Effect of a Recliner Chair with Rocking Motions on Sleep Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recliner Chair
2.2. Experimental Protocols
2.3. Sleep Stage Estimation
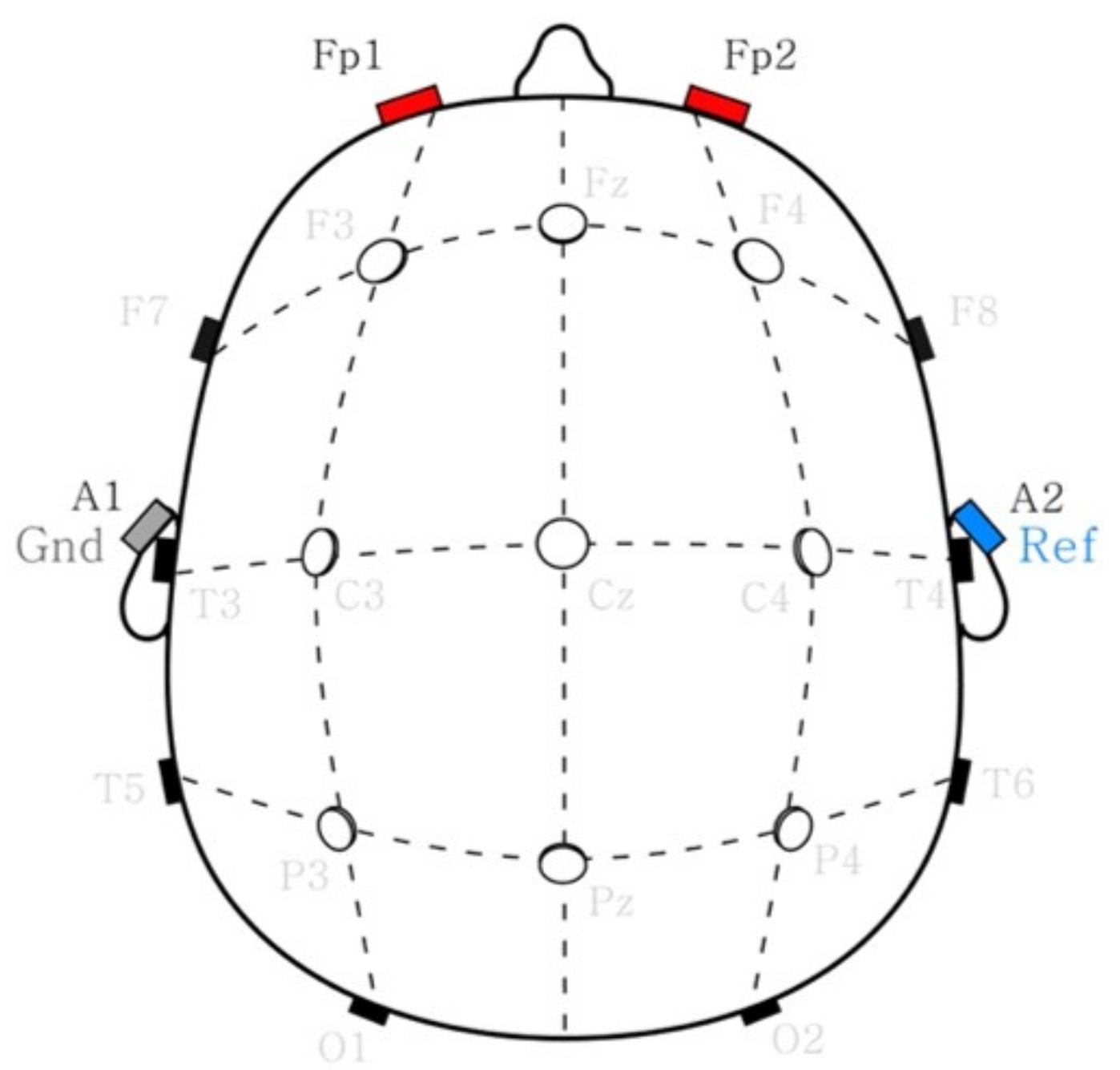
2.4. Sleep Spindle Estimation
2.5. Sleep Parameters
2.6. Self-Reported Sleep Quality Analysis
3. Results
3.1. Sleep Stage Automation Evaluation
3.2. Sleep Parameter Analysis
3.3. Sleep Spindle Analysis
3.4. Qualitative Sleep Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Härmä, M. Ageing, physical fitness and shiftwork tolerance. Appl. Ergon. 1996, 27, 25–29. [Google Scholar] [CrossRef]
- Shepard, J.W.; Buysse, D.J.; Chesson, A.L.; Dement, W.C.; Goldberg, R.; Guilleminault, C.; Harris, C.D.; Iber, C.; Mignot, E.; Mitler, M.M.; et al. History of the development of sleep medicine in the United States. J. Clin. Sleep Med. 2005, 1, 61–82. [Google Scholar] [CrossRef]
- Hsu, H.C.; Lin, M.H. Exploring quality of sleep and its related factors among menopausal women. J. Nurs. Res. JNR 2005, 13, 153–164. [Google Scholar] [CrossRef]
- Pilcher, J.J.; Ginter, D.R.; Sadowsky, B. Sleep quality versus sleep quantity: Relationships between sleep and measures of health, well-being and sleepiness in college students. J. Psychosom. Res. 1997, 42, 583–596. [Google Scholar] [CrossRef]
- Iliescu, E.A.; Coo, H.; McMurray, M.H.; Meers, C.L.; Quinn, M.M.; Singer, M.A.; Hopman, W.M. Quality of sleep and health-related quality of life in haemodialysis patients. Nephrol. Dial. Transplant. 2003, 18, 126–132. [Google Scholar] [CrossRef] [Green Version]
- LeBourgeois, M.K.; Giannotti, F.; Cortesi, F.; Wolfson, A.R.; Harsh, J. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics 2005, 115, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Léger, D.; Guilleminault, C.; Bader, G.; Lévy, E.; Paillard, M. Medical and socio-professional impact of insomnia. Sleep 2002, 25, 621–625. [Google Scholar] [CrossRef] [Green Version]
- Medic, G.; Wille, M.; Hemels, M.E. Short-and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Womack, S.D.; Hook, J.N.; Reyna, S.H.; Ramos, M. Sleep loss and risk-taking behavior: A review of the literature. Behav. Sleep Med. 2013, 11, 343–359. [Google Scholar] [CrossRef]
- Terzano, M.G.; Parrino, L.; Cirignotta, F.; Ferini-Strambi, L.; Gigli, G.; Rudelli, G.; Sommacal, S. Studio Morfeo: Insomnia in primary care, a survey conducted on the Italian population. Sleep Med. 2004, 5, 67–75. [Google Scholar] [CrossRef]
- Ganz, F.D. Sleep and immune function. Crit. Care Nurse 2012, 32, e19–e25. [Google Scholar] [CrossRef] [Green Version]
- Michal, M.; Wiltink, J.; Kirschner, Y.; Schneider, A.; Wild, P.S.; Münzel, T.; Blettner, M.; Schulz, A.; Lackner, K.; Pfeiffer, N.; et al. Complaints of sleep disturbances are associated with cardiovascular disease: Results from the Gutenberg Health Study. PLoS ONE 2014, 9, e104324. [Google Scholar]
- Hayashi, M.; Ito, S.; Hori, T. The effects of a 20-min nap at noon on sleepiness, performance and EEG activity. Int. J. Psychophysiol. 1999, 32, 173–180. [Google Scholar] [CrossRef]
- Andrechuk, C.R.S.; Ceolim, M.F. Sleep quality and adverse outcomes for patients with acute myocardial infarction. J. Clin. Nurs. 2016, 25, 223–230. [Google Scholar] [CrossRef]
- BaHammam, A. Sleep quality of patients with acute myocardial infarction outside the CCU environment: A preliminary study. Med. Sci. Monit. 2006, 12, CR168–CR172. [Google Scholar]
- Fredriksson-Larsson, U.; Alsén, P.; Karlson, B.W.; Brink, E. Fatigue two months after myocardial infarction and its relationships with other concurrent symptoms, sleep quality and coping strategies. J. Clin. Nurs. 2015, 24, 2192–2200. [Google Scholar] [CrossRef]
- Adams, S.K.; Kisler, T.S. Sleep quality as a mediator between technology-related sleep quality, depression, and anxiety. Cyberpsychol. Behav. Soc. Netw. 2013, 16, 25–30. [Google Scholar] [CrossRef]
