Clustering Accelerometer Activity Patterns from the UK Biobank Cohort
Abstract
:1. Introduction
2. Materials and Methods
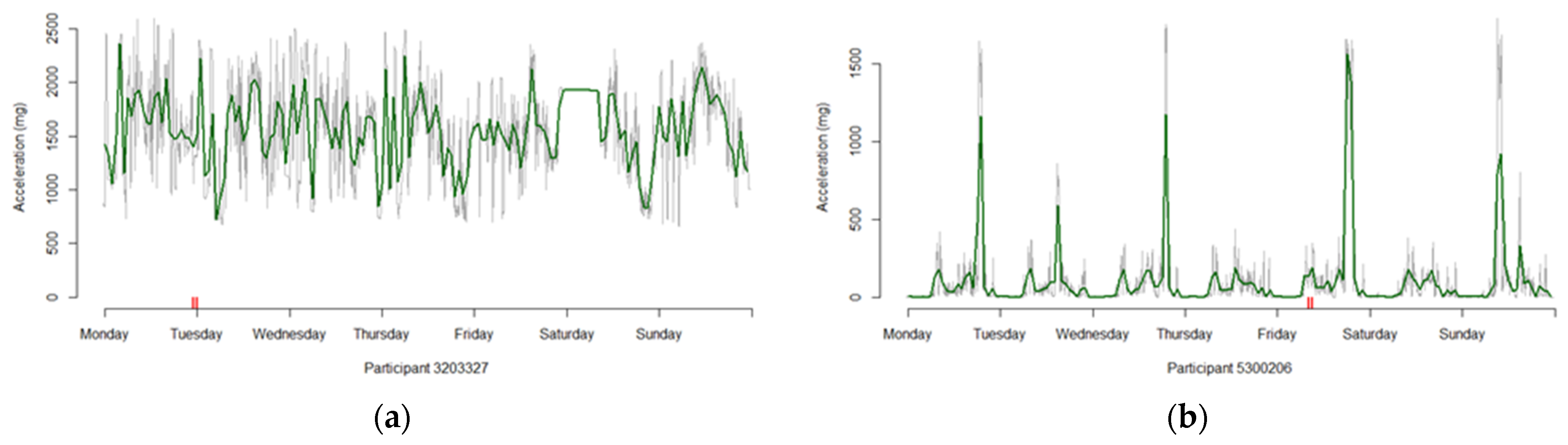
2.1. Activity Profile Selection
2.2. Clustering Algorithm Choice
2.3. Model Specification and Validation
2.4. Application of Activity Profiles to Health Outcomes
3. Results
3.1. Selection of Profiles
3.2. Comparison of Distance Metrics
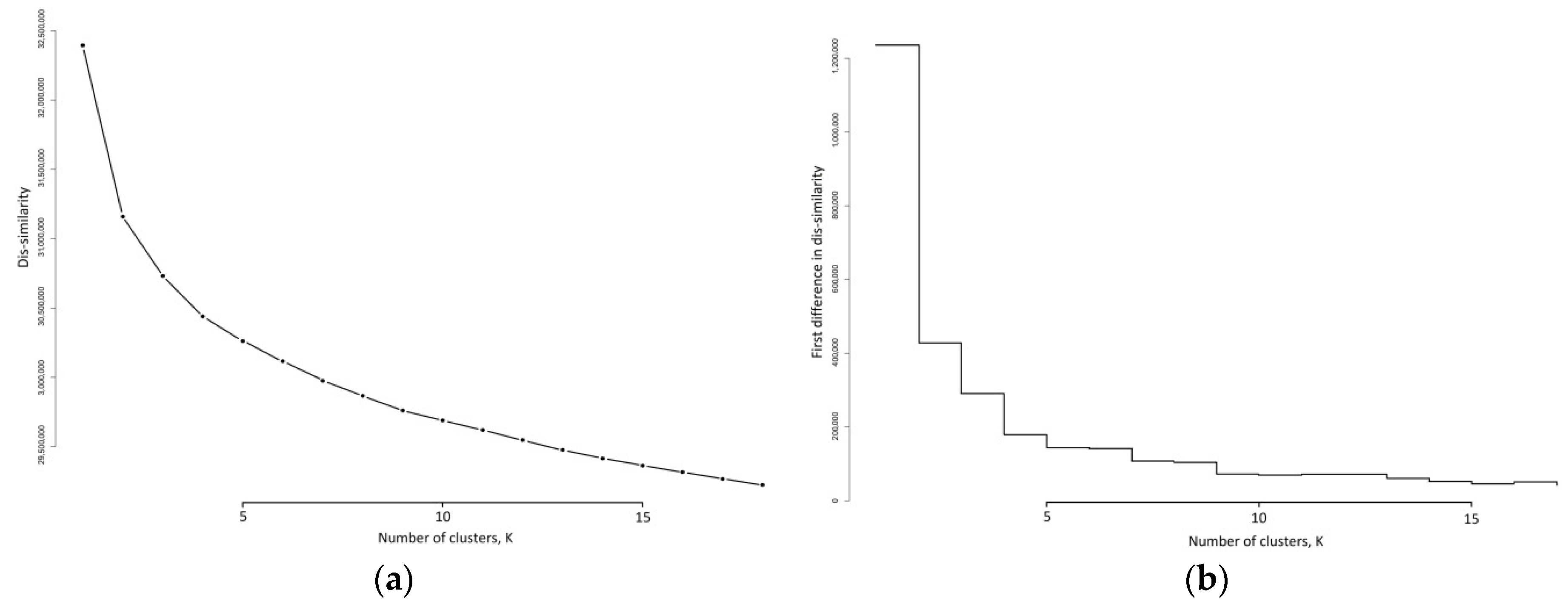
3.3. K-Medoids Clustering
3.4. Nine Cluster Solution Verification
3.5. Nine Cluster Solution Validation
3.6. Obesity Outcomes
3.7. COVID-19 Test Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Schrempft, S.; Jackowska, M.; Hamer, M.; Steptoe, A. Associations between social isolation, loneliness, and objective physical activity in older men and women. BMC Public Health 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.N.; Cohen, D.A. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48,440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Public Health England. Physical Activity: Applying All Our Health. 2019. Available online: https://www.gov.uk/government/publications/physical-activity-applying-all-our-health/physical-activity-applying-all-our-health (accessed on 28 October 2021).
- Rowlands, A.V.; Kloecker, D.E.; Chudasama, Y.; Davies, M.J.; Dawkins, N.P.; Edwardson, C.L.; Gillies, C.; Khunti, K.; Razieh, C.; Islam, N.; et al. Association of timing and balance of physical activity and rest/sleep with risk of COVID-19: A UK Biobank Study. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Sallis, J.F.; Saelens, B.E. Assessment of physical activity by self-report: Status, limitations, and future directions. Res. Q. Exerc. Sport 2000, 71 (Suppl. 2), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Gorber, S.C.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [Green Version]
- Doherty, A.; Jackson, D.; Hammerla, N.; Plötz, T.; Olivier, P.; Granat, M.H.; White, T.; Van Hees, V.T.; Trenell, M.I.; Owen, C.G.; et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS ONE 2017, 12, e0169649. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.; Strain, T.; Kim, Y.; Sharp, S.J.; Westgate, K.; Wijndaele, K.; Gonzales, T.; Wareham, N.J.; Brage, S. Estimating physical activity from self-reported behaviours in large-scale population studies using network harmonisation: Findings from UK Biobank and associations with disease outcomes. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, P.C.; Musicha, C.; Rowlands, A.V.; Davies, M.; Khunti, K.; Razieh, C.; Timmins, I.; Zaccardi, F.; Codd, V.; Nelson, C.P.; et al. Causal Associations of Self-Reported Walking Pace with Telomere Length in 405,981 middle-aged adults: A UK Biobank study. medRxiv 2021. [Google Scholar]
