Feasibility and First Results of Heart Failure Monitoring Using the Wearable Cardioverter–Defibrillator in Newly Diagnosed Heart Failure with Reduced Ejection Fraction
Abstract
:1. Introduction
2. Methods
2.1. WCD Data
2.2. Statistics
3. Results
3.1. Patient Characteristics
3.2. WCD-Use
3.3. Heart Failure Parameters at Baseline and after Three Months
3.4. Early Predictors of LVEF Improvement
4. Discussion
- Patients with newly diagnosed HFrEF show a decrease in heart rate, as well as an increase in heart rate variability approximate and step count during the first three months of heart failure treatment.
- A higher delta of heart rate or step count between the first and last seven days of usage correlates with a higher delta of the heart rate variability approximate.
- A delta of heart rate variability approximately >23 ms within the first 45 days of WCD wear time was an independent predictor of LVEF improvement.
4.1. Heart Rate
4.2. Heart Rate Variability
4.3. Step Count
4.4. Predictors of LVEF Improvement and Clinical Application
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duncker, D.; Haghikia, A.; König, T.; Hohmann, S.; Gutleben, K.-J.; Westenfeld, R.; Oswald, H.; Klein, H.; Bauersachs, J.; Hilfiker-Kleiner, D.; et al. Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function-value of the wearable cardioverter/defibrillator. Eur. J. Heart Fail. 2014, 16, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.; König, T.; Hohmann, S.; Bauersachs, J.; Veltmann, C. Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: Insights from the PROLONG study. Clin. Cardiol. 2017, 40, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Wranicz, J.; Malik, R.; Morin, D.P.; Zweibel, S.; Buxton, A.E.; Elayi, C.S.; Chung, E.H.; et al. Wearable Cardioverter–Defibrillator after Myocardial Infarction. N. Engl. J. Med. 2018, 379, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deneke, T.; Bosch, R.; Eckardt, L.; Nowak, B.; Schwab, J.O.; Sommer, P.; Veltmann, C.; Helms, T.M. Der tragbare Kardioverter/Defibrillator (WCD)—Indikationen und Einsatz. Der Kardiol. 2019, 13, 292–304. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2018, 138, e91–e220. [Google Scholar]
- Duncker, D.; Westenfeld, R.; Konrad, T.; Pfeffer, T.; Correia de Freitas, C.A.; Pfister, R.; Thomas, D.; Fürnkranz, A.; Andrié, R.P.; Napp, A.; et al. Risk for life-threatening arrhythmia in newly diagnosed peripartum cardiomyopathy with low ejection fraction: A German multi-centre analysis. Clin. Res. Cardiol. 2017, 106, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Epstein, A.E.; Abraham, W.T.; Bianco, N.R.; Kern, K.B.; Mirro, M.; Rao, S.V.; Rhee, E.K.; Solomon, S.D.; Szymkiewicz, S.J. Wearable Cardioverter-Defibrillator Use in Patients Perceived to Be at High Risk Early Post-Myocardial Infarction. J. Am. Coll. Cardiol. 2013, 62, 2000–2007. [Google Scholar] [CrossRef] [Green Version]
