Non-Contact Spirometry Using a Mobile Thermal Camera and AI Regression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermal Image Acquisition System
2.2. Respiration Rate Measurement
2.3. Spirometry Measurements
2.3.1. Subject
2.3.2. Regression Models
- −
- Decision Trees Regressor
- −
- Random Forest Regressor
- −
- Gradient and Adaptive Boosting Regressors
2.3.3. Performance Measures
3. Results
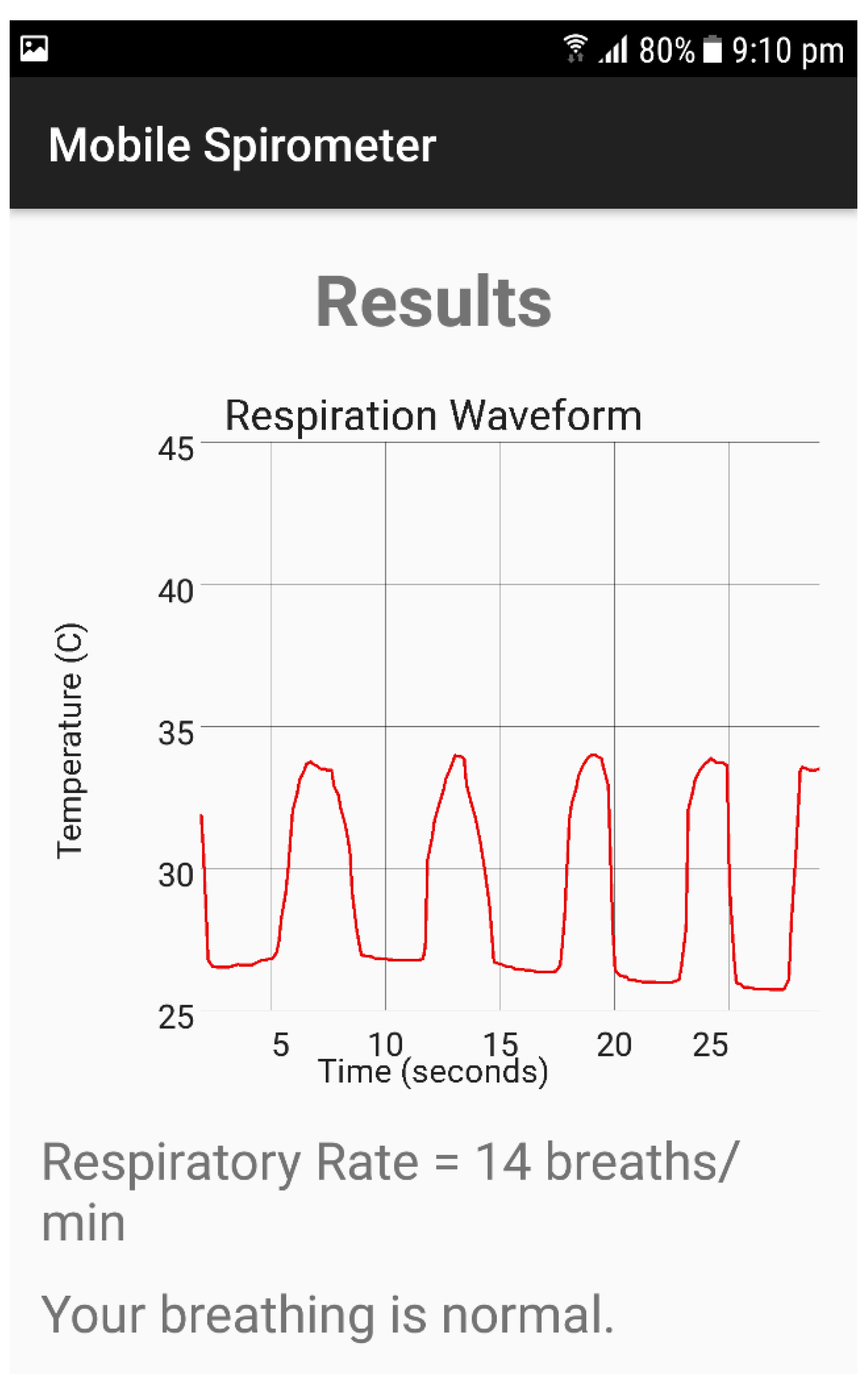
3.1. Respiration Signal and Respiration Rate Measurements
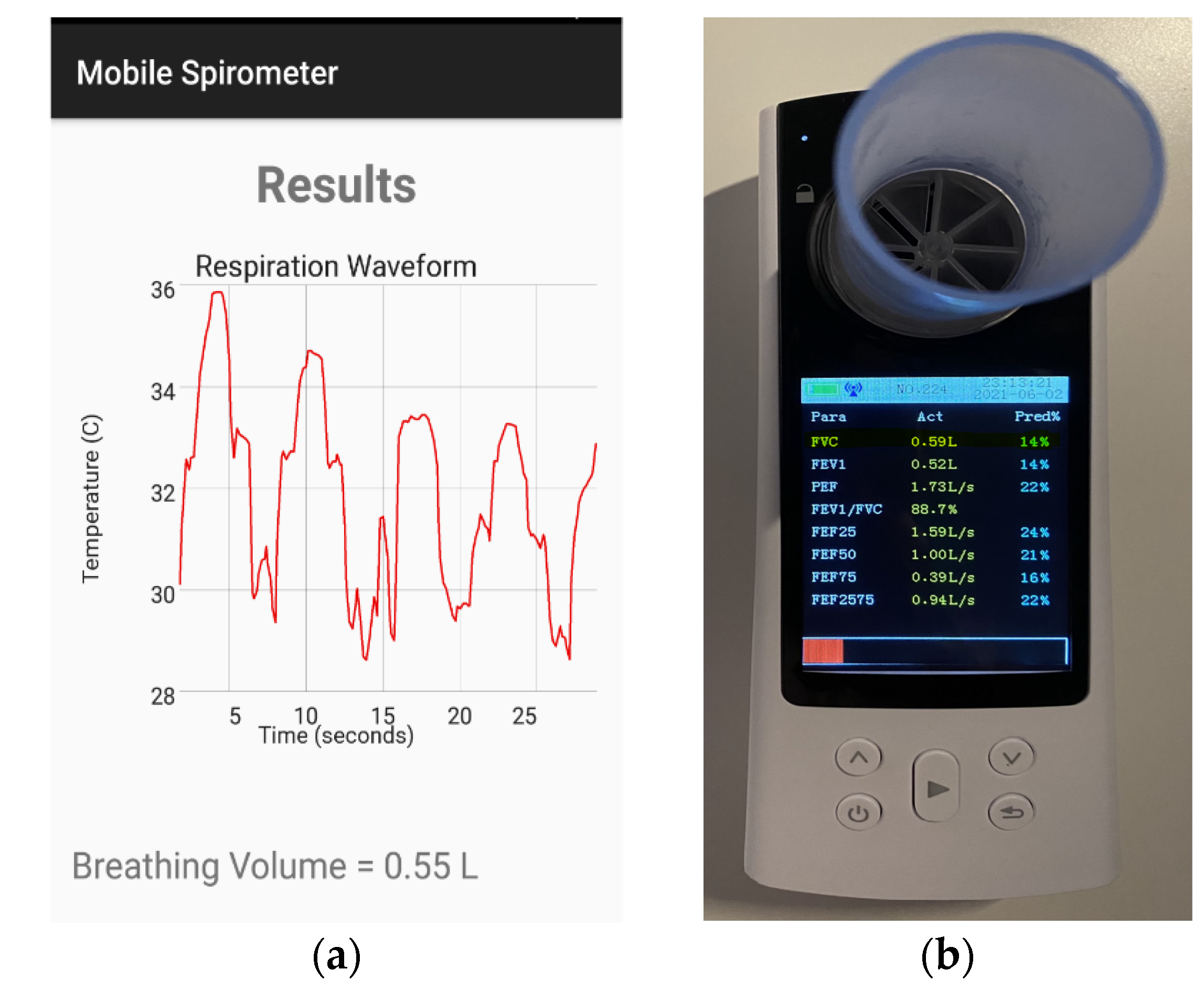
3.2. Spirometry Measurement System
3.3. Spirometry System Testing
4. Discussion
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Athanazio, R. Airway disease: Similarities and differences between asthma, COPD and bronchiectasis. Clinics 2012, 67, 1335–1343. [Google Scholar] [CrossRef]
- Ponce, M.C.; Sharma, S. Pulmonary Function Tests; Springer: Berlin/Heidelberg, Germany, 2021; pp. 11–20. [Google Scholar]
- Mannino, D.M.; Buist, A.S.; Vollmer, W.M. Chronic obstructive pulmonary disease in the older adult: What defines abnormal lung function? Thorax 2007, 62, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güder, G.; Brenner, S.; Angermann, C.E.; Ertl, G.; Held, M.; Sachs, A.P.; Lammers, J.W.; Zanen, P.; Hoes, A.W.; Störk, S.; et al. GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study. Respir. Res. 