- Kim, Y.; White, T.; Wijndaele, K.; Sharp, S.J.; Wareham, N.J.; Brage, S. Adiposity and grip strength as long-term predictors of objectively measured physical activity in 93,015 adults: The UK Biobank study. Int. J. Obes. 2017, 41, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, R.; Doherty, A.; Smith-Byrne, K.; Rahimi, K.; Bennett, D.; Woodward, M.; Walmsley, R.; Dwyer, T. Accelerometer measured physical activity and the incidence of cardiovascular disease: Evidence from the UK Biobank cohort study. PLoS Med. 2021, 18, e1003487. [Google Scholar]
- Walmsley, R.; Chan, S.; Smith-Byrne, K.; Ramakrishnan, R.; Woodward, M.; Rahimi, K.; Dwyer, T.; Bennett, D.; Doherty, A. Reallocation of time between device-measured movement behaviours and risk of incident cardiovascular disease. Br. J. Sports Med. 2021. [Google Scholar] [CrossRef]
- O’Donnell, J.; Smith-Byrne, K.; Velardo, C.; Conrad, N.; Salimi-Khorshidi, G.; Doherty, A.; Dwyer, T.; Tarassenko, L.; Rahimi, K. Self-reported and objectively measured physical activity in people with and without chronic heart failure: UK Biobank analysis. Open Heart 2020, 7, e001099. [Google Scholar] [CrossRef] [Green Version]
- McDonald, L.; Oguz, M.; Carroll, R.; Thakkar, P.; Yang, F.; Dhalwani, N.; Cox, A.; Merinopoulou, E.; Malcolm, B.; Mehmud, F.; et al. Comparison of accelerometer-derived physical activity levels between individuals with and without cancer: A UK Biobank study. Future Oncol. 2019, 15, 3763–3774. [Google Scholar] [CrossRef]
- Guo, W.; Fensom, G.K.; Reeves, G.K.; Key, T.J. Physical activity and breast cancer risk: Results from the UK Biobank prospective cohort. Br. J. Cancer 2020, 122, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Zebin, T.; Peek, N.; Casson, A.J. Physical activity based classification of serious mental illness group participants in the UK Biobank using ensemble dense neural networks. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Manhattan, NY, USA, 2019. [Google Scholar]