- Zishiri, E.T.; Williams, S.; Cronin, E.M.; Blackstone, E.H.; Ellis, S.G.; Roselli, E.E.; Smedira, N.G.; Gillinov, A.M.; Glad, J.A.; Tchou, P.J.; et al. Early Risk of Mortality after Coronary Artery Revascularization in Patients with Left Ventricular Dysfunction and Potential Role of the Wearable Cardioverter Defibrillator. Circ. Arrhythmia Electrophysiol. 2013, 6, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.; Goldenberg, I.; Moss, A.J.; Klein, H.; Huang, D.T.; Bianco, N.R.; Szymkiewicz, S.J.; Zareba, W.; Brenyo, A.; Buber, J.; et al. Wearable Defibrillator in Congenital Structural Heart Disease and Inherited Arrhythmias. Am. J. Cardiol. 2011, 108, 1632–1638. [Google Scholar] [CrossRef]
- Opreanu, M.; Wan, C.; Singh, V.; Salehi, N.; Ahmad, J.; Szymkiewicz, S.J.; Thakur, R.K. Wearable cardioverter-defibrillator as a bridge to cardiac transplantation: A national database analysis. J. Hear. Lung Transplant. 2015, 34, 1305–1309. [Google Scholar] [CrossRef]
- Salehi, N.; Nasiri, M.; Bianco, N.R.; Opreanu, M.; Singh, V.; Satija, V.; Jhand, A.S.; Karapetyan, L.; Safadi, A.R.; Surapaneni, P.; et al. The Wearable Cardioverter Defibrillator in Nonischemic Cardiomyopathy: A US National Database Analysis. Can. J. Cardiol. 2016, 32, 1247.e1–1247.e6. [Google Scholar] [CrossRef]
- Wäßnig, N.K.; Günther, M.; Quick, S.; Pfluecke, C.; Rottstädt, F.; Szymkiewicz, S.J.; Ringquist, S.; Strasser, R.H.; Speiser, U. Experience With the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation 2016, 134, 635–643. [Google Scholar] [CrossRef]
- Erath, J.W.; Vamos, M.; Benz, A.P.; Hohnloser, S.H. Usefulness of the WCD in patients with suspected tachymyopathy. Clin. Res. Cardiol. 2018, 107, 70–75. [Google Scholar] [CrossRef]
- Duncker, D.; Veltmann, C. The Wearable Cardioverter/Defibrillator—Toy or Tool? J. Atr. Fibrillation 2016, 8, 1367. [Google Scholar]
- Duncker, D.; Veltmann, C. Role of the Wearable Defibrillator in Newly Diagnosed Heart Failure. Curr. Heart Fail. Rep. 2018, 15, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncker, D.; König, T.; Hohmann, S.; Bauersachs, J.; Veltmann, C. Avoiding Untimely Implantable Cardioverter/Defibrillator Implantation by Intensified Heart Failure Therapy Optimization Supported by the Wearable Cardioverter/Defibrillator—The PROLONG Study. J. Am. Heart Assoc. 2017, 6, e004512. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Leisse, J.; Brunn, J.; Zormpas, C.; Hohmann, S.; Hillmann HA, K.; Eiringhaus, J.; Bauersachs, J.; Veltmann, C.; Duncker, D. Extended follow-up after wearable cardioverter-defibrillator period: The PROLONG-II study. ESC Hear. Fail. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Szymkiewicz, S.J.; Shao, M.; Zishiri, E.; Niebauer, M.J.; Lindsay, B.D.; Tchou, P.J. Aggregate National Experience With the Wearable Cardioverter-Defibrillator. J. Am. Coll. Cardiol. 2010, 56, 194–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutyifa, V.; Moss, A.J.; Klein, H.; Biton, Y.; McNitt, S.; MacKecknie, B.; Zareba, W.; Goldenberg, I. Use of the Wearable Cardioverter Defibrillator in High-Risk Cardiac Patients. Circulation 2015, 132, 1613–1619. [Google Scholar] [CrossRef]
- Veltmann, C.; Winter, S.; Duncker, D.; Wäßnig, N.K.; Geller, J.C.; Erath, J.W.; Goeing, O.; Perings, C.; Ulbrich, M.; Roser, M.; et al. Protected risk stratification with the wearable cardioverter-defibrillator: Results from the WEARIT-II-EUROPE registry. Clin. Res. Cardiol. 2020, 110, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Zylla, M.M.; Hillmann HA, K.; Proctor, T.; Kieser, M.; Scholz, E.; Zitron, E.; Katus, H.A.; Thomas, D. Use of the wearable cardioverter-defibrillator (WCD) and WCD-based remote rhythm monitoring in a real-life patient cohort. Heart Vessels 2018, 33, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Kannel, C.; Paffenbarger, R.S.; Cupples, L.A. Heart rate and cardiovascular mortality: The Framingham study. Am. Heart J. 1987, 113, 1489–1494. [Google Scholar] [CrossRef]
- Greenland, P.; Daviglus, M.L.; Dyer, A.R.; Liu, K.; Huang, C.F.; Goldberger, J.J.; Stamler, J. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: The Chicago Heart Association Detection Project in Industry. Am. J. Epidemiol. 1999, 149, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Kristal-Boneh, E.; Silber, H.; Harari, G.; Froom, P. The association of resting heart rate with cardiovascular, cancer and all-cause mortality. Eight year follow-up of 3527 male Israeli employees (the CORDIS Study). Eur. Heart J. 2000, 21, 116–124. [Google Scholar] [CrossRef]
- Saxena, A.; Minton, D.; Lee, D.; Sui, X.; Fayad, R.; Lavie, C.J.; Blair, S.N. Protective role of resting heart rate on all-cause and cardiovascular disease mortality. Mayo Clin. Proc. 2013, 88, 1420–1426. [Google Scholar] [CrossRef] [Green Version]
- Kotecha, D.; Flather, M.D.; Altman, D.G.; Holmes, J.; Rosano, G.; Wikstrand, J.; Packer, M.; Coats, A.J.S.; Manzano, L.; Böhm, M.; et al. Heart Rate and Rhythm and the Benefit of Beta-Blockers in Patients with Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarson, A. Significance of reduction in heart rate in cardiovascular disease. Clin. Cardiol. 1998, 21, II3-7. [Google Scholar] [PubMed]
- Hjalmarson, Å.; Gilpin, E.A.; Kjekshus, J.; Schieman, G.; Nicod, P.; Henning, H.; Ross, J. Influence of heart rate on mortality after acute myocardial infarction. Am. J. Cardiol. 1990, 65, 547–553. [Google Scholar] [CrossRef]