- Demirci, K.; Akgönül, M.; Akpinar, A. Relationship of smartphone use severity with sleep quality, depression, and anxiety in university students. J. Behav. Addict. 2015, 4, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Ness, T.E.B.; Saksvik-Lehouillier, I. The relationships between life satisfaction and sleep quality, sleep duration and variability of sleep in university students. J. Eur. Psychol. Stud. 2018, 9, 28–39. [Google Scholar] [CrossRef]
- Nicholson, A.; Stone, B.M. Influence of back angle on the quality of sleep in seats. Ergonomics 1987, 30, 1033–1041. [Google Scholar] [CrossRef]
- Roach, G.D.; Matthews, R.; Naweed, A.; Kontou, T.G.; Sargent, C. Flat-out napping: The quantity and quality of sleep obtained in a seat during the daytime increase as the angle of recline of the seat increases. Chronobiol. Int. 2018, 35, 872–883. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Sharp, D.J.; Thornton, A.T. The effects of posture on obstructive sleep apnea. Am. Rev. Respir. Dis. 1986, 133, 662–666. [Google Scholar]
- Martin, S.E.; Marshall, I.; Douglas, N.J. The effect of posture on airway caliber with the sleep-apnea/hypopnea syndrome. Am. J. Respir. Crit. Care Med. 1995, 152, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Neill, A.M.; Angus, S.M.; Sajkov, D.; McEVOY, R.D. Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1997, 155, 199–204. [Google Scholar] [CrossRef] [PubMed]
- de Barros Souza, F.J.F.; Genta, P.R.; de Souza Filho, A.J.; Wellman, A.; Lorenzi-Filho, G. The influence of head-of-bed elevation in patients with obstructive sleep apnea. Sleep Breath. 2017, 21, 815–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, B.A.; Sodhi, J.S.; Zargar, S.A.; Javid, G.; Yattoo, G.N.; Shah, A.; Gulzar, G.M.; Khan, M.A. Effect of bed head elevation during sleep in symptomatic patients of nocturnal gastroesophageal reflux. J. Gastroenterol. Hepatol. 2012, 27, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM manual for the scoring of sleep and associated events. Rules Terminol. Tech. Specif. Darien Ill. Am. Acad. Sleep Med. 2012, 176, 2012. [Google Scholar]
- Mendonça, F.; Mostafa, S.S.; Morgado-Dias, F.; Ravelo-Garcia, A.G.; Penzel, T. A review of approaches for sleep quality analysis. IEEE Access 2019, 7, 24527–24546. [Google Scholar] [CrossRef]
- De Gennaro, L.; Ferrara, M. Sleep spindles: An overview. Sleep Med. Rev. 2003, 7, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Wagner, M.H. Sleep Medicine Pearls E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- JSSR; Hori, T.; Sugita, Y.; Koga, E.; Shirakawa, S.; Inoue, K.; Uchida, S.; Kuwahara, H.; Kousaka, M.; Kobayashi, T.; et al. Proposed supplements and amendments to ‘A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects’, the Rechtschaffen & Kales (1968) standard. Psychiatry Clin. Neurosci. 2001, 55, 305–310. [Google Scholar]
- Fogel, S.M.; Smith, C.T. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci. Biobehav. Rev. 2011, 35, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Hennies, N.; Ralph, M.A.L.; Kempkes, M.; Cousins, J.N.; Lewis, P.A. Sleep spindle density predicts the effect of prior knowledge on memory consolidation. J. Neurosci. 2016, 36, 3799–3810. [Google Scholar] [CrossRef] [Green Version]
- Schönauer, M.; Pöhlchen, D. Sleep spindles. Curr. Biol. 2018, 28, R1129–R1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüthi, A. Sleep spindles: Where they come from, what they do. Neuroscientist 2014, 20, 243–256. [Google Scholar] [CrossRef]