2012, 13, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, E.T.; Guppy, M.; Straus, S.E.; Bell, K.J.L.; Glasziou, P. Rate of normal lung function decline in ageing adults: A systematic review of prospective cohort studies. BMJ Open 2019, 9, e028150. [Google Scholar] [CrossRef]
- Droitcour, A.D.; Boric-Lubecke, O.; Kovacs, G.T.A. Signal-to-Noise Ratio in Doppler Radar System for Heart and Respiratory Rate Measurements. IEEE Trans. Microw. Theory Tech. 2009, 57, 2498–2507. [Google Scholar] [CrossRef]
- Shao, D.; Yang, Y.; Liu, C.; Tsow, F.; Yu, H.; Tao, N. Noncontact Monitoring Breathing Pattern, Exhalation Flow Rate and Pulse Transit Time. IEEE Trans. Biomed. Eng. 2014, 61, 2760–2767. [Google Scholar] [CrossRef]
- Madhav, K.V.; Ram, M.R.; Krishna, E.H.; Komalla, N.R.; Reddy, K.A. Robust Extraction of Respiratory Activity from PPG Signals Using Modified MSPCA. IEEE Trans. Instrum. Meas. 2013, 62, 1094–1106. [Google Scholar] [CrossRef]
- Mostov, K.; Liptsen, E.; Boutchko, R. Medical applications of shortwave FM radar: Remote monitoring of cardiac and respiratory motion. Med. Phys. 2010, 37, 1332–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.; Chen, D.; Tsai, W. Image-Based Motion-Tolerant Remote Respiratory Rate Evaluation. IEEE Sens. J. 2016, 16, 3263–3271. [Google Scholar] [CrossRef]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef] [Green Version]
