Quasi-Simultaneous Sensitive Detection of Two Gas Species by Cavity-Ringdown Spectroscopy with Two Lasers
Abstract
:1. Introduction
2. Experimental System
3. Methods
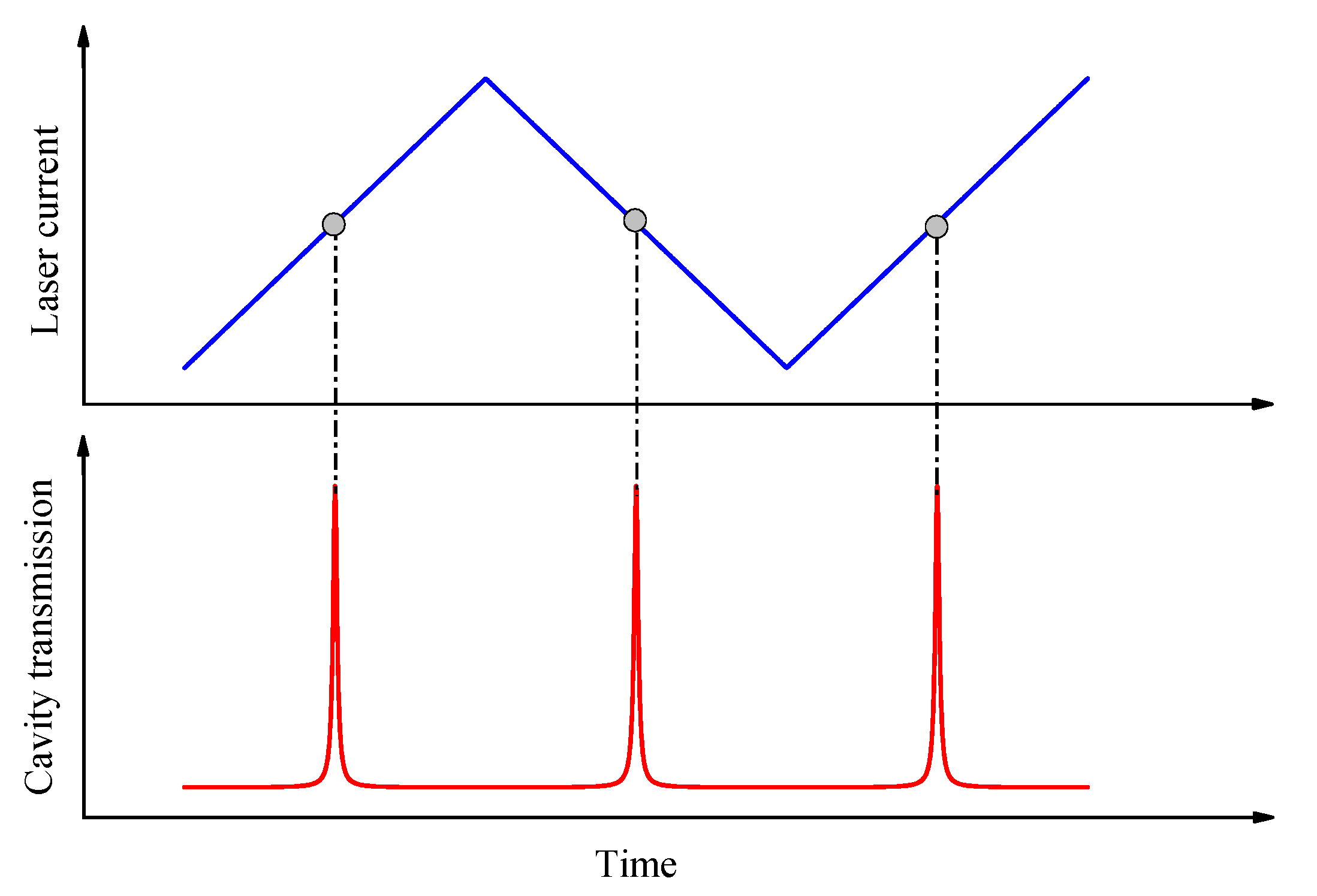
3.1. Step-Scanning Laser Frequency for Achieving Cavity Optical Resonances
3.2. Simultaneous Multi-Wavelength Operation with Two or More Lasers
4. Results and Discussion
4.1. Measurements of the Cavity’s FSR Serve as a Frequency Scale of Spectral Scans
4.2. Instrument Sensitivity
4.3. Large Range of Absorption Measurements
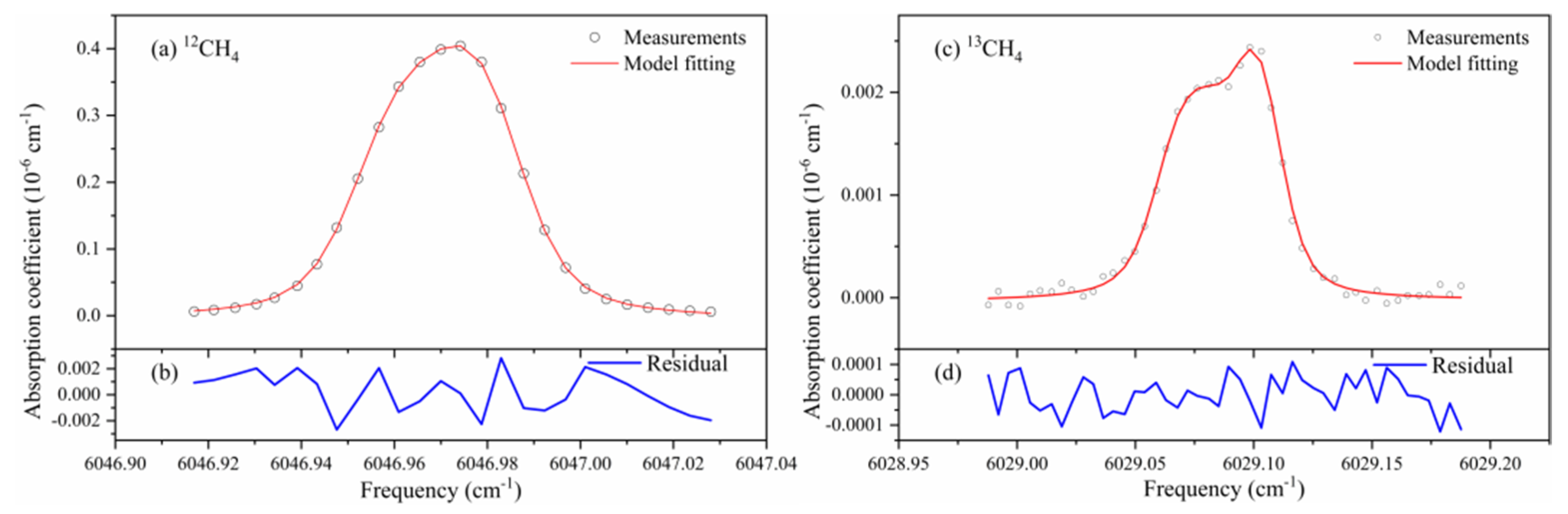
4.4. CH4 Isotope Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yung, Y.L.; Chen, P.; Nealson, K.; Atreya, S.; Beckett, P.; Blank, J.G.; Ehlmann, B.; Eiler, J.; Etiope, G.; Ferry, J.G.; et al. Methane on Mars and habitability: Challenges and responses. Astrobiology 2018, 18, 1221–1242. [Google Scholar] [CrossRef] [PubMed]
- Wuebbles, D.J.; Hayhoe, K. Atmospheric methane and global change. Earth-Sci. Rev. 2002, 57, 177–210. [Google Scholar] [CrossRef]
- MacDonald, G.J. The future of methane as an energy resource. Annu. Rev. Energy 1990, 15, 53–83. [Google Scholar] [CrossRef]
- Ferry, J.G. Enzymology of one-carbon metabolism in methanogenic pathways. FEMS Microbiol. Rev. 1999, 23, 13–38. [Google Scholar] [CrossRef]
- Stolper, D.A.; Martini, A.M.; Clog, M.; Douglas, P.M.; Shusta, S.S.; Valentine, D.L.; Sessions, A.L.; Eiler, J.M. Distinguishing and understanding thermogenic and biogenic sources of methane using multiply substituted isotopologues. Geochim. Et Cosmochim. Acta 2015, 161, 219–247. [Google Scholar] [CrossRef] [Green Version]
- Yarnes, C. δ13C and δ2H measurement of methane from ecological and geological sources by gas chromatography/ combustion/ pyrolysis isotope-ratio mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1036–1044. [Google Scholar] [CrossRef]
- Brass, M.; Röckmann, T. Continuous-flow isotope ratio mass spectrometry method for carbon and hydrogen isotope measurements on atmospheric methane. Atmos. Meas. Tech. 2010, 3, 1707–1721. [Google Scholar] [CrossRef] [Green Version]
- Sigrist, M.W.; Bartlome, R.; Marinov, D.; Rey, J.M.; Vogler, D.E.; Wächter, H. Trace gas monitoring with infrared laser-based detection schemes. Appl. Phys. B 2008, 90, 289–300. [Google Scholar] [CrossRef]
- Dong, L.; Tittel, F.K.; Li, C.; Sanchez, N.P.; Wu, H.; Zheng, C.; Yu, Y.; Sampaolo, A.; Griffin, R.J. Compact TDLAS based sensor design using interband cascade lasers for mid-IR trace gas sensing. Opt. Express 2016, 24, A528–A535. [Google Scholar] [CrossRef]