- Dennison, C.A.; Legge, S.E.; Bracher-Smith, M.; Menzies, G.; Escott-Price, V.; Smith, D.J.; Doherty, A.R.; Owen, M.J.; O’Donovan, M.C.; Walters, J.T. Association of genetic liability for psychiatric disorders with accelerometer-assessed physical activity in the UK Biobank. PLoS ONE 2021, 16, e0249189. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.B.; Rosenbaum, S.; Ward, P.B.; Firth, J.A.; Sarris, J.; Yung, A.R. The Validity and Value of Self-reported Physical Activity and Accelerometry in People With Schizophrenia: A Population-Scale Study of the UK Biobank. Schizophr. Bull. 2018, 44, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Kandola, A.A.; del Pozo Cruz, B.; Osborn, D.P.J.; Stubbs, B.; Choi, K.W.; Hayes, J.F. Impact of replacing sedentary behaviour with other movement behaviours on depression and anxiety symptoms: A prospective cohort study in the UK Biobank. BMC Med. 2021, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Khunti, K.K.; Zaccardi, F.; Rowlands, A.V.; Yates, T.; Gillies, C.L.; Davies, M.J.; Dhalwani, N.N. Physical activity, multimorbidity, and life expectancy: A UK Biobank longitudinal study. BMC Med. 2019, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Leroux, A.; Xu, S.; Kundu, P.; Muschelli, J.; Smirnova, E.; Chatterjee, N.; Crainiceanu, C. Quantifying the Predictive Performance of Objectively Measured Physical Activity on Mortality in the UK Biobank. J. Gerontol. Ser. A 2021, 76, 1486–1494. [Google Scholar] [CrossRef]
- Gill, J.M.R. Linking volume and intensity of physical activity to mortality. Nat. Med. 2020, 26, 1332–1334. [Google Scholar] [CrossRef]
- Millard, L.A.C.; Tilling, K.; Gaunt, T.R.; Carslake, D.; Lawlor, D.A. Association of changing physical activity intensity and bout length with mortality: A study of 79,507 participants in UK Biobank. medRxiv 2020. [Google Scholar] [CrossRef]
- Jones, S.E.; van Hees, V.T.; Mazzotti, D.R.; Marques-Vidal, P.; Sabia, S.; van der Spek, A.; Dashti, H.S.; Engmann, J.; Kocevska, D.; Tyrrell, J.; et al. Genetic studies of accelerometer-based sleep measures yield new insights into human sleep behaviour. Nat. Commun. 2019, 10, 1585. [Google Scholar] [CrossRef]
- Zhu, G.; Catt, M.; Cassidy, S.; Birch-Machin, M.; Trenell, M.; Hiden, H.; Woodman, S.; Anderson, K.N. Objective sleep assessment in >80,000 UK mid-life adults: Associations with sociodemographic characteristics, physical activity and caffeine. PLoS ONE 2019, 14, e0226220. [Google Scholar] [CrossRef] [PubMed]
- Nikbakhtian, S.; Reed, A.B.; Obika, B.D.; Morelli, D.; Cunningham, A.C. Accelerometer-Derived Sleep Onset Timing and Cardiovascular Disease Incidence: A UK Biobank Cohort Study. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3857637 (accessed on 28 October 2021).
