A Flexible Chemosensor Based on Colorimetric and Fluorescent Dual Modes for Rapid and Sensitive Detection of Hypochlorite Anion
Abstract
:1. Introduction
2. Results and Discussion
2.1. NMR Spectra
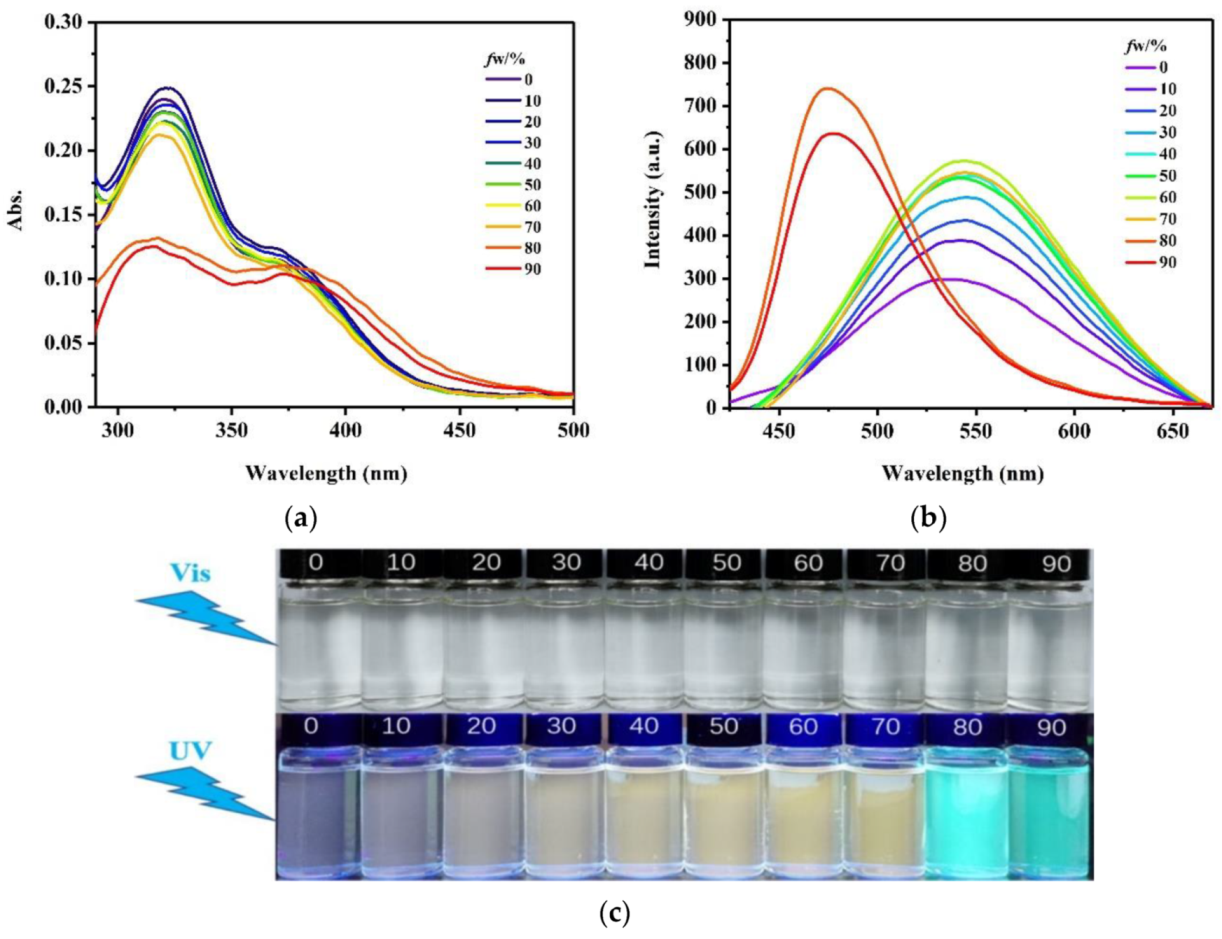
2.2. Aggregation-Induced Fluorescent Behavior
2.3. Titration Experiment of Probe TPE4A
2.4. Paper-Based Sensor
2.5. Possible Mechanism of Probe TPE4A
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gross, S.; Gammon, S.T.; Moss, B.L.; Rauch, D.; Harding, J.; Heinecke, J.W.; Ratner, L.; Piwnica-Worms, D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009, 15, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Reja, S.I.; Bhalla, V.; Sharma, A.; Kaur, G.; Kumar, M. A highly selective fluorescent probe for hypochlorite and its endogenous imaging in living cells. Chem. Commun. 2014, 50, 11911–11914. [Google Scholar] [CrossRef]
- Gui, S.L.; Huang, Y.Y.; Hu, F.; Jin, Y.L.; Zhang, G.X.; Yan, L.S.; Zhang, D.Q.; Zhao, R. Fluorescence turn-on chemosensor for highly selective and sensitive detection and bioimaging of Al3+ in living cells based on ion-induced aggregation. Anal. Chem. 2015, 87, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.R.; Hu, T.L.; Sun, T.; Li, T.D.; Chi, H.; Niu, Q.F. A colorimetric and fluorometric oligothiophene-indenedione-based sensor for rapid and highly sensitive detection of cyanide in real samples and bioimaging in living cells. Dyes Pigments 2019, 163, 667–674. [Google Scholar] [CrossRef]
- Xu, H.; Wu, S.L.; Lin, N.J.; Lu, Y.; Xiao, J.; Wang, Y.W.; Peng, Y. A NIR fluorescent probe for rapid turn-on detection and bioimaging of hypochlorite anion. Sens. Actuators B Chem. 2021, 346, 130484. [Google Scholar] [CrossRef]
- Pattison, D.I.; Davies, M.J. Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: Implications for the prevention of hypochlorous-acid-mediated damage. Biochemistry 2006, 45, 8152–8162. [Google Scholar] [CrossRef]
- Kohler, A.T.; Rodtoff, A.C.; Labahn, M.; Reinhardt, M.; Truyen, U.; Speck, S. Efficacy of sodium hypochlorite against multidrug-resistant Gram-negative bacteria. J. Hosp. Inf. 2018, 100, E40–E46. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A. Use of Hypochlorite Solution as Disinfectant during COVID-19 Outbreak in India: From the Perspective of Human Health and Atmospheric Chemistry. Aerosol Air Qual. Res. 2020, 20, 1516–1519. [Google Scholar] [CrossRef]