- He, Y.; Orr, B.J. Ringdown and cavity-enhanced absorption spectroscopy using a continuous-wave tunable diode laser and a rapidly swept optical cavity. Chem. Phys. Lett. 2000, 319, 131–137. [Google Scholar] [CrossRef]
- Orr, B.J.; He, Y. Rapidly swept continuous-wave cavity-ringdown spectroscopy. Chem. Phys. Lett. 2011, 512, 1–20. [Google Scholar] [CrossRef]
- Bergamaschi, P.; Schupp, M.; Harris, G.W. High-precision direct measurements of 13CH4/12CH4 and 12CH3D/12CH4 ratios in atmospheric methane sources by means of a long-path tunable diode laser absorption spectrometer. Appl. Opt. 1994, 33, 7704–7716. [Google Scholar] [CrossRef] [Green Version]
- Durry, G.; Li, J.S.; Vinogradov, I.; Titov, A.; Joly, L.; Cousin, J.; Decarpenterie, T.; Amarouche, N.; Liu, X.; Parvitte, B.; et al. Near infrared diode laser spectroscopy of C2H2, H2O, CO2 and their isotopologues and the application to TDLAS, a tunable diode laser spectrometer for the martian PHOBOS-GRUNT space mission. Appl. Phys. B 2010, 99, 339–351. [Google Scholar] [CrossRef]
- Deng, H.; Yang, C.; Wang, W.; Shan, C.; Xu, Z.; Chen, B.; Yao, L.; Hu, M.; Kan, R.; He, Y. Near infrared heterodyne radiometer for continuous measurements of atmospheric CO2 column concentration. Infrared Phys. Technol. 2019, 101, 39–44. [Google Scholar] [CrossRef]
- Li, X.; Yuan, F.; Hu, M.; Chen, B.; He, Y.; Yang, C.; Shi, L.; Kan, R. Compact open-path sensor for fast measurements of CO2 and H2O using scanned-wavelength modulation spectroscopy with 1f-phase method. Sensors 2020, 20, 1910. [Google Scholar] [CrossRef] [Green Version]
- Berden, G.; Richard, E. (Eds.) Cavity Ring-Down Spectroscopy: Techniques and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Chen, H.; Winderlich, J.; Gerbig, C.; Hoefer, A.; Rella, C.W.; Crosson, E.R.; Van Pelt, A.D.; Steinbach, J.; Kolle, O.; Beck, V.; et al. High-accuracy continuous airborne measurements of greenhouse gases (CO2 and CH4) using the cavity ring-down spectroscopy (CRDS) technique. Atmos. Meas. Tech. 2010, 3, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Hu, M.; He, Y.; Chen, B.; Yao, L.; Xu, Z.; Kan, R. Development of an in situ analysis system for methane dissolved in seawater based on cavity ringdown spectroscopy. Rev. Sci. Instrum. 2020, 91, 083106. [Google Scholar] [CrossRef] [PubMed]