- Al-Naji, A.; Al-Askery, A.J.; Gharghan, S.K.; Chahl, J. A system for monitoring breathing activity using an ultrasonic radar detection with low power consumption. J. Sens. Actuator Netw. 2019, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.P.; Young, M.S.; Tai, C.C. Noninvasive respiratory monitoring system based on the piezoceramic transducer’s pyroelectric effect. Rev. Sci. Instrum. 2008, 79, 35103. [Google Scholar] [CrossRef]
- Murthy, R.; Pavlidis, I. Noncontact measurement of breathing function. IEEE Eng. Med. Biol. Mag. 2006, 25, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Murthy, J.N.; Van Jaarsveld, J.; Fei, J.; Pavlidis, I.; Harrykissoon, R.I.; Lucke, J.F.; Faiz, S.; Castriotta, R.J. Thermal Infrared Imaging: A Novel Method to Monitor Airflow During Polysomnography. Sleep 2009, 32, 1521–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, G.B.; Duffy, N.D. A video-based optical system for rapid measurements of chest wall movement. Physiol. Meas. 2001, 22, 489–503. [Google Scholar] [CrossRef]
- Chon, K.H.; Dash, S.; Ju, K. Estimation of Respiratory Rate From Photoplethysmogram Data Using Time–Frequency Spectral Estimation. IEEE Trans. Biomed. Eng. 2009, 56, 2054–2063. [Google Scholar] [CrossRef]
- Teledyne FLIR. Available online: https://www.flir.com/products/flir-one-pro/ (accessed on 25 October 2021).
