Effects of Touchscreen Media Use on Toddlers’ Sleep: Insights from Longtime ECG Monitoring
Abstract
:1. Introduction
- To develop suitable methods to gain physiological indicators of sleep quality in young children from ECG data, which were recorded with wearable, undisturbing devices.
- These novel methods are applied in a highly topical context, that is, to study the impact of touchscreen media use on the quality of sleep in toddlers; thus, obtaining indication of their practical relevance.
2. Materials and Methods
2.1. Participants
2.2. Acquisition of ParentReported Data
2.2.1. Sleep Habits and Quality of Sleep
- Nighttime sleep (how many hours did your child usually sleep at night within the last 4 weeks?)
- Sleep onset latency (how long did it usually take for your child to fall asleep, in the evenings, on average, within the last 4 weeks?)
2.2.2. Toddlers’ Media Use
2.3. Acquisition and Processing of ECG Data
2.3.1. Quantification of Sleep Quality
- Duration of sleep according to the activity log: plausibility of this information was checked based on the activity data from the 3D acceleration sensor (position detection and variance of the signal) as well as the corresponding heart rate change.
- Validity and variance of the data from the 3D acceleration sensor: a 2-min segment is marked as representative of a “resting period” when the variances of the acceleration signals fall below an empirically determined threshold. Individual limits were determined based on the variance distributions of the sensors during the 32 h period of data collection, and evaluation of their sensitivity and specificity by using, e.g., entries in the activity log, such as “nap” or “afternoon nap”).
- Validity of ECG and EDR (ECG-derived respiration): a 2-min segment is marked as representative of a “resting period” when a valid determination of the respiration pattern in the ECG signal is possible for an empirically determined proportion of data (due to the given population of toddlers this threshold was set to two thirds).
- Validity of ECG and the quotient of heart rate variability (HRV; using the time domain parameters SDNN and RMSSD): the interaction of the two branches (sympathetic and parasympathetic) of the autonomic nervous system was used as an indicator for activity or rest. Individual limits were determined based on the variance distributions of the variable during the 32 h period of data collection, and evaluation of their sensitivity and specificity by using, e.g., entries in the activity log, such as “nap” or “afternoon nap”).
- These criteria for the determination of the 2-min “resting periods” must be fulfilled for at least 10 consecutive minutes, to fully meet the “rest” criterion.
- dHR sleep—average heart rate during restless sleep periods minus average heart rate during restful sleep periods (beats per minutes, bpm);
