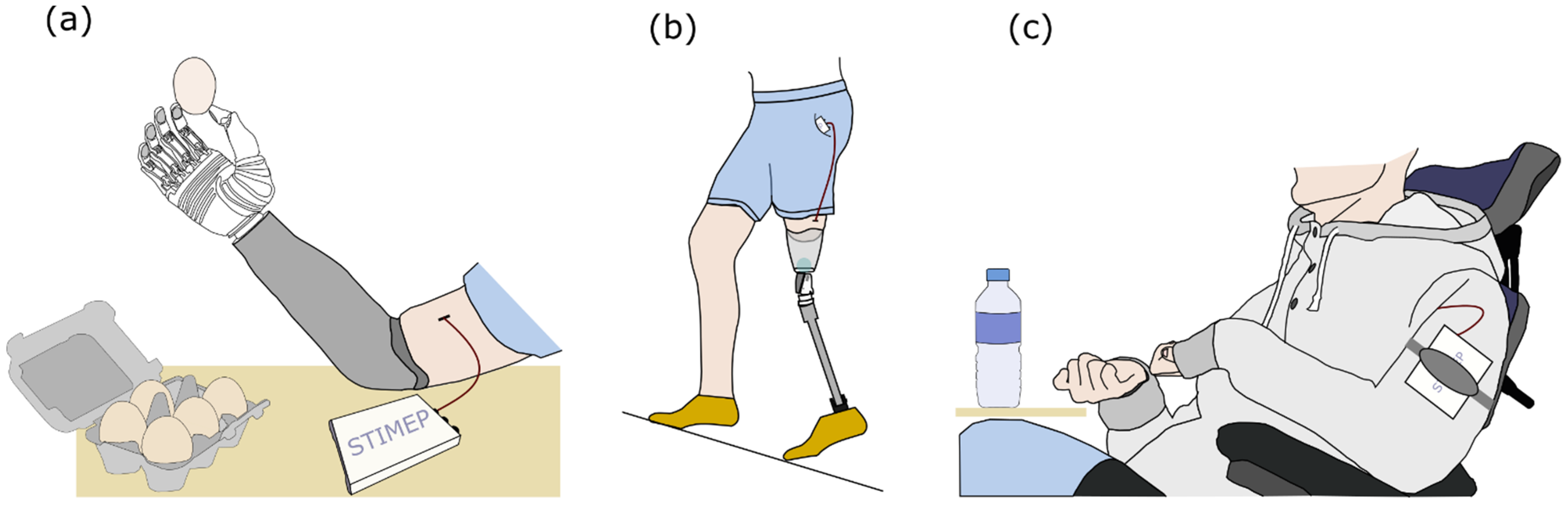
2.1. General Description of the Stimulation Device—STIMEP Platform
2.2. In Vivo Validation of the Stimulation Device and Selectivity Study
2.2.1. Experimental Set-Up
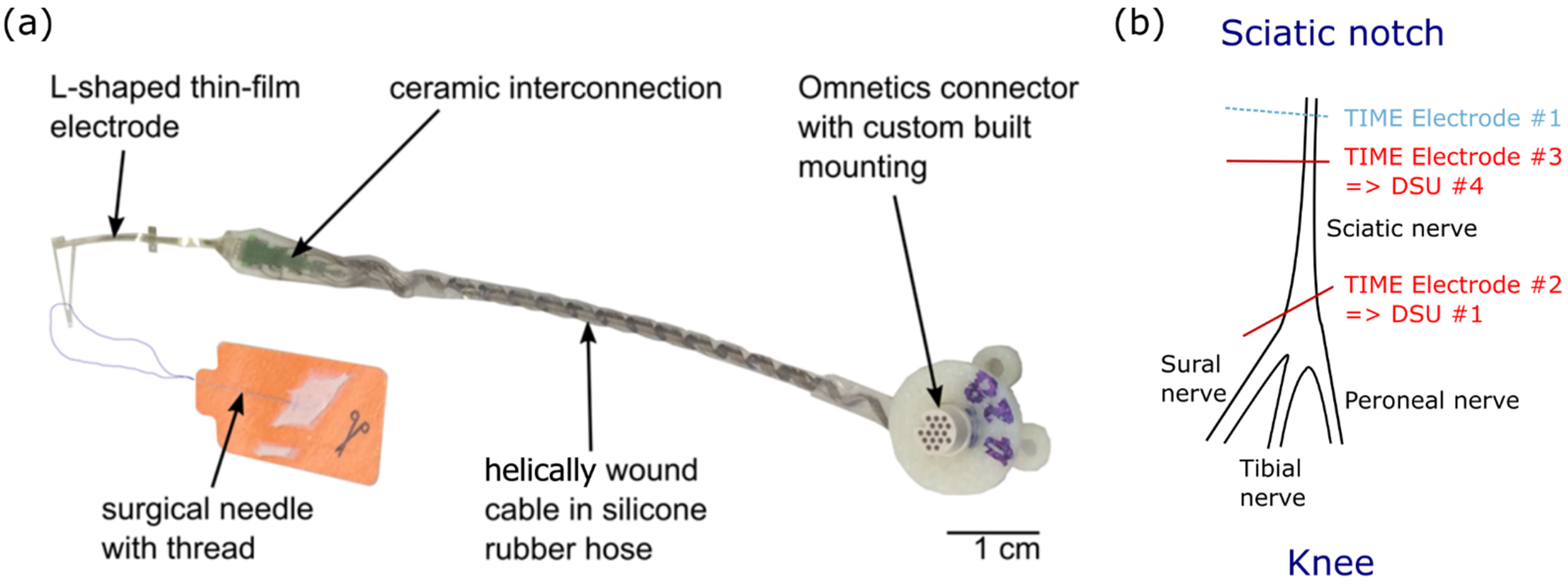
2.2.2. TIME Implants
2.2.3. Stimulation Paradigms
- Protocol 1: Scanning with intensity for different pulse widths using a single TIME implant
- Protocol 2: Stimulation using two DSU simultaneously to drive two TIME implants
- Protocol 3: Implementation of two stimulation waveforms
2.2.4. Signal Processing
3. Results
3.1. STIMEP Successfully Delivered Finely Tuned Stimulations That Potentiate Intrafascicular Stimulation Selectivity
3.2. STIMEP Successfully Drove Two TIME Implants Simultaneously and Increased Spatial Selectivity
3.3. STIMEP Generated Complex Waveforms and Underlines the Relative Impact of Polarity on Stimulation Selectivity Using TIME Implants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wyndaele, M.; Wyndaele, J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide liter-ature survey? Spinal Cord 2006, 44, 523–529. [Google Scholar] [CrossRef]
- Snoek, G.J.; Ijzerman, M.J.; Hermens, H.J.; Maxwell, D.; Biering-Sørensen, F. Survey of the needs of patients with spinal cord injury: Impact and priority for improvement in hand function in tetraplegics. Spinal Cord 2004, 42, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.D. Targeting Recovery: Priorities of the Spinal Cord-Injured Population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef]
- Glenn, W.W.; Phelps, M.L.; Elefterleades, J.A.; Dentz, B.; Hogan, J.F. Twenty years of experience in phrenic nerve stimulation to pace the diaphragm. Pacing Clin. Electrophysiol. 1986, 9, 780–784. [Google Scholar] [CrossRef]
- Possover, M.; Schurch, B.; Henle, K. New strategies of pelvic nerves stimulation for recovery of pelvic visceral functions and locomotion in paraplegics. Neurourol. Urodyn. 2010, 29, 1433–1438. [Google Scholar] [CrossRef]
- Brindley, G.S. The first 500 patients with sacral anterior root stimulator implants: General description. Spinal Cord 1994, 32, 795–805. [Google Scholar] [CrossRef] [Green Version]
- Andersen, I.S.; Rijkhoff, N.J.M.; Vukovic, A.; Buntzen, S.; Djurhuus, J.C.; Laurberg, S. Anorectal motility responses to selective stimulation of the ventral sacral nerve roots in an experimental model. Br. J. Surg. 2005, 92, 1513–1519. [Google Scholar] [CrossRef]
- Guiho, T.; Delleci, C.; Azevedo-Coste, C.; Fattal, C.; Guiraud, D.; Vignes, J.R.; Bauchet, L. Impact of direct epispinal stimulation on bladder and bowel functions in pigs: A feasibility study. Neurourol. Urodyn. 2017, 37, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.B.; Seki, K.; Jackson, A. Reanimating the arm and hand with intraspinal microstimulation. J. Neural Eng. 2011, 8, 054001. [Google Scholar] [CrossRef]