- Contec Medical. Available online: https://contecmed.eu/products/sp80b-bluetooth-digital-spirometer-lung-function-breathing-pulmonary-diagnostic?_pos=1&_sid=28dfd9d6c&_ss=r (accessed on 24 October 2021).
- Breiman, L.; Friedman, J.; Stone, C.J.; Olshen, R.A. Classification and Regression Trees; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Hastie, J.F.T. Robert Tibshirani. In The Elements of Statistical Learning; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Geurts, P.; Ernst, D.; Wehenkel, L. Extremely randomized trees. Mach. Learn. 2006, 63, 3–42. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.H. Greedy Function Approximation: A Gradient Boosting Machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Devore, J.L. Probability and Statistics for Engineering and the Sciences, 8th ed.; Cengage Learning: Boston, MA, USA, 2011. [Google Scholar]
- Scikit-Learn, Machine learning in Python. Available online: https://scikit-learn.org/stable/ (accessed on 25 October 2021).
- Fraiwan, L.; AlKhodari, M.; Ninan, J.; Mustafa, B.; Saleh, A.; Ghazal, M. Diabetic foot ulcer mobile detection system using smart phone thermal camera: A feasibility study. Biomed. Eng. Online 2017, 16, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Naji, A.A.; Chahl, J. Detection of Cardiopulmonary Activity and Related Abnormal Events Using Microsoft Kinect Sensor. Sensors 2018, 18, 920. [Google Scholar] [CrossRef] [Green Version]
- Schoun, B.; Transue, S.; Halbower, A.C.; Choi, M.-H. Non-contact tidal volume measurement through thin medium thermal imaging. Smart Health 2018, 9–10, 37–49. [Google Scholar] [CrossRef]
- L-Khalidi, F.Q.A.; Saatchi, R.; Burke, D.; Elphick, H. Facial tracking method for noncontact respiration rate monitoring. In Proceedings of the 2010 7th International Symposium on Communication Systems, Networks Digital Signal Processing (CSNDSP 2010), Newcastle Upon Tyne, UK, 21–23 July 2010; pp. 751–754. [Google Scholar] [CrossRef]
- Brieva, J.; Ponce, H. A Contactless Respiratory Rate Estimation Method Using a Hermite Magnification Technique and Convolutional Neural Networks. Appl. Sci. 2020, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, C.; Yang, Y.; Tsow, F.; Shao, D.; Tao, N. Noncontact spirometry with a webcam. J. Biomed. Opt. 2021, 22, 57002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, C.B.; Yu, X.; Czaplik, M.; Blazek, V.; Leonhardt, S. Remote monitoring of breathing dynamics using infrared thermography. Biomed. Opt. Express 2015, 6, 1373–1384. [Google Scholar] [CrossRef] [PubMed]











| Performance Measure/ Regression Method | Random Forest | Gradient Boosting | Adaptive Boosting | Decision Trees |
|---|---|---|---|---|
| R-square | 0.9409 | 0.9948 | 0.9558 | 0.9998 |
| Mean absolute error | 0.1886 | 0.0532 | 0.1724 | 0.0023 |
| Mean squared error | 0.0670 | 0.0049 | 0.0423 | 0.0002 |
| Root mean squared error | 0.2484 | 0.0687 | 0.2054 | 0.0088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraiwan, L.; Khasawneh, N.; Lweesy, K.; Elbalki, M.; Almarzooqi, A.; Abu Hamra, N. Non-Contact Spirometry Using a Mobile Thermal Camera and AI Regression. Sensors 2021, 21, 7574. https://doi.org/10.3390/s21227574
Fraiwan L, Khasawneh N, Lweesy K, Elbalki M, Almarzooqi A, Abu Hamra N. Non-Contact Spirometry Using a Mobile Thermal Camera and AI Regression. Sensors. 2021; 21(22):7574. https://doi.org/10.3390/s21227574
Chicago/Turabian StyleFraiwan, Luay, Natheer Khasawneh, Khaldon Lweesy, Mennatalla Elbalki, Amna Almarzooqi, and Nada Abu Hamra. 2021. "Non-Contact Spirometry Using a Mobile Thermal Camera and AI Regression" Sensors 21, no. 22: 7574. https://doi.org/10.3390/s21227574