- Lyall, L.M.; Wyse, C.A.; Graham, N.; Ferguson, A.; Lyall, D.M.; Cullen, B.; Morales, C.A.C.; Biello, S.M.; Mackay, D.; Ward, J.; et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: A cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry 2018, 5, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Pocuca, N.; Farrell, M.; McNicholas, P.D. Defying the Circadian Rhythm: Clustering Participant Telemetry in the UK Biobank Data. arXiv 2020, arXiv:2011.08350. [Google Scholar]
- Smith, L.; Panter, J.; Ogilvie, D. Characteristics of the environment and physical activity in midlife: Findings from UK Biobank. Prev. Med. 2019, 118, 150–158. [Google Scholar] [CrossRef]
- Hajna, S.; White, T.; Panter, J.; Brage, S.; Wijndaele, K.; Woodcock, J.; Ogilvie, D.; Imamura, F.; Griffin, S.J. Driving status, travel modes and accelerometer-assessed physical activity in younger, middle-aged and older adults: A prospective study of 90 810 UK Biobank participants. Int. J. Epidemiol. 2019, 48, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wijndaele, K.; Sharp, S.J.; Strain, T.; Pearce, M.; White, T.; Wareham, N.; Brage, S. Specific physical activities, sedentary behaviours and sleep as long-term predictors of accelerometer-measured physical activity in 91,648 adults: A prospective cohort study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Wrobel, J.; Muschelli, J.; Leroux, A. Diurnal Physical Activity Patterns across Ages in a Large UK Based Cohort: The UK Biobank Study. Sensors 2021, 21, 1545. [Google Scholar] [CrossRef]
- Woodbridge, S.P.; Aung, N.; Paiva, J.M.; Sanghvi, M.M.; Zemrak, F.; Fung, K.; Petersen, S.E. Physical activity and left ventricular trabeculation in the UK Biobank community-based cohort study. Heart 2019, 105, 990–998. [Google Scholar] [CrossRef] [Green Version]
- Celis-Morales, C.A.; Lyall, D.M.; Petermann, F.; Anderson, J.; Ward, J.; Iliodromiti, S.; Mackay, D.F.; Welsh, P.; Bailey, M.E.; Pell, J.; et al. Do physical activity, commuting mode, cardiorespiratory fitness and sedentary behaviours modify the genetic predisposition to higher BMI? Findings from a UK Biobank study. Int. J. Obes. 2019, 43, 1526–1538. [Google Scholar]
- Schneider, C.V.; Zandvakili, I.; Thaiss, C.A.; Schneider, K.M. Physical activity is associated with reduced risk of liver disease in the prospective UK Biobank cohort. JHEP Rep. 2021, 3, 100263. [Google Scholar] [CrossRef]
- Williamson, J.R.; Telfer, B.; Mullany, R.; Friedl, K.E. Detecting Parkinson’s Disease from Wrist-Worn Accelerometry in the UK Biobank. Sensors 2021, 21, 2047. [Google Scholar] [CrossRef] [PubMed]
- Stiles, V.H.; Metcalf, B.S.; Knapp, K.M.; Rowlands, A.V. A small amount of precisely measured high-intensity habitual physical activity predicts bone health in pre- and post-menopausal women in UK Biobank. Int. J. Epidemiol. 2017, 46, 1847–1856. [Google Scholar] [CrossRef] [Green Version]
- Barker, J.; Smith Byrne, K.; Doherty, A.; Foster, C.; Rahimi, K.; Ramakrishnan, R.; Woodward, M.; Dwyer, T. Physical activity of UK adults with chronic disease: Cross-sectional analysis of accelerometer-measured physical activity in 96,706 UK Biobank participants. Int. J. Epidemiol. 2019, 48, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.; Fuller, H.; Chau, J.; Catt, M.; Bauman, A.; Trenell, M.I. Accelerometer-derived physical activity in those with cardio-metabolic disease compared to healthy adults: A UK Biobank study of 52,556 participants. Acta Diabetol. 2018, 55, 975–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strain, T.; Wijndaele, K.; Dempsey, P.C.; Sharp, S.J.; Pearce, M.; Jeon, J.; Lindsay, T.; Wareham, N.; Brage, S. Wearable-device-measured physical activity and future health risk. Nat. Med. 2020, 26, 1385–1391. [Google Scholar] [CrossRef]
- Pan, F.; Byrne, K.S.; Ramakrishnan, R.; Ferreira, M.; Dwyer, T.; Jones, G. Association between musculoskeletal pain at multiple sites and objectively measured physical activity and work capacity: Results from UK Biobank study. J. Sci. Med. Sport 2019, 22, 444–449. [Google Scholar] [CrossRef]
- World Health Organisation. Obesity and Overweight. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 October 2021).