- Stangeland, L.; Grong, K.; Vik-Mo, H.; Andersen, K.S.; Lekven, J. Is reduced cardiac performance the only mechanism for myocardial infarct size reduction during beta adrenergic blockade? Cardiovasc. Res. 1986, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Clements, I.P.; Miller, W.L.; Olson, L.J. Resting heart rate and cardiac function in dilated cardiomyopathy. Int. J. Cardiol. 1999, 72, 27–37. [Google Scholar] [CrossRef]
- Calé, R.; Mendes, M.; Brito, J.; Sousa, P.; Carmo, P.; Almeida, S.; Gomes, R.; Ferreira, A.; Santos, K.R.; Cavaco, D.; et al. Resting heart rate is a powerful predictor of arrhythmic events in patients with dilated cardiomyopathy and implantable cardioverter-defibrillator. Rev. Port. Cardiol. 2011, 30, 199–212. [Google Scholar]
- Optimizing Beta Blocker Dosage in Women While Using the Wearable Cardioverter Defibrillator (OPT-BB WOMEN). Available online: https://clinicaltrials.gov/ct2/show/NCT04504188 (accessed on 28 October 2021).
- Rashba, E.J.; Estes NA, M.; Wang, P.; Schaechter, A.; Howard, A.; Zareba, W.; Couderc, J.-P.; Perkiomaki, J.; Levine, J.; Kadish, A. Preserved heart rate variability identifies low-risk patients with nonischemic dilated cardiomyopathy: Results from the DEFINITE trial. Heart Rhythm 2006, 3, 281–286. [Google Scholar] [CrossRef]
- Swearingen, A.; Schafer, J.; De Lurgio, D.; Stone, J. SDANN as a Predictor of Heart Failure Hospitalizations in Cardiac Resynchronization Therapy Patients. J. Card. Fail. 2006, 12, S112–S113. [Google Scholar] [CrossRef]
- Soares-Miranda, L.; Sattelmair, J.; Chaves, P.; Duncan, G.E.; Siscovick, D.S.; Stein, P.K.; Mozaffarian, D. Physical Activity and Heart Rate Variability in Older Adults. Circulation 2014, 129, 2100–2110. [Google Scholar] [CrossRef] [Green Version]
- Masroor, S.; Bhati, P.; Verma, S.; Khan, M.; Hussain, M.E. Heart Rate Variability following Combined Aerobic and Resistance Training in Sedentary Hypertensive Women: A Randomised Control Trial. Indian Heart J. 2018, 70, S28–S35. [Google Scholar] [CrossRef]
- Sandercock GR, H.; Bromley, P.D.; Brodie, D.A. Effects of Exercise on Heart Rate Variability: Inferences from Meta-Analysis. Med. Sci. Sport. Exerc. 2005, 37, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.; Ding, W.Y.; Etheridge, S.; Noseworthy, P.A.; Veltmann, C.; Yao, X.; Bunch, T.J.; Gupta, D. Smart Wearables for Cardiac Monitoring-Real-World Use beyond Atrial Fibrillation. Sensors 2021, 21, 2539. [Google Scholar] [CrossRef] [PubMed]
- Saint-Maurice, P.F.; Troiano, R.P.; Bassett, D.R.; Graubard, B.I.; Carlson, S.A.; Shiroma, E.J.; Fulton, J.E.; Matthews, C.E. Association of Daily Step Count and Step Intensity with Mortality among US Adults. JAMA—J. Am. Med. Assoc. 2020, 323, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Jamé, S.; Kutyifa, V.; Polonsky, B.; McNitt, S.; Al-Ahmad, A.; Moss, A.J.; Zareba, W.; Wang, P.J. Predictive value of device-derived activity level for short-term outcomes in MADIT-CRT. Heart Rhythm 2017, 14, 1081–1086. [Google Scholar] [CrossRef]