- Yver Kwok, C.; Laurent, O.; Guemri, A.; Philippon, C.; Wastine, B.; Rella, C.W.; Vuillemin, C.; Truong, F.; Delmotte, M.; Kazan, V.; et al. Comprehensive laboratory and field testing of cavity ring-down spectroscopy analyzers measuring H2O, CO2, CH4 and CO. Atmos. Meas. Tech. 2015, 8, 3867–3892. [Google Scholar] [CrossRef] [Green Version]
- Butler, T.J.A.; Miller, J.L.; Orr-Ewing, A.J. Cavity ring-down spectroscopy measurements of single aerosol particle extinction. I. The effect of position of a particle within the laser beam on extinction. J. Chem. Phys. 2007, 126, 174302. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.J.A.; Mellon, D.; Kim, J.; Litman, J.; Orr-Ewing Andrew, J. Optical-feedback cavity ring-down spectroscopy measurements of extinction by aerosol particles. J. Phys. Chem. A 2009, 113, 3963–3972. [Google Scholar] [CrossRef]
- Guo, R.; Teng, J.; Cao, K.; Dong, H.; Cui, W.; Zhang, T. Comb-assisted, Pound-Drever-Hall locked cavity ring-down spectrometer for high-performance retrieval of transition parameters. Opt. Express 2019, 27, 31850–31863. [Google Scholar] [CrossRef]
- Long, D.A.; Wojtewicz, S.; Miller, C.E.; Hodges, J.T. Frequency-agile, rapid scanning cavity ring-down spectroscopy (FARS-CRDS) measurements of the (30012)←(00001) near-infrared carbon dioxide band. J. Quant. Spectrosc. Radiat. Transf. 2015, 161, 35–40. [Google Scholar] [CrossRef]
- Leshchishina, O.; Kassi, S.; Gordon, I.E.; Rothman, L.S.; Wang, L.; Campargue, A. High sensitivity CRDS of the a1Δg− X3Σg− band of oxygen near 1.27 μm: Extended observations, quadrupole transitions, hot bands and minor isotopologues. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 2236–2245. [Google Scholar] [CrossRef] [Green Version]
- Crosson, E.R.; Ricci, K.N.; Richman, B.A.; Chilese, F.C.; Owano, T.G.; Provencal, R.A.; Todd, M.W.; Glasser, J.; Kachanov, A.A.; Paldus, B.A.; et al. Stable isotope ratios using cavity ring-down spectroscopy: Determination of 13C/12C for carbon dioxide in human breath. Anal. Chem. 2002, 74, 2003–2007. [Google Scholar] [CrossRef]
- Sahay, P.; Scherrer, S.T.; Wang, C. Measurements of the weak UV absorptions of isoprene and acetone at 261–275 nm using cavity ringdown spectroscopy for evaluation of a potential portable ringdown breath analyzer. Sensors 2013, 13, 8170–8187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritsch, T.; Hering, P.; Mürtz, M. Infrared laser spectroscopy for online recording of exhaled carbon monoxide—A progress report. J. Breath Res. 2007, 1, 014002. [Google Scholar] [CrossRef]