- Sleep duration—duration of sleep at night based on the ECG data (hours, see above, slightly corrected, if necessary, according to the activity log).
2.4. Study Design and Procedure
3. Results
3.1. Prediction of the Quality of Sleep
3.2. Relationships between Parent-Reported and Objective Data of Sleep Quality
4. Discussion
4.1. The Use of Wearable Biomedical Sensing Technology in Very Young Children
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
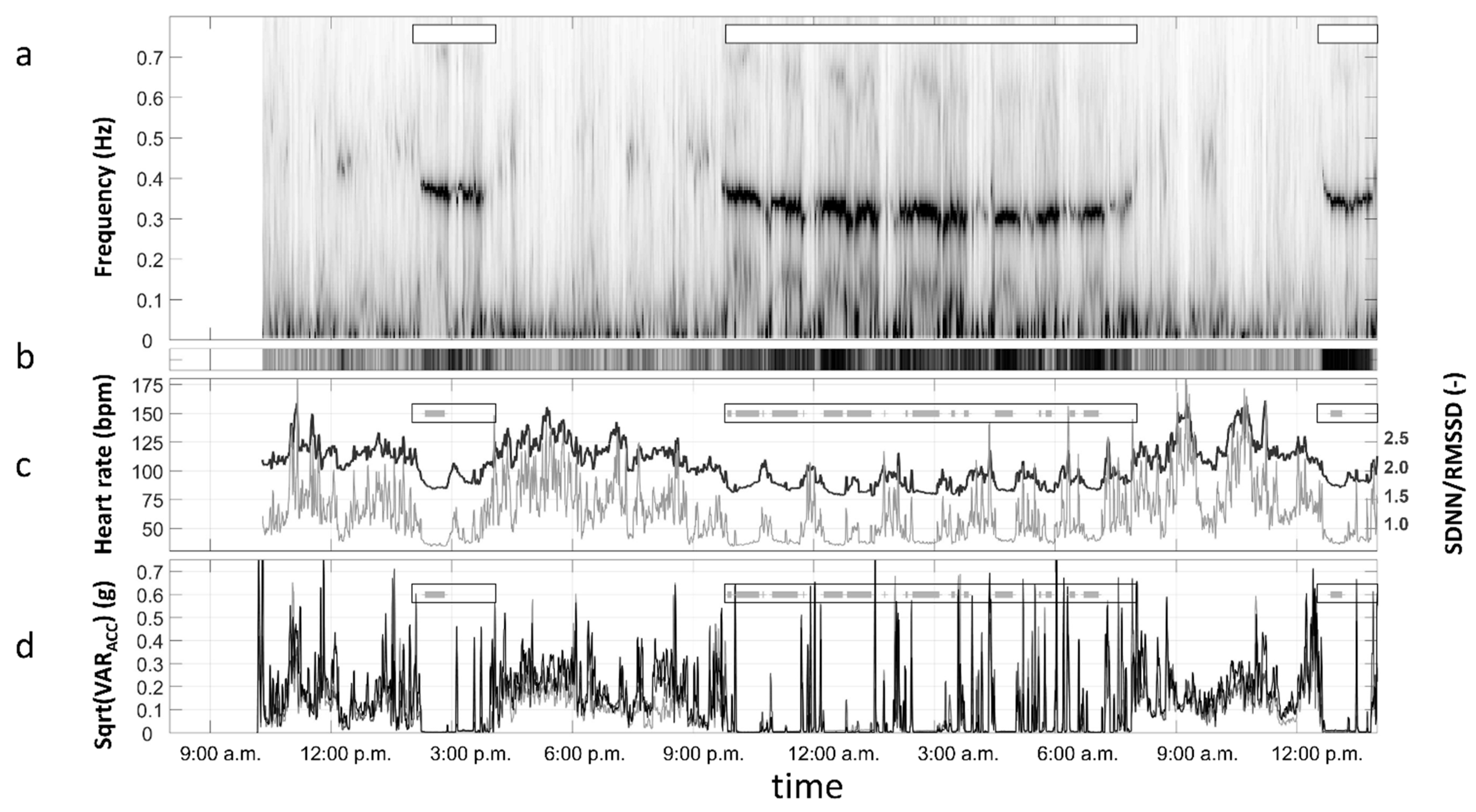
Data Preprocessing
References
- Izmailova, E.S.; Wagner, J.A.; Perakslis, E.D. Wearable devices in clinical trials: Hype and hypothesis. Clin. Pharmacol. Ther. 2018, 104, 42–52. [Google Scholar] [CrossRef]
- Friedrich, M.; Mölle, M.; Friederici, A.D.; Born, J. Sleep-dependent memory consolidation in infants protects new episodic memories from existing semantic memories. Nat. Commun. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gais, S.; Born, J. Declarative memory consolidation: Mechanisms acting during human sleep. Learn. Mem. 2004, 11, 679–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Herman, A.B.; West, G.B.; Poe, G.; Savage, V.M. Unraveling why we sleep: Quantitative analysis reveals abrupt transition from neural reorganization to repair in early development. Sci. Adv. 2020, 6, eaba0398. [Google Scholar] [CrossRef]
- Ednick, M.; Cohen, A.P.; McPhail, G.L.; Beebe, D.; Simakajornboon, N.; Amin, R.S. A review of the effects of sleep during the first year of life on cognitive, psychomotor, and temperament development. Sleep 2009, 32, 1449–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassiakos, Y.R.; Radesky, J.; Christakis, D.; Moreno, M.A.; Cross, C. Children and Adolescents and Digital Media. Pediatrics 2016, 138, e20162593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, R.; Kirkorian, H.; Radesky, J.; Coyne, S.; Nichols, D.; Blanchfield, O.; Rusnak, S.; Stockdale, L.; Ribner, A.; Durnzes, J.; et al. Beyond screen time: A synergistic approach to a more comprehensive assessment of family media exposure during early childhood. Front. Psychol. 2020, 11, 1283. [Google Scholar] [CrossRef]
- Livingstone, S.; Marsh, J.; Plowman, L.; Ottovordemgentschenfelde, S.; Fletcher-Watson, B. Young Children (0–8) and Digital Technology: A Qualitative Exploratory Study—National Report-UK; Joint Research Centre: Luxembourg, 2014. [Google Scholar]
- Cristia, A.; Seidl, A. Parental reports on touch screen use in early childhood. PLoS ONE 2015, 10, e0128338. [Google Scholar] [CrossRef]
- Marsh, J.; Plowman, L.; Yamada-Rice, D.; Bishop, J.C.; Lahmar, J.; Scott, F.; Davenport, A.; Davis, S.; French, K.; Piras, M.; et al. Exploring Play and Creativity in Pre-Schoolers’ Use of Apps: Final Project Report. Available online: http://www.techandplay.org/reports/TAP_Final_Report.pdf (accessed on 28 September 2021).