- Jarrin, D.C.; McGrath, J.J.; Silverstein, J.E.; Drake, C. Objective and subjective socioeconomic gradients exist for sleep quality, sleep latency, sleep duration, weekend oversleep, and daytime sleepiness in adults. Behav. Sleep Med. 2013, 11, 144–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, R.; Schapiro, A.C.; Manoach, D.S.; Stickgold, R. Individual differences in frequency and topography of slow and fast sleep spindles. Front. Hum. Neurosci. 2017, 11, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mölle, M.; Bergmann, T.O.; Marshall, L.; Born, J. Fast and slow spindles during the sleep slow oscillation: Disparate coalescence and engagement in memory processing. Sleep 2011, 34, 1411–1421. [Google Scholar] [CrossRef]
- Omlin, X.; Crivelli, F.; Näf, M.; Heinicke, L.; Skorucak, J.; Malafeev, A.; Guerrero, A.F.; Riener, R.; Achermann, P. The effect of a slowly rocking bed on sleep. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crivelli, F.; Omlin, X.; Rauter, G.; Von Zitzewitz, J.; Achermann, P.; Riener, R. Somnomat: A novel actuated bed to investigate the effect of vestibular stimulation. Med. Biol. Eng. Comput. 2016, 54, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Abe, A. Short daytime naps in a car seat to counteract daytime sleepiness: The effect of backrest angle. Sleep Biol. Rhythm. 2008, 6, 34–41. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Hoch, C.C.; Yeager, A.L.; Kupfer, D.J. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 1991, 14, 331–338. [Google Scholar] [PubMed]
- Cho, C.H.; Yoon, H.K.; Kang, S.G.; Kim, L.; Lee, E.I.; Lee, H.J. Impact of exposure to dim light at night on sleep in female and comparison with male subjects. Psychiatry Investig. 2018, 15, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhrström, E.; Skånberg, A. Sleep disturbances from road traffic and ventilation noise—laboratory and field experiments. J. Sound Vib. 2004, 271, 279–296. [Google Scholar] [CrossRef]
- Berglund, B.; Lindvall, T. Community Noise; Center for Sensory Research, Stockholm University and Karolinska Institute: Stockholm, Sweden, 1995. [Google Scholar]
- Caddick, Z.A.; Gregory, K.; Arsintescu, L.; Flynn-Evans, E.E. A review of the environmental parameters necessary for an optimal sleep environment. Build. Environ. 2018, 132, 11–20. [Google Scholar] [CrossRef]
- Krishnan, K.K.; Soman, K. CNN based classification of motor imaginary using variational mode decomposed EEG-spectrum image. Biomed. Eng. Lett. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Chen, Y.; Qiu, T. Multifractional Property Analysis of Human Sleep Electroencephalogram Signals. In Fractional Processes and Fractional-Order Signal Processing: Techniques and Applications; Springer: London, UK, 2012; pp. 243–250. [Google Scholar] [CrossRef]
- Casson, A.J. Wearable EEG and beyond. Biomed. Eng. Lett. 2019, 9, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Seo, W.; Kim, S.; Park, S.M. Electrogastrogram: Demonstrating Feasibility in Mental Stress Assessment Using Sensor Fusion. IEEE Sens. J. 2021, 21, 14503–14514. [Google Scholar] [CrossRef]
- Brito, M.A.; Li, D.; Mashour, G.A.; Pal, D. State-dependent and bandwidth-specific effects of ketamine and propofol on electroencephalographic complexity in rats. Front. Syst. Neurosci. 2020, 14, 50. [Google Scholar] [CrossRef]
- Lee, H.; Kim, G.; Kim, J.; Kang, Y.; Park, C. Optimal Design of Convolutional Neural Network for EEG-based Authentication. IEIE Trans. Smart Process. Comput. 2021, 10, 199–203. [Google Scholar] [CrossRef]
- Piskorowski, J. Digital notch filter with time-varying quality factor for the reduction of powerline interference. In Proceedings of the 2010 IEEE International Symposium on Circuits and Systems, Paris, France, 30 May–2 June 2010; pp. 2706–2709. [Google Scholar]
- Agnew, H., Jr.; Webb, W.B.; Williams, R.L. The first night effect: An Eeg studyof sleep. Psychophysiology 1966, 2, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Morley, A.; Hill, L.; Kaditis, A. 10–20 System EEG Placement; European Respiratory Society, European Respiratory Society: Lausanne, Brussels; Sheffield, UK, 2016. [Google Scholar]