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef] [Green Version]
- Galante, J.; Adamska, L.; Young, A.; Young, H.; Littlejohns, T.J.; Gallacher, J.; Allen, N. The acceptability of repeat Internet-based hybrid diet assessment of previous 24-h dietary intake: Administration of the Oxford WebQ in UK Biobank. Br. J. Nutr. 2016, 115, 681–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskar, A.; Katikireddi, V.; Niedzwiedz, C.; Mutambudzi, M.; Flowers, P.; Hunt, K.; Demou, E. P65 How Are Occupational Histories Associated with Self-Rated Health in Middle-Aged Adults? A Cross-Sectional Analysis of Retrospective UK Biobank Data; BMJ Publishing Group Ltd.: London, UK, 2020. [Google Scholar]
- Davis, K.A.; Coleman, J.R.; Adams, M.; Allen, N.; Breen, G.; Cullen, B.; Dickens, C.; Fox, E.; Graham, N.; Holliday, J.; et al. Mental health in UK Biobank—Development, implementation and results from an online questionnaire completed by 157 366 participants: A reanalysis. BJPsych Open 2020, 6, e18. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Alcantara, J.; Leal-Martín, J.; Mañas, A.; Ara, I.; Glynn, N.W.; Shiroma, E.J. Calibration and Cross-Validation of Accelerometer Cut-Points to Classify Sedentary Time and Physical Activity from Hip and Non-Dominant and Dominant Wrists in Older Adults. Sensors 2021, 21, 3326. [Google Scholar] [CrossRef] [PubMed]
- Willetts, M.; Hollowell, S.; Aslett, L.; Holmes, C.; Doherty, A. Statistical machine learning of sleep and physical activity phenotypes from sensor data in 96,220 UK Biobank participants. Sci. Rep. 2018, 8, 7961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berndt, D.J.; Clifford, J. Using dynamic time warping to find patterns in time series. In KDD Workshop; Association for the Advancement of Artificial Intelligence: Palo Alto, CA, USA, 1994. [Google Scholar]
- Kate, R.J. Using dynamic time warping distances as features for improved time series classification. Data Min. Knowl. Discov. 2016, 30, 283–312. [Google Scholar] [CrossRef]
- Müller, M. Dynamic Time Warping. In Information Retrieval for Music and Motion; Springer: Berlin/Heidelberg, Germany, 2007; pp. 69–84. [Google Scholar]
- Genolini, C.; Ecochard, R.; Benghezal, M.; Driss, T.; Andrieu, S.; Subtil, F. kmlShape: An efficient method to cluster longitudinal data (time-series) according to their shapes. PLoS ONE 2016, 11, e0150738. [Google Scholar]
- Rdusseeun, L.; Kaufman, P. Clustering by means of medoids. In Proceedings of the Statistical Data Analysis Based on the L1 Norm Conference, Neuchatel, Switzerland, 31 August 1987. [Google Scholar]
- Schubert, E.; Rousseeuw, P.J. Faster k-medoids clustering: Improving the PAM, CLARA, and CLARANS algorithms. In International Conference on Similarity Search and Applications; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Mouselimis, L. ClusterR: Gaussian Mixture Models, K-Means, Mini-Batch-Kmeans, K-Medoids and Affinity Propagation Clustering. 2020. Available online: https://mlampros.github.io/ClusterR/ (accessed on 28 October 2021).
- Everitt, B.; Landau, S.; Leese, M. Cluster Analysis, 4th ed.; Arnold: London, UK, 2001. [Google Scholar]
- Gale, C.G.; Singleton, A.; Bates, A.G.; Longley, P.A. Creating the 2011 area classification for output areas (2011 OAC). J. Spat. Inf. Sci. 2016, 12, 1–27. [Google Scholar] [CrossRef]
- CACI. The Wellbeing Acorn User Guide. 2021. Available online: http://caci.co.uk/wp-content/uploads/2021/07/Wellbeing_Acorn_User_Guide.pdf (accessed on 28 October 2021).