- Jin, H.; Gu, M.; Hua, W.; Fan, X.-H.; Niu, H.-X.; Ding, L.-G.; Wang, J.; Xue, C.; Zhang, S. Predictors of super-response to cardiac resynchronization therapy: The significance of heart failure medication, pre-implant left ventricular geometry and high percentage of biventricular pacing. J. Geriatr. Cardiol. 2017, 14, 737–742. [Google Scholar]
- Joshi, K.; Alam, I.; Ruden, E.; Gradus-Pizlo, I.; Mahenthiran, J.; Kamalesh, M.; Feigenbaum, H.; Sawada, S. Effect of improvement in left ventricular ejection fraction on long-term survival in revascularized patients with ischaemic left ventricular systolic dysfunction. Eur. J. Echocardiogr. 2011, 12, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Böhm, M.; Boll, H.; Kim, S.S.; Koehler, K.; Lücke, S.; et al. Telemedical Interventional Monitoring in Heart Failure (TIM-HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: Study design. Eur. J. Heart Fail. 2010, 12, 1354–1362. [Google Scholar] [CrossRef]
- Koehler, J.; Stengel, A.; Hofmann, T.; Wegscheider, K.; Koehler, K.; Sehner, S.; Rose, M.; Deckwart, O.; Anker, S.D.; Koehler, F.; et al. Telemonitoring in patients with chronic heart failure and moderate depressed symptoms: Results of the Telemedical Interventional Monitoring in Heart Failure (TIM-HF) study. Eur. J. Heart Fail. 2021, 23, 186–194. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Winkler, S.; Vettorazzi, E.; Polze, A.; Stangl, K.; Hartmann, O.; et al. Telemedical Interventional Management in Heart Failure II (TIM-HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: Study design and description. Eur. J. Heart Fail. 2018, 20, 1485–1493. [Google Scholar] [CrossRef] [Green Version]




| Patient Characteristics | Baseline (n = 276) | 3-Month Follow-Up (n = 271) |
|---|---|---|
| NYHA functional class (mean ± SD) | 2.6 ± 0.8 | 2.0 ± 0.6 |
| LVEF (%; mean ± SD) | 25.3 ± 8.5 | 34.1 ± 10.3 |
| Beta-blocker (n, %) | 260 (94.2) | 255 (94.1) |
| % target dose (mean ± SD) | 51.6 ± 28.0 | 62.5 ± 29.7 |
| Renin–angiotensin system inhibitor (n, %) | 264 (95.7) | 265 (97.8) |
| % target dose (mean ± SD) | 47.9 ± 28.4 | 61.8 ± 31.7 |
| Mineralocorticoid receptor antagonist (n, %) | 233 (84.4) | 235 (86.7) |
| % target dose (mean ± SD) | 46.1 ± 23.4 | 54.2 ± 28.9 |
| Diuretics (n, %) | 217 (78.6) | 213 (78.6) |
| Digitalis (n, %) | 25 (9.1) | 20 (7.4) |
| Ivabradine (n, %) | 57 (20.7) | 48 (17.7) |
| Parameter | First Seven Days of Usage | Last Seven Days of Usage | p-Value |
|---|---|---|---|
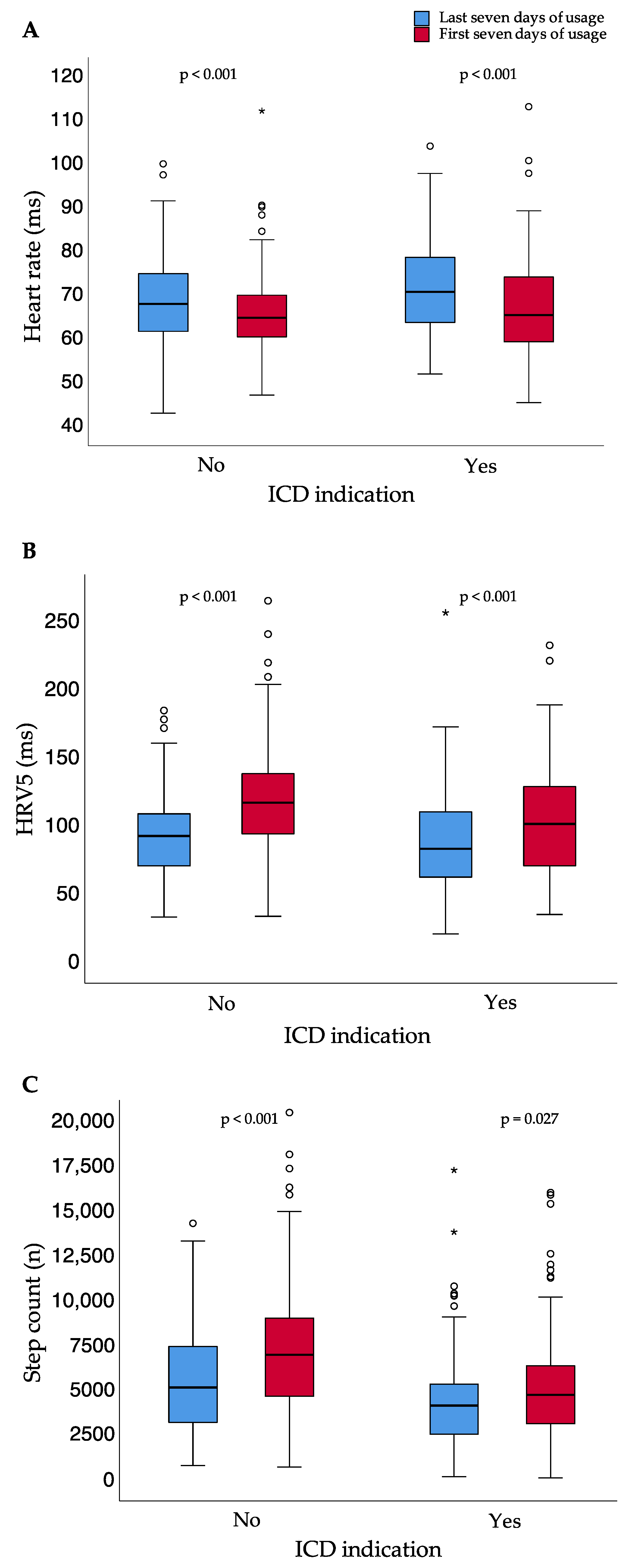
| Heart rate (bpm; median; (IQR)) | 69.5 (62.0–76.8) | 65.9 (60.4–72.2) | <0.001 |
| Step count per day (n; median; (IQR)) | 4657 (2778–6918) | 5562 (3890–8446) | <0.001 |