- Gutmann, B.; Mierau, A.; Hülsdünker, T.; Hildebrand, C.; Przyklenk, A.; Hollmann, W.; Strüder, H.K. Effects of physical exercise on individual resting state EEG alpha peak frequency. Neural Plast. 2015, 2015, 717312. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Åkerstedt, T.; Gillberg, M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.W.; Gottlieb, D.J.; Redline, S.; Norman, R.G.; Dodge, R.R.; Shahar, E.; Surovec, S.; Nieto, F.J. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep 1998, 21, 749–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacourse, K.; Delfrate, J.; Beaudry, J.; Peppard, P.; Warby, S.C. A sleep spindle detection algorithm that emulates human expert spindle scoring. J. Neurosci. Methods 2019, 316, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Vallat, R.; Walker, M.P. A universal, open-source, high-performance tool for automated sleep staging. bioRxiv 2021. [Google Scholar] [CrossRef]
- Supratak, A.; Dong, H.; Wu, C.; Guo, Y. DeepSleepNet: A model for automatic sleep stage scoring based on raw single-channel EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1998–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.Q.; Cui, L.; Mueller, R.; Tao, S.; Kim, M.; Rueschman, M.; Mariani, S.; Mobley, D.; Redline, S. The National Sleep Research Resource: Towards a sleep data commons. J. Am. Med. Inform. Assoc. 2018, 25, 1351–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, T.; Palta, M.; Dempsey, J.; Peppard, P.E.; Nieto, F.J.; Hla, K.M. Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 2009, 108, 246. [Google Scholar]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, W.; Kim, N.; Kim, S.; Lee, C.; Park, S.M. Deep ECG-respiration network (DeepER net) for recognizing mental stress. Sensors 2019, 19, 3021. [Google Scholar] [CrossRef] [Green Version]
- Senyurek, V.Y.; Imtiaz, M.H.; Belsare, P.; Tiffany, S.; Sazonov, E. A CNN-LSTM neural network for recognition of puffing in smoking episodes using wearable sensors. Biomed. Eng. Lett. 2020, 10, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.H.; Ho, Z.J.; Ng, C.H.; Goh, Y.L. Enhanced CNN-based plant growing-stage classification using additional information carried in an additional channel. IEIE Trans. Smart Process. Comput. 2019, 8, 171–177. [Google Scholar] [CrossRef]
- Han, S.; Lee, W.; Eom, H.; Kim, J.; Park, C. Detection of arrhythmia using 1D convolution neural network with LSTM model. IEIE Trans. Smart Process. Comput. 2020, 9, 261–265. [Google Scholar] [CrossRef]
- Venkatramaphanikumar, S.; Bhattacharyya, D. Review on the Usage of Deep Learning Models in Multi-modal Sentiment Analysis. IEIE Trans. Smart Process. Comput. 2020, 9, 435–444. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Chatburn, A.; Coussens, S.; Lushington, K.; Kennedy, D.; Baumert, M.; Kohler, M. Sleep spindle activity and cognitive performance in healthy children. Sleep 2013, 36, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schabus, M.; Gruber, G.; Parapatics, S.; Sauter, C.; Klösch, G.; Anderer, P.; Klimesch, W.; Saletu, B.; Zeitlhofer, J. Sleep spindles and their significance for declarative memory consolidation. Sleep 2004, 27, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Clemens, Z.; Fabó, D.; Halász, P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience 2005, 132, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Lustenberger, C.; Wehrle, F.; Tüshaus, L.; Achermann, P.; Huber, R. The multidimensional aspects of sleep spindles and their relationship to word-pair memory consolidation. Sleep 2015, 38, 1093–1103. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, D. Sleep spindles as facilitators of memory formation and learning. Neural Plast. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Cairney, S.A.; El Marj, N.; Staresina, B.P. Memory consolidation is linked to spindle-mediated information processing during sleep. Curr. Biol. 2018, 28, 948–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, C.A.; Chilakamarri, P.; Thurm, A.E.; Swedo, S.E.; Holmes, G.L.; Buckley, A.W. Spindle activity in young children with autism, developmental delay, or typical development. Neurology 2018, 91, e112–e122. [Google Scholar] [CrossRef] [PubMed]