- Dahnke, H.; Kleine, D.; Urban, W.; Hering, P.; Mürtz, M. Isotopic ratio measurement of methane in ambient air using mid-infrared cavity leak-out spectroscopy. Appl. Phys. B 2001, 72, 121–125. [Google Scholar] [CrossRef]
- Maity, A.; Pal, M.; Banik, G.D.; Maithani, S.; Pradhan, M. Cavity ring-down spectroscopy using an EC-QCL operating at 7.5 µm for direct monitoring of methane isotopes in air. Laser Phys. Lett. 2017, 14, 115701. [Google Scholar] [CrossRef]
- Chen, Y.; Lehmann, K.K.; Kessler, J.; Sherwood Lollar, B.; Lacrampe Couloume, G.; Onstott, T.C. Measurement of the 13C/12C of atmospheric CH4 using near-infrared (NIR) cavity ring-down spectroscopy. Anal. Chem. 2013, 85, 11250–11257. [Google Scholar] [CrossRef]
- Rella, C.W.; Hoffnagle, J.; He, Y.; Tajima, S. Local-and regional-scale measurements of CH4, δ13CH4, and C2H6 in the Uintah Basin using a mobile stable isotope analyzer. Atmos. Meas. Tech. 2015, 8, 4539–4559. [Google Scholar] [CrossRef] [Green Version]
- Maher, D.T.; Santos, I.R.; Leuven, J.R.F.W.; Oakes, J.M.; Erler, D.V.; Carvalho, M.C.; Eyre, B.D. Novel use of cavity ring-down spectroscopy to investigate aquatic carbon cycling from microbial to ecosystem scales. Environ. Sci. Technol. 2013, 47, 12938–12945. [Google Scholar] [CrossRef]
- Schulz, K.J.; Simpson, W.R. Frequency-matched cavity ring-down spectroscopy. Chem. Phys. Lett. 1998, 297, 523–529. [Google Scholar] [CrossRef]
- He, Y.; Orr, B.J. Rapidly swept, continuous-wave cavity ringdown spectroscopy with optical heterodyne detection: Single- and multi-wavelength sensing of gases. Appl. Phys. B 2002, 75, 267–280. [Google Scholar] [CrossRef]
- Romanini, D.; Kachanov, A.A.; Sadeghi, N.; Stoeckel, F. CW cavity ring down spectroscopy. Chem. Phys. Lett. 1997, 264, 316–322. [Google Scholar] [CrossRef]
- Kassi, S.; Campargue, A. Cavity ring down spectroscopy with 5 × 10−13 cm−1 sensitivity. J. Chem. Phys. 2012, 137, 234201. [Google Scholar] [CrossRef]
- Paldus, B.A.; Kachanov, A.A. An historical overview of cavity-enhanced methods. Can. J. Phys. 2005, 83, 975–999. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.R.; Zhou, Z.Y.; Chen, J.; Liu, A.W.; Hu, S.M. Ultrasensitive, self-calibrated cavity ring-down spectrometer for quantitative trace gas analysis. Appl. Opt. 2014, 53, 7716–7723. [Google Scholar] [CrossRef]