- Notthoff, N.; Reisch, P.; Gerstorf, D. Individual Characteristics and Physical Activity in Older Adults: A Systematic Review. Gerontology 2017, 63, 443–459. [Google Scholar] [CrossRef] [Green Version]
- van Uffelen, J.G.; Khan, A.; Burton, N.W. Gender differences in physical activity motivators and context preferences: A population-based study in people in their sixties. BMC Public Health 2017, 17, 624. [Google Scholar] [CrossRef]
- O’donoghue, G.; Perchoux, C.; Mensah, K.; Lakerveld, J.; Van Der Ploeg, H.; Bernaards, C.; Chastin, S.F.; Simon, C.; O’gorman, D.; Nazare, J.A. A systematic review of correlates of sedentary behaviour in adults aged 18-65 years: A socio-ecological approach. BMC Public Health 2016, 16, 163. [Google Scholar] [CrossRef] [Green Version]
- Pontin, F.; Lomax, N.; Clarke, G.; Morris, M.A. Socio-demographic determinants of physical activity and app usage from smartphone data. Soc. Sci. Med. 2021, 284, 114235. [Google Scholar] [CrossRef]
- Pontin, F.; Lomax, N.; Clarke, G.; Morris, M.A. Characterisation of temporal patterns in step count behaviour from smartphone app data: An unsupervised machine learning approach. Int. J. Environ. Res. Public Health 2021, 18, 11476. [Google Scholar] [CrossRef]
- Hemmingsson, E.; Ekelund, U. Is the association between physical activity and body mass index obesity dependent? Int. J. Obes. 2007, 31, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Butland, B.; Jebb, S.; Kopelman, P.; McPherson, K. Tackling Obesities: Future Choices-Project Report. 2007. Available online: https://www.gov.uk/government/publications/reducing-obesity-future-choices (accessed on 28 October 2021).
- Yates, T.; Razieh, C.; Zaccardi, F.; Davies, M.J.; Khunti, K. Obesity and risk of COVID-19: Analysis of UK biobank. Prim. Care Diabetes 2020, 14, 566–567. [Google Scholar] [CrossRef]
- Christensen, R.A.; Sturrock, S.L.; Arneja, J.; Brooks, J.D. Measures of Adiposity and Risk of Testing Positive for SARS-CoV-2 in the UK Biobank Study. J. Obes. 2021, 2021, 8837319. [Google Scholar] [CrossRef]
- Niedzwiedz, C.L.; O’Donnell, C.A.; Jani, B.D.; Demou, E.; Ho, F.K.; Celis-Morales, C.; Nicholl, B.I.; Mair, F.S.; Welsh, P.; Sattar, N.; et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: Prospective cohort study using UK Biobank. BMC Med. 2020, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chadeau-Hyam, M.; Bodinier, B.; Elliott, J.; Whitaker, M.D.; Tzoulaki, I.; Vermeulen, R.; Kelly-Irving, M.; Delpierre, C.; Elliott, P. Risk factors for positive and negative COVID-19 tests: A cautious and in-depth analysis of UK biobank data. Int. J. Epidemiol. 2020, 49, 1454–1467. [Google Scholar] [CrossRef] [PubMed]
- NHS Digital. COVID-19—High Risk Shielded Patient List Identification Methodology. 2020. Available online: https://digital.nhs.uk/coronavirus/shielded-patient-list/methodology (accessed on 28 October 2021).
- Mitze, T.; Kosfeld, R. The propagation effect of commuting to work in the spatial transmission of COVID-19. J. Geogr. Syst. 2021, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.V.; Dempsey, P.C.; Gillies, C.; Kloecker, D.E.; Razieh, C.; Chudasama, Y.; Islam, N.; Zaccardi, F.; Lawson, C.; Norris, T.; et al. Association between accelerometer-assessed physical activity and severity of COVID-19 in UK Biobank. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 997–1007. [Google Scholar] [CrossRef]
- Hicks, J.L.; Althoff, T.; Kuhar, P.; Bostjancic, B.; King, A.C.; Leskovec, J.; Delp, S.L. Best practices for analyzing large-scale health data from wearables and smartphone apps. NPJ Digit. Med. 2019, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.; Catt, M.; Cassidy, S.; Bacardit, J.; Darke, P.; Butterfield, S.; Alshabrawy, O.; Trenell, M.; Missier, P. Using Wearable Activity Trackers to Predict Type 2 Diabetes: Machine Learning–Based Cross-sectional Study of the UK Biobank Accelerometer Cohort. JMIR Diabetes 2021, 6, e23364. [Google Scholar] [CrossRef]
- Jones, P.; Mirkes, E.M.; Yates, T.; Edwardson, C.L.; Catt, M.; Davies, M.J.; Khunti, K.; Rowlands, A.V. Towards a Portable Model to Discriminate Activity Clusters from Accelerometer Data. Sensors 2019, 19, 4504. [Google Scholar] [CrossRef] [Green Version]
- Althoff, T.; Sosic, R.; Hicks, J.L.; King, A.C.; Delp, S.L.; Leskovec, J. Large-scale physical activity data reveal worldwide activity inequality. Nature 2017, 547, 336–339. [Google Scholar] [CrossRef]
- Wheeler, B. Documentation of Environmental Indicators Attributed to Participants Based on Home Location Grid References. 2017. Available online: https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/App15374Docs.pdf (accessed on 28 October 2021).