- Capogrosso, M.; Milekovic, T.; Borton, D.; Wagner, F.; Moraud, E.M.; Mignardot, J.-B.; Buse, N.; Gandar, J.; Barraud, Q.; Xing, D.; et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nat. Cell Biol. 2016, 539, 284–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushahwar, V.K.; Collins, D.F.; Prochazka, A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Exp. Neurol. 2000, 163, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.J.; Pinault, G.C.J.; Neville, J.J.; Syed, I.; Davis, J.A., Jr.; Jean-Claude, J.; Triolo, R.J. Fascicular anatomy of human femoral nerve: Implications for neural prostheses using nerve cuff electrodes. J. Rehabil. Res. Dev. 2009, 46, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.D.; Badia, J.; Pascual-Font, A.; Rodríguez-Baeza, A.; Navarro, X. Fascicular Topography of the Human Median Nerve for Neuroprosthetic Surgery. Front. Neurosci. 2016, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Catalan, M.; Håkansson, B.; Brånemark, R. An osseointegrated human-machine gateway for long-term sensory feed-back and motor control of artificial limbs. Sci. Transl. Med. 2014, 6, 257re6. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.W.; Schiefer, M.A.; Keith, M.W.; Anderson, J.R.; Tyler, J.; Tyler, D.J. A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 2014, 6, 257ra138. [Google Scholar] [CrossRef] [Green Version]
- Raspopovic, S.; Capogrosso, M.; Petrini, F.M.; Bonizzato, M.; Rigosa, J.; Di Pino, G.; Carpaneto, J.; Controzzi, M.; Boretius, T.; Fernandez, E.; et al. Restoring Natural Sensory Feedback in Real-Time Bidirectional Hand Prostheses. Sci. Transl. Med. 2014, 6, 222ra19. [Google Scholar] [CrossRef]
- Grill, W.; Mortimer, J. Stimulus waveforms for selective neural stimulation. IEEE Eng. Med. Boil. Mag. 1995, 14, 375–385. [Google Scholar] [CrossRef]
- Veraart, C.; Grill, W.; Mortimer, J. Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans. Biomed. Eng. 1993, 40, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Nannini, N.; Horch, K. Muscle recruitment with intrafascicular electrodes. IEEE Trans. Biomed. Eng. 1991, 38, 769–776. [Google Scholar] [CrossRef]
- Tyler, D.; Durand, D. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2002, 10, 294–303. [Google Scholar] [CrossRef]
- Lawrence, S.; Dhillon, G.; Jensen, W.; Yoshida, K.; Horch, K. Acute peripheral nerve recording Characteristics of polymer-based longitudinal intrafascicular electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 345–348. [Google Scholar] [CrossRef]
- Badia, J.; Boretius, T.; Pascual-Font, A.; Udina, E.; Stieglitz, T.; Navarro, X. Biocompatibility of chronically implanted trans-verse intrafascicular multichannel electrode (TIME) in the rat sciatic nerve. IEEE Trans. Biomed. Eng. 2011, 58, 2324–2332. [Google Scholar] [CrossRef]