- Merikanto, I.; Kuula, L.; Makkonen, T.; Salmela, L.; Räikkönen, K.; Pesonen, A.K. Autistic traits are associated with decreased activity of fast sleep spindles during adolescence. J. Clin. Sleep Med. 2019, 15, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Latreille, V.; Carrier, J.; Lafortune, M.; Postuma, R.B.; Bertrand, J.A.; Panisset, M.; Chouinard, S.; Gagnon, J.F. Sleep spindles in Parkinson’s disease may predict the development of dementia. Neurobiol. Aging 2015, 36, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Kam, K.; Parekh, A.; Sharma, R.A.; Andrade, A.; Lewin, M.; Castillo, B.; Bubu, O.M.; Chua, N.J.; Miller, M.D.; Mullins, A.E.; et al. Sleep oscillation-specific associations with Alzheimer’s disease CSF biomarkers: Novel roles for sleep spindles and tau. Mol. Neurodegener. 2019, 14, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendt, S.L.; Welinder, P.; Sorensen, H.B.; Peppard, P.E.; Jennum, P.; Perona, P.; Mignot, E.; Warby, S.C. Inter-expert and intra-expert reliability in sleep spindle scoring. Clin. Neurophysiol. 2015, 126, 1548–1556. [Google Scholar] [CrossRef] [Green Version]
- Uygun, D.S.; Katsuki, F.; Bolortuya, Y.; Aguilar, D.D.; McKenna, J.T.; Thankachan, S.; McCarley, R.W.; Basheer, R.; Brown, R.E.; Strecker, R.E.; et al. Validation of an automated sleep spindle detection method for mouse electroencephalography. Sleep 2019, 42, zsy218. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K. Artificial neural network and wavelet based automated detection of sleep spindles, REM sleep and wake states. J. Med. Syst. 2008, 32, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.; Selesnick, I.W.; Osorio, R.S.; Varga, A.W.; Rapoport, D.M.; Ayappa, I. Multichannel sleep spindle detection using sparse low-rank optimization. J. Neurosci. Methods 2017, 288, 1–16. [Google Scholar] [CrossRef]
- Chambon, S.; Thorey, V.; Arnal, P.J.; Mignot, E.; Gramfort, A. A deep learning architecture to detect events in EEG signals during sleep. In Proceedings of the 2018 IEEE 28th International Workshop on Machine Learning for Signal Processing (MLSP), Aalborg, Denmark, 17–20 September 2018; pp. 1–6. [Google Scholar]
- Vallat, R.; Walker, M.P. An open-source, high-performance tool for automated sleep staging. Elife 2021, 10, e70092. [Google Scholar] [CrossRef]
- Hannesson, H.K. Evaluation of an Accessible Automatic Sleep Spindle Detector. Ph.D. Thesis, Frontiers in Human Neuroscience, Aalborg, Switzerland, 2015; p. 353. [Google Scholar]
- Sejdić, E.; Djurović, I.; Jiang, J. Time–frequency feature representation using energy concentration: An overview of recent advances. Digit. Signal Process. 2009, 19, 153–183. [Google Scholar] [CrossRef]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson correlation coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- Lim, Y.; Parker, S. FIR filter design over a discrete powers-of-two coefficient space. IEEE Trans. Acoust. Speech Signal Process. 1983, 31, 583–591. [Google Scholar]
- Choi, S.H.; Kwon, H.B.; Jin, H.W.; Yoon, H.; Lee, M.H.; Lee, Y.J.; Park, K.S. Long Short-Term Memory Networks for Unconstrained Sleep Stage Classification Using Polyvinylidene Fluoride Film Sensor. IEEE J. Biomed. Health Inform. 2020, 24, 3606–3615. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Qi, X.; Wang, H.; Liu, Z.; Wang, G.; Yan, X. A Multi-Class Automatic Sleep Staging Method Based on Long Short-Term Memory Network Using Single-Lead Electrocardiogram Signals. IEEE Access 2019, 7, 85959–85970. [Google Scholar] [CrossRef]