- Sevtsuk, A.; Ratti, C. Does urban mobility have a daily routine? Learning from the aggregate data of mobile networks. J. Urban Technol. 2010, 17, 41–60. [Google Scholar] [CrossRef]
- Jiang, S.; Ferreira, J.; González, M.C. Clustering daily patterns of human activities in the city. Data Min. Knowl. Discov. 2012, 25, 478–510. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Koutsopoulos, H.N.; Zhao, J. Discovering latent activity patterns from transit smart card data: A spatiotemporal topic model. Transp. Res. Part C Emerg. Technol. 2020, 116, 102627. [Google Scholar] [CrossRef]


| Characteristic | Clustering Sample | Accelerometer Sample | UK Biobank |
|---|---|---|---|
| Female | 56.5% | 56.2% | 54.4% |
| Younger, 40 to 54 | 39.6% | 39.1% | 38.8% |
| White/British | 96.6% | 96.4% | 94.1% |
| In paid employment or self-employed | 61.8% | 62.3% | 57.4% |
| Non-Car/motor vehicle commute | 39.9% | 40.0% | 63.0% |
| College/University | 42.9% | 43.1% | 32.1% |
| School qualifications | 37.5% | 37.4% | 37.3% |
| Income of more than £31,000 | 54.8% | 55.1% | 43.9% |
| Least 20% deprived | 44.5% | 44.2% | 39.9% |
| Healthy BMI | 38.7% | 38.6% | 32.3% |
| Excellent/good health | 81.5% | 81.3% | 73.8% |
| No Long standing illness | 70.1% | 70.1% | 65.6% |
| Very/Fairly easy to get up | 82.2% | 82.1% | 81.0% |
| Definitely/more a ‘morning’ person | 56.5% | 56.2% | 55.3% |
| Spring/summer accelerometer wear | 48.8% | 49.2% | NA |
| N | 91,533 | 103,332 | 500,028 |
| Characteristic | Active 9 to 5 | Active | Morning Movers | Get Up and Active | Live for the Weekend | Moderates | Leisurely 9 to 5 | Sedate | Inactive | Clustering Sample |
|---|---|---|---|---|---|---|---|---|---|---|
| Female | 61.1% | 60.2% | 59.5% | 61.2% | 56.0% | 58.2% | 49.5% | 52.8% | 45.3% | 56.5% |
| Younger, 40 to 54 | 67.8% | 46.4% | 32.6% | 31.6% | 49.6% | 35.8% | 66.1% | 18.0% | 26.2% | 39.6% |
| White/British | 95.4% | 97.2% | 97.5% | 97.2% | 96.3% | 95.9% | 94.1% | 98.0% | 96.5% | 96.6% |
| In paid employment or self-employed | 81.6% | 65.3% | 55.8% | 56.3% | 71.1% | 59.2% | 85.8% | 44.7% | 47.8% | 61.8% |
| Non-car/motor vehicle commute | 46.6% | 40.6% | 36.5% | 41.3% | 39.2% | 39.3% | 41.9% | 34.4% | 36.3% | 39.9% |
| Attended college/university | 46.9% | 41.9% | 39.2% | 44.3% | 46.1% | 44.8% | 46.5% | 37.4% | 39.6% | 42.9% |
| School qualifications | 39.3% | 40.6% | 38.8% | 36.2% | 37.6% | 36.7% | 38.0% | 36.8% | 35.2% | 37.5% |
| Income of more than GBP 31,000/year | 66.0% | 56.3% | 52.3% | 52.9% | 62.4% | 51.7% | 67.2% | 44.7% | 43.6% | 54.8% |
| Lives in east 20% deprived neighbourhood | 43.4% | 46.5% | 47.7% | 47.3% | 45.2% | 41.6% | 39.1% | 46.8% | 38.1% | 44.5% |
| Healthy BMI | 55.8% | 54.1% | 43.5% | 42.5% | 41.1% | 33.6% | 33.7% | 27.2% | 20.1% | 38.7% |
| Excellent/good health | 89.8% | 88.7% | 86.5% | 84.9% | 85.0% | 77.4% | 80.4% | 76.6% | 60.9% | 81.5% |
| No long standing illness | 81.5% | 77.7% | 74.6% | 71.4% | 74.9% | 66.3% | 72.7% | 62.5% | 48.1% | 70.1% |