- Wang, L.; Long, L.L.; Zhou, L.P.; Wu, Y.J.; Zhang, C.; Han, Z.X.; Wang, J.L.; Da, Z.L. A ratiometric fluorescent probe for highly selective and sensitive detection of hypochlorite based on the oxidation of N-alkylpyridinium. RSC Adv. 2014, 4, 59535–59540. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Tang, C.; Dong, G.; Zhao, P.; Peng, D.; Wang, T.; Du, L.; Li, M. Multiple rapid-responsive probes towards hypochlorite detection based on dioxetane luminophore derivatives. J. Pharm. Anal. 2021, in press. [Google Scholar] [CrossRef]
- Jin, L.; Xu, M.Y.; Jiang, H.; Wang, W.L.; Wang, Q.M. A simple fluorescein derived colorimetric and fluorescent ‘off-on’ sensor for the detection of hypochlorite. Anal. Methods 2018, 10, 4562–4569. [Google Scholar] [CrossRef]
- Dongare, P.R.; Gore, A.H. Recent advances in colorimetric and fluorescent chemosensors for ionic species: Design, principle and optical signalling mechanism. ChemistrySelect 2021, 6, 5657–5669. [Google Scholar] [CrossRef]
- Ma, C.G.; Zhong, G.Y.; Zhao, Y.; Zhang, P.; Fu, Y.Q.; Shen, B.X. Recent development of synthetic probes for detection of hypochlorous acid/hypochlorite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118545. [Google Scholar] [CrossRef]
- Song, Z.-G.; Yuan, Q.; Lv, P.; Chen, K. Research progress of small molecule fluorescent probes for detecting hypochlorite. Sensors 2021, 21, 6326. [Google Scholar] [CrossRef]
- Zhu, B.C.; Xu, Y.H.; Liu, W.Q.; Shao, C.X.; Wu, H.F.; Jiang, H.L.; Du, B.; Zhang, X.L. A highly selective colorimetric probe for fast and sensitive detection of hypochlorite in absolute aqueous solution. Sens. Actuators B Chem. 2014, 191, 473–478. [Google Scholar] [CrossRef]
- Li, J.F.; Huo, F.J.; Yin, C.X. A selective colorimetric and fluorescent probe for the detection of ClO− and its application in bioimaging. RSC Adv. 2014, 4, 44610–44613. [Google Scholar] [CrossRef]
- Yu, S.Y.; Hsu, C.Y.; Chen, W.C.; Wei, L.F.; Wu, S.P. A hypochlorous acid turn-on fluorescent probe based on HOCl-promoted oxime oxidation and its application in cell imaging. Sens. Actuators B Chem. 2014, 196, 203–207. [Google Scholar] [CrossRef]
- Venkatesan, P.; Wu, S.P. A turn-on fluorescent probe for hypochlorous acid based on the oxidation of diphenyl telluride. Analyst 2015, 140, 1349–1355. [Google Scholar] [CrossRef]
- Chen, W.C.; Venkatesan, P.; Wu, S.P. A highly selective turn-on fluorescent probe for hypochlorous acid based on hypochlorous acid-induced oxidative intramolecular cyclization of boron dipyrromethene-hydrazone. Anal. Chim. Acta 2015, 882, 68–75. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Z.; Long, S.R.; Du, J.J.; Fan, J.L.; Peng, X.J. Synthesis of an ultrasensitive BODIPY-derived fluorescent probe for detecting HOCl in live cells. Nat. Protoc. 2018, 13, 2348–2361. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, A.; Kim, C. A simple hydrazine-based probe bearing anthracene moiety for the highly selective detection of hypochlorite. Inorg. Chem. Commun. 2019, 101, 1–5. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, J.; You, X.; Wang, C.; Li, Z.; Xie, W. A light-up probe for detection of adenosine in urine samples by a combination of an AIE molecule and an aptamer. Sensors 2017, 17, 2246. [Google Scholar] [CrossRef] [Green Version]
- Rha, C.J.; Lee, H.; Kim, C. Development of an azo-naphthol-based probe for detecting hypochlorite (ClO−) via color change in aqueous solution. Inorg. Chem. Commun. 2020, 121, 108244. [Google Scholar] [CrossRef]
- Lee, S.C.; Park, S.; So, H.; Lee, G.; Kim, K.-T.; Kim, C. An acridine-based fluorescent sensor for monitoring ClO− in water samples and zebrafish. Sensors 2020, 20, 4764. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhuang, Y.P.; Li, X.; Agren, H.; Yu, L.; Ding, J.D.; Zhu, L.L. Selective dual-channel imaging on cyanostyryl-modified azulene systems with unimolecularly tunable visible-near infrared luminescence. Chem. Eur. J. 2017, 23, 7642–7647. [Google Scholar] [CrossRef]
- Fang, H.; Gan, Y.T.; Wang, S.R.; Tao, T. A selective and colorimetric chemosensor for fluoride based on dimeric azulene boronate ester. Inorg. Chem. Commun. 2018, 95, 17–21. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, K.; Kwon, J.E.; Park, H.; Lee, S.; Kim, S.; Park, S.Y. Dual-color fluorescent nanoparticles showing perfect color-specific photoswitching for bioimaging and super-resolution microscopy. Nat. Commun. 2019, 10, 3089. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.B.; Li, B.; Yang, D.D.; Liu, C.; Feng, S.; Chen, M.L.; Sun, Y.; Tian, Y.N.; Su, X.; Wang, X.M.; et al. A flexible ultrasensitive optoelectronic sensor array for neuromorphic vision systems. Nat. Commun. 2021, 12, 1798. [Google Scholar] [CrossRef]
- Kim, S.; Yun, T.G.; Kang, C.; Son, M.J.; Kang, J.G.; Kim, I.H.; Lee, H.J.; An, C.H.; Hwang, B. Facile fabrication of paper-based silver nanostructure electrodes for flexible printed energy storage system. Mater. Des. 2018, 151, 1–7. [Google Scholar] [CrossRef]
- Jiao, J.J.; Li, Z.J.; Qiao, Z.W.; Li, X.; Liu, Y.; Dong, J.Q.; Jiang, J.W.; Cui, Y. Design and self-assembly of hexahedral coordination cages for cascade reactions. Nat. Commun. 2018, 9, 4423. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Z.; Diercks, C.S.; Ma, Y.H.; Lyu, H.; Zhu, C.H.; Alshmimri, S.A.; Alshihri, S.; Yaghi, O.M. 3D covalent organic frameworks of interlocking 1D square ribbons. J. Am. Chem. Soc. 2019, 141, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Tao, H.; Zhao, S.J.; Yang, K.; Cao, Q.Y.; Lan, M.H. A tetraphenylethylene-based aggregation-induced emission probe for fluorescence turn-on detection of lipopolysaccharide in injectable water with sensitivity down to picomolar. Ind. Eng. Chem. Res. 2020, 59, 8252–8258. [Google Scholar] [CrossRef]
- Zhang, S.S.; Huang, Y.P.; Kong, L.; Zhang, X.J.; Yang, J.X. Aggregation-induced emission-active tetraphenylethylene derivatives containing arylimidazole unit for reversible mechanofluorochromism and selective detection of picric acid. Dyes Pigments 2020, 181, 108574. [Google Scholar] [CrossRef]
- Ding, Y.; Li, X.; Li, T.; Zhu, W.; Xie, Y. α-Monoacylated and α,α′- and α,β′-diacylated dipyrrins as highly sensitive fluorescence “turn-on” zn2+ probes. J. Org. Chem. 2013, 78, 5328–5338. [Google Scholar] [CrossRef]
- Xiong, K.M.; Huo, F.J.; Yin, C.X.; Chu, Y.Y.; Yang, Y.T.; Chao, J.B.; Zheng, A.M. A novel recognition mechanism supported by experiment and theoretical calculation for hypochlorites recognition and its practical application. Sens. Actuators B Chem. 2016, 224, 307–314. [Google Scholar] [CrossRef]
- Cheng, X.H.; Jia, H.Z.; Long, T.; Feng, J.; Qin, J.G.; Li, Z. A “turn-on” fluorescent probe for hypochlorous acid: Convenient synthesis, good sensing performance, and a new design strategy by the removal of C=N isomerization. Chem. Commun. 2011, 47, 11978–11980. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, J.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Schlegel, H.B.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Tao, T.; Zhao, Y.; Huang, W. A Flexible Chemosensor Based on Colorimetric and Fluorescent Dual Modes for Rapid and Sensitive Detection of Hypochlorite Anion. Sensors 2021, 21, 8082. https://doi.org/10.3390/s21238082
Wu Q, Tao T, Zhao Y, Huang W. A Flexible Chemosensor Based on Colorimetric and Fluorescent Dual Modes for Rapid and Sensitive Detection of Hypochlorite Anion. Sensors. 2021; 21(23):8082. https://doi.org/10.3390/s21238082
Chicago/Turabian StyleWu, Qin, Tao Tao, Yunxia Zhao, and Wei Huang. 2021. "A Flexible Chemosensor Based on Colorimetric and Fluorescent Dual Modes for Rapid and Sensitive Detection of Hypochlorite Anion" Sensors 21, no. 23: 8082. https://doi.org/10.3390/s21238082
APA StyleWu, Q., Tao, T., Zhao, Y., & Huang, W. (2021). A Flexible Chemosensor Based on Colorimetric and Fluorescent Dual Modes for Rapid and Sensitive Detection of Hypochlorite Anion. Sensors, 21(23), 8082. https://doi.org/10.3390/s21238082