- Geng, D.; Yang, D.; Cai, M.; Zheng, L. A novel microwave treatment for sleep disorders and classification of sleep stages using multi-scale entropy. Entropy 2020, 22, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, Z.; Lan, K.; Liu, X.; Zhang, Z.; Li, P.; Cao, D.; Zheng, J.; Pan, J. Sleep Stage Classification Using Bidirectional LSTM in Wearable Multi-sensor Systems. IEEE Conf. Comput. Commun. Work. 2019, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.S.; Lin, C.L.; Ko, L.W.; Liu, S.Y.; Sua, T.P.; Lin, C.T. A hierarchical classification system for sleep stage scoring via forehead EEG signals. In Proceedings of the 2013 IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB), Singapore, 16–19 April 2013; pp. 1–5. [Google Scholar] [CrossRef]
- Penzel, T.; Kantelhardt, J.W.; Grote, L.; Peter, J.H.; Bunde, A. Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans. Biomed. Eng. 2003, 50, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackowska, M.; Dockray, S.; Hendrickx, H.; Steptoe, A. Psychosocial factors and sleep efficiency: Discrepancies between subjective and objective evaluations of sleep. Psychosom. Med. 2011, 73, 810–816. [Google Scholar] [CrossRef]
- Most, E.I.; Aboudan, S.; Scheltens, P.; Van Someren, E.J. Discrepancy between subjective and objective sleep disturbances in early-and moderate-stage Alzheimer disease. Am. J. Geriatr. Psychiatry 2012, 20, 460–467. [Google Scholar] [CrossRef]
- Perrault, A.A.; Khani, A.; Quairiaux, C.; Kompotis, K.; Franken, P.; Muhlethaler, M.; Schwartz, S.; Bayer, L. Whole-night continuous rocking entrains spontaneous neural oscillations with benefits for sleep and memory. Curr. Biol. 2019, 29, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Mônico-Neto, M.; Dáttilo, M.; Ribeiro, D.A.; Lee, K.S.; de Mello, M.T.; Tufik, S.; Antunes, H.K.M. REM sleep deprivation impairs muscle regeneration in rats. Growth Factors 2017, 35, 12–18. [Google Scholar] [CrossRef]
- Chennaoui, M.; Vanneau, T.; Trignol, A.; Arnal, P.; Gomez-Merino, D.; Baudot, C.; Perez, J.; Pochettino, S.; Eirale, C.; Chalabi, H. How does sleep help recovery from exercise-induced muscle injuries? J. Sci. Med. Sport 2021. [CrossRef] [PubMed]
- Eide, P.K.; Vinje, V.; Pripp, A.H.; Mardal, K.A.; Ringstad, G. Sleep deprivation impairs molecular clearance from the human brain. Brain 2021, 144, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.; McClintick, J.; Costlow, C.; Fortner, M.; White, J.; Gillin, J.C. Partial night sleep deprivation reduces natural killer and celhdar immune responses in humans. FASEB J. 1996, 10, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Coronado, E.G.; Pérez-Torres, A.; Pantaleón-Martínez, A.M.; Velazquéz-Moctezuma, J.; Rodriguez-Mata, V.; Morales-Montor, J. Innate immunity modulation in the duodenal mucosa induced by REM sleep deprivation during infection with Trichinella spirallis. Sci. Rep. 2017, 7, 1–14. [Google Scholar]
- Almondes, K.M.d.; Agudelo, H.A.M.; Correa, U.J. Impact of Sleep Deprivation on Emotional Regulation and the Immune System of Healthcare Workers as a Risk Factor for COVID 19: Practical Recommendations From a Task Force of the Latin American Association of Sleep Psychology. Front. Psychol. 2021, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Hancock, P.; Larner, A. Diagnostic utility of the Pittsburgh Sleep Quality Index in memory clinics. Int. J. Geriatr. Psychiatry 2009, 24, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Potvin, O.; Lorrain, D.; Forget, H.; Dubé, M.; Grenier, S.; Préville, M.; Hudon, C. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep 2012, 35, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.Y.; Han, H.J.; Shin, D.J.; Park, H.M.; Lee, Y.B.; Park, K.H. Sleep problems associated with behavioral and psychological symptoms as well as cognitive functions in Alzheimer’s disease. J. Clin. Neurol. 2014, 10, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.V.; Montazar, E.; Rezaei, S.; Hosseini, S.P. Sleep Quality and Cognitive Function in the Elderly Population. J. Sleep Sci. 2020, 5, 20–27. [Google Scholar] [CrossRef]