| Very/fairly easy to get up | 84.9% | 81.1% | 85.4% | 85.1% | 84.6% | 72.5% | 82.7% | 84.4% | 75.1% | 82.2% |
| Definitely/more a morning person | 65.7% | 52.5% | 60.2% | 60.5% | 61.7% | 38.8% | 63.1% | 56.6% | 44.6% | 56.5% |
| Spring/summer accelerometer wear | 54.4% | 52.3% | 49.5% | 48.4% | 51.0% | 47.0% | 49.7% | 44.4% | 44.9% | 48.8% |
| N (%) | 8313 (9.1%) | 5975 (6.5%) | 10,758 (11.8%) | 15,154 (16.6%) | 12,395 (13.5%) | 11,037 (12.1%) | 8064 (8.8%) | 13,050 (14.3%) | 6787 (7.4%) | 91,533 (100%) |
| Active 9 to 5 | Active | Morning Movers | Get Up and Active | Live for the Weekend | Moderates | Leisurely 9 to 5 | Sedate | Inactive | |
|---|---|---|---|---|---|---|---|---|---|
| Healthy | 13.1% (+4.0%) | 9.1% (+2.6%) | 13.2% (+1.5%) | 18.2% (+1.6%) | 14.4% (+0.8%) | 10.5% (−1.6%) | 7.7% (−1.1%) | 10.0% (−4.2%) | 3.8% (−3.6%) |
| Overweight | 7.6% (−1.5%) | 5.8% (−0.7%) | 12.0% (+0.3%) | 16.8% (+0.3%) | 13.8% (+0.2%) | 12.6% (+0.6%) | 8.8% (0.0%) | 15.6% (+1.4%) | 7.0% (−0.4%) |
| Obese | 4.0% (−5.0%) | 2.8% (−3.7%) | 8.4% (−3.4%) | 12.9% (−3.7%) | 11.4% (−2.1%) | 14.1% (+2.1%) | 11.1% (+2.3%) | 19.9% (+5.7%) | 15.3% (+7.9%) |
| All | 9.1% | 6.5% | 11.8% | 16.6% | 13.5% | 12.1% | 8.8% | 14.3% | 7.4% |
| COVID-19 Outcomes | Active 9 to 5 | Active | Morning Movers | Get Up and Active | Live for the Weekend | Moderates | Leisurely 9 to 5 | Sedate | Inactive | Clustering Sample | Wearable Sample | UK Biobank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alive on 23 March 2021 | 8215 | 5891 | 10,534 | 14,801 | 12,153 | 10,696 | 7897 | 12,455 | 6205 | 88,847 | 100,292 | 465,472 |
| % Participants alive | 98.8% | 98.6% | 97.9% | 97.7% | 98.0% | 96.9% | 97.9% | 95.4% | 91.4% | 97.1% | 97.1% | 93.1% |
| % Participants alive and tested | 16.1% | 14.4% | 16.1% | 16.6% | 17.3% | 17.9% | 17.1% | 18.0% | 20.8% | 17.1% | 17.2% | 18.6% |
| % Participants alive and tested positive | 3.7% | 2.9% | 2.8% | 2.4% | 3.1% | 2.8% | 4.0% | 2.3% | 3.0% | 2.9% | 3.0% | 3.7% |
| Positive rate | 23.20% | 20.4% | 17.2% | 14.6% | 17.8% | 15.7% | 23.19% | 12.8% | 14.4% | 17.0% | 17.2% | 20.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, S.; Lomax, N.; Morris, M.; Pontin, F.; Birkin, M. Clustering Accelerometer Activity Patterns from the UK Biobank Cohort. Sensors 2021, 21, 8220. https://doi.org/10.3390/s21248220
Clark S, Lomax N, Morris M, Pontin F, Birkin M. Clustering Accelerometer Activity Patterns from the UK Biobank Cohort. Sensors. 2021; 21(24):8220. https://doi.org/10.3390/s21248220
Chicago/Turabian StyleClark, Stephen, Nik Lomax, Michelle Morris, Francesca Pontin, and Mark Birkin. 2021. "Clustering Accelerometer Activity Patterns from the UK Biobank Cohort" Sensors 21, no. 24: 8220. https://doi.org/10.3390/s21248220
APA StyleClark, S., Lomax, N., Morris, M., Pontin, F., & Birkin, M. (2021). Clustering Accelerometer Activity Patterns from the UK Biobank Cohort. Sensors, 21(24), 8220. https://doi.org/10.3390/s21248220