- Boretius, T.; Badia, J.; Pascual-Font, A.; Schuettler, M.; Navarro, X.; Yoshida, K.; Stieglitz, T. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 2010, 26, 62–69. [Google Scholar] [CrossRef]
- Badia, J.; Boretius, T.; Andreu, D.; Azevedo-Coste, C.; Stieglitz, T.; Navarro, X. Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicles. J. Neural. Eng. 2011, 8, 036023. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.C.; Grill, W.M. Extracellular Stimulation of Central Neurons: Influence of Stimulus Waveform and Frequency on Neuronal Output. J. Neurophysiol. 2002, 88, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Qing, K.Y.; Ward, M.P.; Irazoqui, P.P. Burst-Modulated Waveforms Optimize Electrical Stimuli for Charge Efficiency and Fiber Selectivity. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Dali, M.; Picq, C.; Rossel, O.; Maciejasz, P.; Malbert, C.-H.; Guiraud, D. Comparison of the efficiency of chopped and non-rectangular electrical stimulus waveforms in activating small vagus nerve fibers. J. Neurosci. Methods 2019, 320, 1–8. [Google Scholar] [CrossRef]
- Shepherd, R.K.; Javel, E. Electrical stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear. Res. 1999, 130, 171–188. [Google Scholar] [CrossRef]
- Guiho, T.; Rossel, O.; Souquet, G.; Alfredo, H.; Laporte, L.; Coste, C.A.; Andreu, D.; Guiraud, D. Toward complex multipolar selective neural stimulation. In Proceedings of the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 22–24 April 2015; pp. 569–572. [Google Scholar]
- Vuckovic, A.; Tosato, M.; Struijk, J.J. A comparative study of three techniques for diameter selective fiber activation in the vagal nerve: Anodal block, depolarizing prepulses and slowly rising pulses. J. Neural Eng. 2008, 5, 275–286. [Google Scholar] [CrossRef]
- Vučković, A.; Rijkhoff, N.J.M. Different pulse shapes for selective large fibre block in sacral nerve roots using a technique of anodal block: An experimental study. Med. Biol. Eng. Comput. 2004, 42, 817–824. [Google Scholar] [CrossRef]
- Grill, W.M.; Mortimer, J.T. Inversion of the current-distance relationship by transient depolarization. IEEE Trans. Biomed. Eng. 1997, 44, 1–9. [Google Scholar] [CrossRef]
- Hennings, K.; Arendt-Nielsen, L.; Andersen, O.K. Orderly activation of human motor neurons using electrical ramp pre-pulses. Clin. Neurophysiol. 2005, 116, 597–604. [Google Scholar] [CrossRef]
- Bhadra, N.; Bhadra, N.; Kilgore, K.; Gustafson, K.J. High frequency electrical conduction block of the pudendal nerve. J. Neural. Eng. 2006, 3, 180. [Google Scholar] [CrossRef] [Green Version]