- Kulkarni, P.M.; Xiao, Z.; Robinson, E.J.; Jami, A.S.; Zhang, J.; Zhou, H.; Henin, S.E.; Liu, A.A.; Osorio, R.S.; Wang, J.; et al. A deep learning approach for real-time detection of sleep spindles. J. Neural Eng. 2019, 16, 036004. [Google Scholar] [CrossRef]
- Mikutta, C.; Feige, B.; Maier, J.G.; Hertenstein, E.; Holz, J.; Riemann, D.; Nissen, C. Phase-amplitude coupling of sleep slow oscillatory and spindle activity correlates with overnight memory consolidation. J. Sleep Res. 2019, 28, e12835. [Google Scholar] [CrossRef] [PubMed]
- Astill, R.G.; Piantoni, G.; Raymann, R.J.; Vis, J.C.; Coppens, J.E.; Walker, M.P.; Stickgold, R.; Van Der Werf, Y.D.; Van Someren, E.J. Sleep spindle and slow wave frequency reflect motor skill performance in primary school-age children. Front. Hum. Neurosci. 2014, 8, 910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, K.R.; Ray, L.; Smith, V.; Smith, C. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J. Sleep Res. 2008, 17, 23–33. [Google Scholar] [CrossRef]
- Ventouras, E.M.; Ktonas, P.Y.; Tsekou, H.; Paparrigopoulos, T.; Kalatzis, I.; Soldatos, C.R. Independent component analysis for source localization of EEG sleep spindle components. Comput. Intell. Neurosci. 2010. [Google Scholar] [CrossRef]
- Ventouras, E.M.; Ktonas, P.Y.; Tsekou, H.; Paparrigopoulos, T.; Kalatzis, I.; Soldatos, C.R. Slow and fast EEG sleep spindle component extraction using Independent Component Analysis. In Proceedings of the 2008 8th IEEE International Conference on BioInformatics and BioEngineering, Athens, Greece, 8–10 October 2008; pp. 1–6. [Google Scholar]
- Ventouras, E.M.; Alevizos, I.; Ktonas, P.Y.; Tsekou, H.; Paparrigopoulos, T.; Kalatzis, I.; Soldatos, C.R.; Nikiforidis, G. Independent components of sleep spindles. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 4002–4005. [Google Scholar]
- Backman, H.; Laajasalo, T.; Saukkonen, S.; Salmi, V.; Kivivuori, J.; Aronen, E.T. Are qualitative and quantitative sleep problems associated with delinquency when controlling for psychopathic features and parental supervision? J. Sleep Res. 2015, 24, 543–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åkerstedt, T.; Hume, K.; Minors, D.; Waterhouse, J. The subjective meaning of good sleep, an intraindividual approach using the Karolinska Sleep Diary. Percept. Mot. Skills 1994, 79, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Frauscher, B.; Ehrmann, L.; Högl, B. Defining muscle activities for assessment of rapid eye movement sleep behavior disorder: From a qualitative to a quantitative diagnostic level. Sleep Med. 2013, 14, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Choilek, S.; Karashima, A.; Motoike, I.; Katayama, N.; Kinoshita, K.; Nakao, M. Subjective sleep quality, quantitative sleep features, and their associations dependent on demographic characteristics, habitual sleep–wake patterns, and distinction of weekdays/weekends. Sleep Biol. Rhythm. 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Gupta, R.; Dahiya, S.; Bhatia, M.S. Effect of depression on sleep: Qualitative or quantitative? Indian J. Psychiatry 2009, 51, 117. [Google Scholar] [CrossRef] [PubMed]








| Wake (%) | N1 (%) | N2 (%) | N3 (%) | REM (%) | |
|---|---|---|---|---|---|
| Accuracy | 94.87 (±4.93) | 92.24 (±4.43) | 88.20 (±5.59) | 95.31 (±3.30) | 93.42 (±3.43) |
| Precision | 85.00 (±12.37) | 46.25 (±14.98) | 80.64 (±13.96) | 93.00 (±6.34) | 85.10 (±16.50) |
| Recall | 89.15 (±14.08) | 48.20 (±18.08) | 89.70 (±5.86) | 76.35 (±14.51) | 81.70 (±12.58) |
| F1 score | 85.60 (±10.54) | 44.00 (±12.90) | 84.45 (±9.43) | 83.05 (±9.44) | 81.40 (±12.26) |
| B Condition | A Condition | H Condition | |
|---|---|---|---|
| TIB [min] | 180 | 180 | 180 |
| TSP [min] | 171.4 (±11.74) | 172.67 (±7.63) | 177.47 (±4.55) |