- Oswald, T.K.; Rumbold, A.R.; Kedzior, S.G.E.; Moore, V.M. Psychological impacts of “screen time” and “green time” for children and adolescents: A systematic scoping review. PLoS ONE 2020, 15, e0237725. [Google Scholar] [CrossRef]
- Garrison, M.M.; Liekweg, K.; Christakis, D.A. Media use and child sleep: The impact of content, timing, and environment. Pediatrics 2011, 128, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Kirkorian, H.L.; Wartella, E.A.; Anderson, D.R. Media and young children’s learning. Future Child. 2008, 18, 39–61. [Google Scholar] [CrossRef]
- Walter-Laager, C.; Brandenberg, K.; Tinguely, L.; Schwarz, J.; Pfiffner, M.R.; Moschner, B. Media-assisted language learning for young children: Effects of a word-learning app on the vocabulary acquisition of two-year-olds. Br. J. Educ. Technol. 2017, 48, 1062–1072. [Google Scholar] [CrossRef]
- Eichen, L.; Hackl-Wimmer, S.; Eglmaier, M.T.W.; Lackner, H.K.; Paechter, M.; Rettenbacher, K.; Rominger, C.; Walther-Laager, C. Families’ digital media use: Intentions, rules, and activities. Br. J. Educ. Technol. 2021, 52, 2162–2177. [Google Scholar] [CrossRef]
- Bellagamba, F.; Presaghi, F.; Di Marco, M.; D’Abundo, E.; Blanchfield, O.; Barr, R. How infant and toddlers’ media use is related to sleeping habits in everyday life in Italy. Front. Psychol. 2021, 12, 589664. [Google Scholar] [CrossRef]
- Cheung, C.H.M.; Bedford, R.; Saez De Urabain, I.R.; Karmiloff-Smith, A.; Smith, T.J. Daily touchscreen use in infants and toddlers is associated with reduced sleep and delayed sleep onset. Sci. Rep. 2017, 7, 46104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chindamo, S.; Buja, A.; DeBattisti, E.; Terraneo, A.; Marini, E.; Gomez Perez, L.J.; Marconi, L.; Baldo, V.; Chiamenti, G.; Doria, M.; et al. Sleep and new media usage in toddlers. Eur. J. Pediatr. 2019, 178, 483–490. [Google Scholar] [CrossRef]
- Ribner, A.D.; McHarg, G.G. Why won’t she sleep? Screen exposure and sleep patterns in young infants. Infant Behav. Dev. 2019, 57, 101334. [Google Scholar] [CrossRef]
- Mindell, J.A.; Williamson, A.A. Benefits of a bedtime routine in young children: Sleep, development, and beyond. Sleep Med. Rev. 2018, 40, 93–108. [Google Scholar] [CrossRef]
- DelRosso, L.M.; Picchietti, D.L.; Spruyt, K.; Bruni, O.; Garcia-Borreguero, D.; Kotagal, S.; Owens, J.A.; Simakajornboon, N.; Ferri, R. Restless sleep in children: A systematic review. Sleep Med. Rev. 2021, 56, 101406. [Google Scholar] [CrossRef]
- El-Sheikh, M.; Sadeh, A.I. Sleep and development: Introduction to the monograph. Monogr. Soc. Res. Child. Dev. 2015, 80, 1–14. [Google Scholar] [CrossRef]
- Jiang, F. Sleep and early brain development. Ann. Nutr. Metab. 2019, 75 (Suppl. S1), 44–53. [Google Scholar] [CrossRef] [PubMed]
- Jenni, O.G.; Molinari, L.; Caflisch, J.A.; Largo, R.H. Sleep duration from ages 1 to 10 years: Variability and stability in comparison with growth. Pediatrics 2007, 120, e769–e776. [Google Scholar] [CrossRef] [Green Version]