- Williams, I.; Brunton, E.; Rapeaux, A.; Liu, Y.; Luan, S.; Nazarpour, K.; Constandinou, T.G. SenseBack—An Implantable System for Bidirectional Neural Interfacing. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Sawan, M.; Hu, Y.; Coulombe, J. Wireless smart implants dedicated to multichannel monitoring and microstimulation. IEEE Circuits Syst. Mag. 2005, 5, 21–39. [Google Scholar] [CrossRef]
- Shon, A.; Chu, J.-U.; Jung, J.; Kim, H.; Youn, I. An Implantable Wireless Neural Interface System for Simultaneous Recording and Stimulation of Peripheral Nerve with a Single Cuff Electrode. Sensors 2017, 18, 1. [Google Scholar] [CrossRef] [Green Version]
- Andreu, D.; Maciejasz, P.; Passama, R.; Cathébras, G.; Souquet, G.; Wauters, L.; Divoux, J.L.; Guiraud, D. Synchronous Multi-Channel Stimulator with Embedded Safety Procedure to Perform 12-Poles Time3h 3d Stimulation, Direct Nerve Stimulation for Induction of Sensation and Treatment of Phantom Limb Pain; River Publishers: Gistrup, Denmark, 2019; pp. 193–216. [Google Scholar]
- Kobetic, R.; Sharma, M.; Triolo, R.; Uhlir, J.; Bieri, C.; Wibowo, M.; Polando, G.; Marsolais, E.; Davis, J.; Ferguson, K. Implanted functional electrical stimulation system for mobility in paraplegia: A follow-up case report. IEEE Trans. Rehabil. Eng. 1999, 7, 390–398. [Google Scholar] [CrossRef]
- Davis, R.; Houdayer, T.; Andrews, B.; Emmons, S.; Patrick, J. Paraplegia: Prolonged Closed-Loop Standing with Implanted Nucleus FES-22 Stimulator and Andrews’ Foot-Ankle Orthosis. Ster. Funct. Neurosurg. 1997, 69, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Arabi, K.; Sawan, M.A. Electronic design of a multichannel programmable implant for neuromuscular electrical stimulation. IEEE Trans. Rehabil. Eng. 1999, 7, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, N.; Weissberg, J.; Loeb, G.E. Flexible communication and control protocol for injectable neuromuscular inter-faces. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 19–27. [Google Scholar] [CrossRef]
- Donaldson, N.D.N.; Perkins, T.; Worley, A. Lumbar Root Stimulation for Restoring Leg Function: Stimulator and Measurement of Muscle Actions. Artif. Organs 1997, 21, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Guiraud, D.; Souquet, G. A distributed architecture for activating the peripheral nervous system. J. Neural Eng. 2009, 6, 026001. [Google Scholar] [CrossRef] [PubMed]
- Guiho, T.; Andreu, D.; López-Alvarez, V.M.; Cvancara, P.; Hiairrassary, A.; Granata, G.; Wauters, L.; Jensen, W.; Divoux, J.L.; Micera, S.; et al. Advanced 56 channels stimulation system to drive intrafascicular electrodes. In Proceedings of the Converging Clinical and Engineering Research on Neurorehabilitation II, International Conference on Neurorehabilitation, Segovia, Spain, 18–21 October 2016; pp. 743–747. [Google Scholar]