| TST ★ [min] | 143.1 (±42.40) | 140.53(±25.2) | 118.7 (±42.50) |
| Sleep Length [min] | |||
| TN1 (N1)★ | 1.3(±0.99) | 1.77(±2.82) | 0.33(±0.48) * |
| TN2 (N2)★ | 78.3(±36.42) | 73.8(±29.92) | 67.2(±31.57) |
| TN3 (N3)★ | 21.8(±20.07) | 41.73(21.51) * | 36.33(±15.20) * |
| TN1+TN2 (Light) | 79.60(±36.37) | 75.56(±29.95) | 67.53(±37.76) |
| TR (REM)★ | 41.70(±43.14) | 23.23(±34.66) | 14.83(±29.91) * |
| Sleep Ratio [%] | |||
| N1% (N1) ★ | 0.89(±0.69) | 1.19(±1.71) | 0.24(±0.40) ** |
| N2% (N2)★ | 55.17(±25.02) | 53.26(±20.26) | 56.40(±15.19) |
| SWS% (N3) ★ | 18.46(±20.52) | 30.58(±15.85) * | 34.08(±15.19) ** |
| N1%+N2% (Light) | 56.05(±25.08) | 54.45(±20.02) | 56.64(±15.28) |
| REM% (REM) ★ | 25.48(±24.57) | 14.97 (±20.44) | 9.27(±16.30) * |
| Sleep Latencies [min] | |||
| To Light (N1+N2) | 21.70(±41.72) | 21.00(±9.79) | 35.60(±15.46) |
| To Deep (N3) | 44.43(±23.85) | 21.03(±10.10) * | 30.23(±30.58) |
| To REM | 16.56(±31.43) | 25.53(±43.35) | 27.00(±47.52) |
| Sleep Indices | |||
| SL% [%] | 57.54(±92.86) | 72.30(±69.66) | 69.15(±48.79) |
| SOL ★ [min] | 36.90(±42.40) | 39.47(±25.2) | 61.30(±42.50) |
| Sleep Efficiency (SE) ★ [%] | 79.50(±23.55) | 78.08(±14.15) | 65.94(±23.61) |
| B Condition | A Condition | H Condition | |
|---|---|---|---|
| Number of Spindle Event | 77.43 (±50.76) | 113.99 (±66.30) ** | 75.33 (±56.83) |
| Spindle Time (s) | 68.32 (±45.00) | 96.22 (±56.00) ** | 64.14 (±52.00) |
| Density (/30 s) | 0.2673 (±0.0240) | 0.1879 (±0.0159) ** | 0.1782 (±0.0207) |
| Duration (s) | 0.83 (±0.28) | 0.83 (±0.29) | 0.83 (±0.30) |
| Fast Spindles | |||
| Amplitude (v) | 56.29 (±35.50) | 60.78 (±41.69) | 65.18 (±59.76) |
| Frequency (Hz) | 12.79 (±0.54) | 12.79 (±0.60) | 12.76 (±0.57) |
| Slow Spindles | |||
| Amplitude (v) | 24.24 (±14.50) | 29.41 (±25.60) | 34.47 (±31.18) |
| Frequency (Hz) | 8.79 (±0.17) | 8.61 (±0.21) | 8.81 (±0.23) |
| B Condition | A Condition | H Condition | |
|---|---|---|---|
| Time in Bed (min) | 180 | 180 | 180 |
| Self-reported Survey Results | |||
| Sleep Latency (min) | 13.13 (±10.29) | 12.53 (±7.92) | 9.60 (±5.78) |
| Wake Time (min) | 30.67 (±19.22) | 28.93 (±15.31) | 31.87 (±16.91) |
| Sleep Time (min) | 149.33 (±19.22) | 151.07 (±15.31) | 148.13 (±16.91) |
| SE (Sleep Efficiency) | 0.83 (±0.11) | 0.84 (±0.06) | 0.82 (±0.09) |
| B Condition | A Condition | H Condition | ||
|---|---|---|---|---|
| Good Sleeper | SL% | 0.82 (±1.15) | 0.60 (±0.49) | 0.73 (±0.51) |
| (PSQI < 5) | SWS% ★ | 0.25 (±0.24) | 0.28 (±0.12) | 0.35 (±0.15) * |
| 9 subject | TN3 (min) ★ | 27.72 (±23.56) | 39.06 (±14.20) | 37.22 (±18.08) |
| Bad Sleeper | SL% | 0.21 (±0.17) | 0.91 (±0.95) | 0.61 (±0.50) |
| (PSQI ≥ 5) | SWS% ★ | 0.09 (±0.06) | 0.34 (±0.22) * | 0.32 (±0.14) * |
| 6 subject | TN3 (min) ★ | 12.92 (±9.04) | 45.75 (±30.67) * | 35.00 (±11.00) * |
| B Condition | A Condition | H Condition | ||
|---|---|---|---|---|
| Good Sleeper (PSQI < 5) | Latency (min) | 15.56 (±12.10) | 15.33 (±9.14) | 9.22 (±4.09) |
| 9 subject | Sleep time (min) | 154.44(±18.37) | 148.22 (±17.09) | 147.22 (±16.41) |
| Bad Sleeper (PSQI ≥ 5) | Latency (min) | 9.50 (±5.96) | 8.33 (±2.58) | 10.17 (±8.13) |
| 6 subject | Sleep time (min) | 141.67 (±19.41) | 155.33 (±12.36) | 149.50 (±19.11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.; Yu, H.; Roh, J.; Lee, J.; Sohn, I.; Kim, S.; Park, C. Effect of a Recliner Chair with Rocking Motions on Sleep Efficiency. Sensors 2021, 21, 8214. https://doi.org/10.3390/s21248214
Baek S, Yu H, Roh J, Lee J, Sohn I, Kim S, Park C. Effect of a Recliner Chair with Rocking Motions on Sleep Efficiency. Sensors. 2021; 21(24):8214. https://doi.org/10.3390/s21248214
Chicago/Turabian StyleBaek, Suwhan, Hyunsoo Yu, Jongryun Roh, Jungnyun Lee, Illsoo Sohn, Sayup Kim, and Cheolsoo Park. 2021. "Effect of a Recliner Chair with Rocking Motions on Sleep Efficiency" Sensors 21, no. 24: 8214. https://doi.org/10.3390/s21248214