- Lampl, M.; Johnson, M.L. Infant growth in length follows prolonged sleep and increased naps. Sleep 2011, 34, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Nathanson, A.I.; Beyens, I. The role of sleep in the relation between young children’s mobile media use and effortful control. Br. J. Dev. Psychol. 2018, 36, 1–21. [Google Scholar] [CrossRef]
- Benita, N.; Gordon-Hacker, A.; Gueron-Sela, N. Sleep through toddlerhood: The distinct roles of overall media use and use of media to regulate child distress. J. Dev. Behav. Pediatr. 2020, 41, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Belenky, G.; Wesensten, N.J.; Thorne, D.R.; Thomas, M.L.; Sing, H.C.; Redmond, D.P.; Russo, M.B.; Balkin, T.J. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J. Sleep Res. 2003, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krystal, A.D.; Edinger, J.D. Measuring sleep quality. Sleep Med. 2008, 9 (Suppl. S1), 10–17. [Google Scholar] [CrossRef]
- Schoch, S.F.; Huber, R.; Kohler, M.; Kurth, S. Which are the central aspects of infant sleep? The dynamics of sleep composites across infancy. Sensors 2020, 20, 7188. [Google Scholar] [CrossRef]
- Sadeh, A., III. Sleep assessment methods. Monogr. Soc. Res. Child. Dev. 2015, 80, 33–48. [Google Scholar] [CrossRef]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, P.; Long, X.; Radha, M.; Haakma, R.; Aarts, R.M.; Rolink, J. Sleep stage classification with ECG and respiratory effort. Physiol. Meas. 2015, 36, 2027–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, G.; Lambertz, M.; Schulz, B.; Langhorst, P.; Krienke, B. Reticular formation of the lower brainstem. A common system for cardio-respiratory and somatomotor functions. Cross-correlation analysis of discharge patterns of neighbouring neurons. J. Auton. Nerv. Syst. 1985, 12, 35–62. [Google Scholar] [CrossRef]
- Markovich, A.N.; Gendron, M.A.; Corkum, P.V. Validating the Children’s Sleep Habit Questionnaire against polysomnography and actigraphy in school-aged children. Front. Psychiatry 2015, 5, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, P.M.; Komatireddy, R.; Haaser, S.; Topol, S.; Sheard, J.; Encinas, J.; Fought, A.J.; Topol, E.J. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am. J. Med. 2014, 127, 95.e11–95.e17. [Google Scholar] [CrossRef] [Green Version]
- Lackner, H.K.; Eglmaier, M.T.W.; Hackl-Wimmer, S.; Paechter, M.; Rominger, C.; Eichen, L.; Rettenbacher, K.; Walter-Laager, C.; Papousek, I. How to use heart rate variability: Quantification of vagal activity in toddlers and adults in long-term ECG. Sensors 2020, 20, 5959. [Google Scholar] [CrossRef]
- Hall, W.A.; Hutton, E.; Brant, R.F.; Collet, J.P.; Gregg, K.; Saunders, R.; Ipsiroglu, O.; Gafni, A.; Triolet, K.; Tse, L.; et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015, 15, 181. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, A.G.; Broyles, S.T.; Chaput, J.P.; Leduc, G.; Boyer, C.; Borghese, M.M.; Tremblay, M.S. Correlates of objectively measured sedentary time and self-reported screen time in Canadian children. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 38. [Google Scholar] [CrossRef] [Green Version]