- Stieglitz, T.; Beutel, H.; Meyer, J.-U. “Microflex”—A New Assembling Technique for Interconnects. J. Intell. Mater. Syst. Struct. 2000, 11, 417–425. [Google Scholar] [CrossRef]
- Boretius, T.; Yoshida, K.; Badia, J.; Harreby, K.; Kundu, A.; Navarro, X.; Jensen, W.; Stieglitz, T. A transverse intrafascicular multichannel electrode (TIME) to treat phantom limb pain Towards human clinical trials. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 282–287. [Google Scholar]
- Mortimer, J.T. Electrical Excitation of Nerve Neural Prostheses. In Neural Prostheses, Fundamental Studies; Agnew, W.F., McCreery, D.B., Eds.; Prentice Hall: Englewood Cliffs, NJ, USA, 1990; pp. 68–83. [Google Scholar]
- Maciejasz, P.; Badia, J.; Boretius, T.; Andreu, D.; Stieglitz, T.; Jensen, W.; Navarro, X.; Guiraud, D. Delaying discharge after the stimulus significantly decreases muscle activation thresholds with small impact on the selectivity: An in vivo study using time. Med. Biol. Eng. Comput. 2015, 53, 371–379. [Google Scholar] [CrossRef]
- Granata, G.; Di Iorio, R.; Romanello, R.; Iodice, F.; Raspopovic, S.; Petrini, F.; Strauss, I.; Valle, G.; Stieglitz, T.; Čvančara, P.; et al. Phantom somatosensory evoked potentials following selective intraneural electrical stimulation in two amputees. Clin. Neurophysiol. 2018, 129, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Rognini, G.; Petrini, F.M.; Raspopovic, S.; Valle, G.; Granata, G.; Strauss, I.; Solcà, M.; Bello-Ruiz, J.; Herbelin, B.; Mange, R.; et al. Multisensory bionic limb to achieve prosthesis embodiment and reduce distorted phantom limb perceptions. J. Neurol. Neurosurg. Psychiatry 2019, 90, 833–836. [Google Scholar] [CrossRef]
- Strauss, I.; Valle, G.; Artoni, F.; D’Anna, E.; Granata, G.; Di Iorio, R.; Guiraud, D.; Stieglitz, T.; Rossini, P.M.; Raspopovic, S.; et al. Characterization of multi-channel intraneural stimulation in transradial amputees. Sci. Rep. 2019, 9, 19258. [Google Scholar] [CrossRef]
- Petrini, F.M.; Valle, G.; Strauss, I.; Granata, G.; Di Iorio, R.; D’Anna, E.; Čvančara, P.; Mueller, M.; Carpaneto, J.; Clemente, F.; et al. Six-Month Assessment of a Hand Prosthesis with Intraneural Tactile Feedback. Ann. Neurol. 2018, 85, 137–154. [Google Scholar] [CrossRef]
- Petrini, F.M.; Bumbasirevic, M.; Valle, G.; Ilic, V.; Mijović, P.; Čvančara, P.; Barberi, F.; Katic, N.; Bortolotti, D.; Andreu, D.; et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat. Med. 2019, 25, 1356–1363. [Google Scholar] [CrossRef] [Green Version]
- Petrini, F.M.; Valle, G.; Bumbasirevic, M.; Barberi, F.; Bortolotti, D.; Cvancara, P.; Hiairrassary, A.; Mijovic, P.; Sverrisson, A.Ö.; Pedrocchi, A.; et al. Enhancing functional abilities and cognitive integration of the lower limb prosthesis. Sci. Transl. Med. 2019, 11, eaav8939. [Google Scholar] [CrossRef]