- He, Y.; Kan, R.; Englich, F.V.; Liu, W.; Orr, B.J. Simultaneous multi-laser, multi-species trace-level sensing of gas mixtures by rapidly swept continuous-wave cavity-ringdown spectroscopy. Opt. Express 2010, 18, 20059–20071. [Google Scholar] [CrossRef]
- Moyer, E.J.; Sayres, D.S.; Engel, G.S.; St. Clair, J.M.; Keutsch, F.N.; Allen, N.T.; Kroll, J.H.; Anderson, J.G. Design considerations in high-sensitivity off-axis integrated cavity output spectroscopy. Appl. Phys. B 2008, 92, 467–474. [Google Scholar] [CrossRef]
- Huang, H.; Lehmann, K.K. Long-term stability in continuous wave cavity ringdown spectroscopy experiments. Appl. Opt. 2010, 49, 1378–1387. [Google Scholar] [CrossRef] [Green Version]
- Werle, P.O.; Mücke, R.; Slemr, F. The limits of signal averaging in atmospheric trace-gas monitoring by tunable diode-laser absorption spectroscopy (TDLAS). Appl. Phys. B 1993, 57, 131–139. [Google Scholar] [CrossRef]
- Russo, S.D.; Zifarelli, A.; Patimisco, P.; Sampaolo, A.; Wei, T.; Wu, H.; Dong, L.; Spagnolo, V. Light-induced thermo-elastic effect in quartz tuning forks exploited as a photodetector in gas absorption spectroscopy. Opt. Express 2020, 28, 19074–19084. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Ma, Y.; He, Y.; Patimisco, P.; Sampaolo, A.; Spagnolo, V. Ppt level carbon monoxide detection based on light-induced thermoelastic spectroscopy exploring custom quartz tuning forks and a mid-infrared QCL. Opt. Express 2021, 29, 25100–25108. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; He, Y.; Chen, B.; Deng, H.; Liu, Y.; Wang, X.; Zhao, Z.; Kan, R. Quasi-Simultaneous Sensitive Detection of Two Gas Species by Cavity-Ringdown Spectroscopy with Two Lasers. Sensors 2021, 21, 7622. https://doi.org/10.3390/s21227622
Ma G, He Y, Chen B, Deng H, Liu Y, Wang X, Zhao Z, Kan R. Quasi-Simultaneous Sensitive Detection of Two Gas Species by Cavity-Ringdown Spectroscopy with Two Lasers. Sensors. 2021; 21(22):7622. https://doi.org/10.3390/s21227622
Chicago/Turabian StyleMa, Guosheng, Yabai He, Bing Chen, Hao Deng, Ying Liu, Xingping Wang, Zhihao Zhao, and Ruifeng Kan. 2021. "Quasi-Simultaneous Sensitive Detection of Two Gas Species by Cavity-Ringdown Spectroscopy with Two Lasers" Sensors 21, no. 22: 7622. https://doi.org/10.3390/s21227622
APA StyleMa, G., He, Y., Chen, B., Deng, H., Liu, Y., Wang, X., Zhao, Z., & Kan, R. (2021). Quasi-Simultaneous Sensitive Detection of Two Gas Species by Cavity-Ringdown Spectroscopy with Two Lasers. Sensors, 21(22), 7622. https://doi.org/10.3390/s21227622