| HRV5 (ms; median; (IQR)) | 89.0 (64.8–110.7) | 111.0 (83.7–134.7) | <0.001 |
| Correlation Analysis for Heart Failure Parameters | Correlation Coefficient (r) | p-Value |
|---|---|---|
| ∆Heart rate/∆HRV5 | 0.382 | <0.001 |
| ∆Heart rate/∆Step count per day | 0.068 | 0.297 |
| ∆Step count per day/∆HRV5 | 0.320 | <0.001 |
| Age/∆Step count per day | −0.256 | <0.001 |
| Age/∆HRV5 | −0.143 | 0.029 |
| Age/∆Heart rate | 0.048 | 0.465 |
| Age/∆LVEF | −0.251 | <0.001 |
| ∆LVEF/∆HRV5 | 0.255 | <0.001 |
| ∆LVEF/∆Step count per day | 0.189 | 0.005 |
| ∆LVEF/∆Heart rate | 0.028 | 0.684 |
| Parameter | AUC | Optimal Cut-Off |
|---|---|---|
| ΔHRV5 | 0.678 | 23 ms |
| ΔCL | 0.566 | 112 ms |
| Δsteps | 0.625 | 1163 steps |
| Parameters | LVEF Improvement < 10% (n = 143) | LVEF Improvement ≥ 10% (n = 118) | p-Value (uni) | Odds Ratio in Multivariate Analysis (95%-CI) | p-Value (Multi) |
|---|---|---|---|---|---|
| Female Sex (n, %) | 39 (27.3%) | 47 (39.8%) | 0.032 | 1.02 (0.51–2.05) | 0.95 |
| Age (mean ± SD) | 60.9 ± 14.3 | 52.6 ± 15.5 | <0.001 | 0.98 (0.96–1.01) per year | 0.130 |
| ICM (n, %) | 69 (48.3%) | 22 (18.6%) | <0.001 | 0.39 (0.18–0.84) | 0.018 |
| Baseline LVEF (%; mean ± SD) | 27.0 ± 8.4 | 22.5 ± 7.1 | <0.001 | 0.92 (0.88–0.96) per percentage point | <0.001 |
| ∆HRV5 (ms; mean ± SD) | 12.9 ± 39.9 | 38.8 ± 42.2 | <0.001 | 2.13 (1.05–4.32) for ∆HRV5 > 23 ms | 0.035 |
| ∆CL (ms; mean ± SD) | 28.1 ± 119.0 | 54.6 ± 154.4 | <0.001 | 1.98 (0.89–4.44) for ∆CL > 112 ms | 0.093 |
| ∆steps (n; mean ± SD) | 1470 ± 3322 | 3287 ± 4057 | 0.002 | 1.94 (0.99–3.86) for ∆steps > 1163 | 0.054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillmann, H.A.K.; Hohmann, S.; Mueller-Leisse, J.; Zormpas, C.; Eiringhaus, J.; Bauersachs, J.; Veltmann, C.; Duncker, D. Feasibility and First Results of Heart Failure Monitoring Using the Wearable Cardioverter–Defibrillator in Newly Diagnosed Heart Failure with Reduced Ejection Fraction. Sensors 2021, 21, 7798. https://doi.org/10.3390/s21237798
Hillmann HAK, Hohmann S, Mueller-Leisse J, Zormpas C, Eiringhaus J, Bauersachs J, Veltmann C, Duncker D. Feasibility and First Results of Heart Failure Monitoring Using the Wearable Cardioverter–Defibrillator in Newly Diagnosed Heart Failure with Reduced Ejection Fraction. Sensors. 2021; 21(23):7798. https://doi.org/10.3390/s21237798
Chicago/Turabian StyleHillmann, Henrike Aenne Katrin, Stephan Hohmann, Johanna Mueller-Leisse, Christos Zormpas, Jörg Eiringhaus, Johann Bauersachs, Christian Veltmann, and David Duncker. 2021. "Feasibility and First Results of Heart Failure Monitoring Using the Wearable Cardioverter–Defibrillator in Newly Diagnosed Heart Failure with Reduced Ejection Fraction" Sensors 21, no. 23: 7798. https://doi.org/10.3390/s21237798