- Dali, M.; William, L.; Tigra, W.; Taillades, H.; Rossel, O.; Coste, C.A.; Guiraud, D. Relevance of selective neural stimulation with a multicontact cuff electrode using multicriteria analysis. PLoS ONE 2019, 14, e0219079. [Google Scholar] [CrossRef] [PubMed]
- Tigra, W.; Dali, M.; William, L.; Fattal, C.; Gelis, A.; Divoux, J.L.; Coulet, B.; Teissier, J.; Guiraud, D.; Coste, C.A. Selective neural electrical stimulation restores hand and forearm movements in individuals with complete tetraplegia. J. Neuroeng. Rehabil. 2020, 17, 66–78. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Value |
|---|---|
| Type of stimulation | Constant current stimulation |
| Number of channels per stimulation unit | 14 active sites (capacitively coupled) 2 references (non coupled) |
| Number of distributed stimulation units (DSU) | 4 (56 active sites in total) |
| Weight Dimensions (W × L × H) | 150 g 81 × 130 × 21 mm3 |
| Pulse width | 2–508 µs (2 µs resolution) |
| Intensity | 10–2540 µA (10 µA resolution) |
| Frequency | 3 ranges: low/mid/high Low range: up to 8 channels per electrode (DSU) -> 4 to 58 Hz Mid range: up to 4 channels per electrode (DSU) -> 4 to 111 Hz High range: up to 2 channels per electrode (DSU) -> 4 to 200 Hz |
| Passive discharge | 150 µs minimum duration |
| Channel capacitive coupling | 330 nF |
| Output voltage | 19 V |
| Powering | USB or external battery |
| Autonomy (external battery) | 8 hours |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guiho, T.; López-Álvarez, V.M.; Čvančara, P.; Hiairrassary, A.; Andreu, D.; Stieglitz, T.; Navarro, X.; Guiraud, D. New Stimulation Device to Drive Multiple Transverse Intrafascicular Electrodes and Achieve Highly Selective and Rich Neural Responses. Sensors 2021, 21, 7219. https://doi.org/10.3390/s21217219
Guiho T, López-Álvarez VM, Čvančara P, Hiairrassary A, Andreu D, Stieglitz T, Navarro X, Guiraud D. New Stimulation Device to Drive Multiple Transverse Intrafascicular Electrodes and Achieve Highly Selective and Rich Neural Responses. Sensors. 2021; 21(21):7219. https://doi.org/10.3390/s21217219
Chicago/Turabian StyleGuiho, Thomas, Victor Manuel López-Álvarez, Paul Čvančara, Arthur Hiairrassary, David Andreu, Thomas Stieglitz, Xavier Navarro, and David Guiraud. 2021. "New Stimulation Device to Drive Multiple Transverse Intrafascicular Electrodes and Achieve Highly Selective and Rich Neural Responses" Sensors 21, no. 21: 7219. https://doi.org/10.3390/s21217219
APA StyleGuiho, T., López-Álvarez, V. M., Čvančara, P., Hiairrassary, A., Andreu, D., Stieglitz, T., Navarro, X., & Guiraud, D. (2021). New Stimulation Device to Drive Multiple Transverse Intrafascicular Electrodes and Achieve Highly Selective and Rich Neural Responses. Sensors, 21(21), 7219. https://doi.org/10.3390/s21217219