- Lackner, H.K.; Weiss, E.M.; Schulter, G.; Hinghofer-Szalkay, H.; Samson, A.C.; Papousek, I. I got it! Transient cardiovascular response to the perception of humor. Biol. Psychol. 2013, 93, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Moody, G.B.; Mark, R.G.; Bump, M.A.; Weinstein, J.S.; Berman, A.D.; Mietus, J.E.; Goldberger, A.L. Clinical validation of the ECG-Derived Respiration (EDR) technique. Comput. Cardiol. 1986, 13, 507–510. [Google Scholar]
- Fonseca, P.; den Teuling, N.; Long, X.; Aarts, R.M. A comparison of probabilistic classifiers for sleep stage classification. Physiol. Meas. 2018, 39, 055001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; SAGE Publications: London, UK, 2009. [Google Scholar]
- Cain, N.; Gradisar, M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Med. 2010, 11, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.B.; Kuppens, P.; Sheeber, L.B. Heart rate responses to parental behavior in depressed adolescents. Biol. Psychol. 2012, 90, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Blascovich, J.; Seery, M.D.; Mugridge, C.A.; Norris, R.K.; Weisbuch, M. Predicting athletic performance from cardiovascular indexes of challenge and threat. J. Exp. Soc. Psychol. 2004, 40, 683–688. [Google Scholar] [CrossRef]
- Papousek, I.; Aydin, N.; Lackner, H.K.; Weiss, E.M.; Bühner, M.; Schulter, G.; Charlesworth, C.; Freudenthaler, H.H. Laughter as a social rejection cue: Gelotophobia and transient cardiac responses to other persons’ laughter and insult. Psychophysiology 2014, 51, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Schwerdtfeger, A. Predicting autonomic reactivity to public speaking: Don’t get fixed on self-report data. Int. J. Psychophysiol. 2004, 52, 217–224. [Google Scholar] [CrossRef]
- Thomas, N.; O’Kane, C. The ethics of participatory research with children. Child. Soc. 1998, 12, 336–348. [Google Scholar] [CrossRef]
- Afifi, A.; Clark, V.A.; Chapman, S.M. Computer-Aided Multivariate Analysis, 4th ed.; Chapman & Hall/CRC: New York, NY, USA, 2004. [Google Scholar]
- Kabali, H.K.; Irigoyen, M.M.; Nunez-Davis, R.; Budacki, J.G.; Mohanty, S.H.; Leister, K.P.; Bonner, R.L. Exposure and use of mobile media devices by young children. Pediatrics 2015, 136, 1044–1050. [Google Scholar] [CrossRef] [Green Version]
- Nygårds, M.E.; Sörnmo, L. Delineation of the QRS complex using the envelope of the e.c.g. Med. Biol. Eng. Comput. 1983, 21, 538–547. [Google Scholar] [CrossRef]
- Bashar, S.K.; Walkey, A.J.; McManus, D.D.; Chon, K.H. VERB: VFCDM-Based Electrocardiogram Reconstruction and Beat Detection Algorithm. IEEE Access 2019, 7, 13856–13866. [Google Scholar] [CrossRef] [PubMed]
- Lackner, H.K.; Weiss, E.M.; Hinghofer-Szalkay, H.; Papousek, I. Cardiovascular effects of acute positive emotional arousal. Appl. Psychophysiol. Biofeedback 2014, 39, 9–18. [Google Scholar] [CrossRef] [PubMed]

| n | M | SD | Min | Max | |
|---|---|---|---|---|---|
| Nighttime sleep (hours) | 53 | 10.21 | 1.06 | 7.50 | 12.50 |
| Sleep onset latency (min) | 54 | 24.31 | 14.44 | 5.00 | 60.00 |
| Sleep time (hours; according to activity log) | 55 | 10.20 | 1.05 | 7.97 | 12.97 |
| Amount of Time | Never | <1 h | >1 h | >2 h | >3 h | Missing |
|---|---|---|---|---|---|---|
| touchscreen media | 34.5 | 34.5 | 21.8 | 1.8 | 7.3 | |
| smartphone | 45.5 | 43.6 | 9.1 | 1.8 | ||
| tablet | 63.6 | 25.5 | 3.6 | 7.3 | ||
| PC/computer/laptop | 87.3 | 7.3 | 1.8 | 3.6 | ||
| TV | 23.6 | 50.9 | 12.7 | 3.6 | 3.6 | 5.5 |
| Variables Derived from ECG Measurements | n | M | SD | Min | Max |
|---|---|---|---|---|---|
| dHR sleep (bpm) | 55 | 7.479 | 3.21 | −0.32 | 15.12 |
| Sleep duration (hours) | 55 | 10.51 | 0.93 | 8.47 | 12.97 |
| Sleep Duration | dHR Sleep | |||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | β | p | B | SE | β | p | |
| Age | −0.013 | 0.028 | −0.068 | 0.655 | −0.246 | 0.088 | −0.378 | 0.007 |
| Gender | −0.379 | 0.288 | −0.199 | 0.195 | −0.397 | 0.901 | −0.059 | 0.661 |
| Touchscreen media use | 0.099 | 0.162 | 0.087 | 0.546 | 1.286 | 0.506 | 0.325 | 0.014 |
| Variables | 1. | 2. | 3. | 4. | 5. | 6. | 7. | |
|---|---|---|---|---|---|---|---|---|
| 1. | Age | - | ||||||
| 2. | Gender | −0.319 * | ||||||
| 3. | Touchscreen media use1 | −0.026 | 0.096 | |||||
| 4. | Nighttime sleep1 | −0.075 | 0.058 | −0.109 | ||||
| 5. | Sleep onset latency1 | 0.243 | 0.084 | 0.165 | −0.441 ** | |||
| 6. | Sleep time1 | 0.071 | −0.257 | 0.036 | 0.162 | −0.038 | ||
| 7. | Sleep duration2 | 0.014 | −0.196 | 0.070 | 0.152 | −0.048 | 0.867 ** | |
| 8. | dHR sleep2 | −0.357 ** | 0.102 | 0.329 * | −0.024 | −0.070 | −0.044 | 0.069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackl-Wimmer, S.; Eglmaier, M.T.W.; Eichen, L.; Rettenbacher, K.; Macher, D.; Walter-Laager, C.; Lackner, H.K.; Papousek, I.; Paechter, M. Effects of Touchscreen Media Use on Toddlers’ Sleep: Insights from Longtime ECG Monitoring. Sensors 2021, 21, 7515. https://doi.org/10.3390/s21227515
Hackl-Wimmer S, Eglmaier MTW, Eichen L, Rettenbacher K, Macher D, Walter-Laager C, Lackner HK, Papousek I, Paechter M. Effects of Touchscreen Media Use on Toddlers’ Sleep: Insights from Longtime ECG Monitoring. Sensors. 2021; 21(22):7515. https://doi.org/10.3390/s21227515
Chicago/Turabian StyleHackl-Wimmer, Sigrid, Marina Tanja Waltraud Eglmaier, Lars Eichen, Karoline Rettenbacher, Daniel Macher, Catherine Walter-Laager, Helmut Karl Lackner, Ilona Papousek, and Manuela Paechter. 2021. "Effects of Touchscreen Media Use on Toddlers’ Sleep: Insights from Longtime ECG Monitoring" Sensors 21, no. 22: 7515. https://doi.org/10.3390/s21227515
APA StyleHackl-Wimmer, S., Eglmaier, M. T. W., Eichen, L., Rettenbacher, K., Macher, D., Walter-Laager, C., Lackner, H. K., Papousek, I., & Paechter, M. (2021). Effects of Touchscreen Media Use on Toddlers’ Sleep: Insights from Longtime ECG Monitoring. Sensors, 21(22), 7515. https://doi.org/10.3390/s21227515







