Wearable GPS and Accelerometer Technologies for Monitoring Mobility and Physical Activity in Neurodegenerative Disorders: A Systematic Review
Abstract
:1. Introduction
1.1. Neurodegenerative Disorders
1.2. Mobility and Physical Activity as an Invaluable Predictor for NDD
1.3. Current Solution to the Mobility Problem
1.4. Processing Tools and Algorithms Used for Data Analysis
1.5. Contribution of This Paper to the Literature
- To identify to what extent GPS and accelerometer-derived measures have been used as biomarkers of mobility and PA in clinical research related to neurogenerative disease;
- To describe the outcome measures reported from these patient populations;
- To identify studies that have established a relationship between neurodegenerative disease and sensor-derived mobility and PA data.
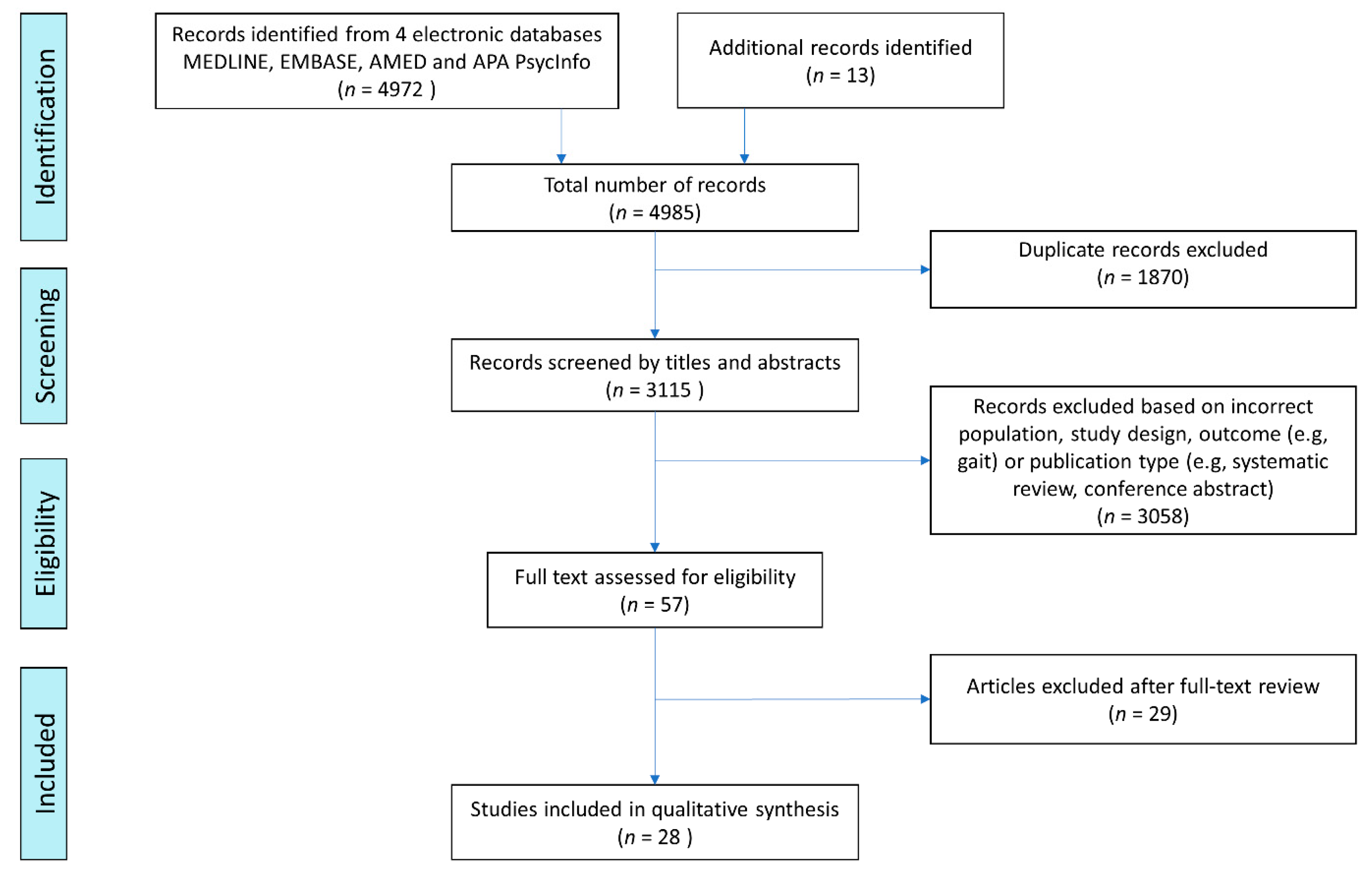
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility
2.3. Study Selection
2.4. Data Extraction and Study Synthesis
3. Results
3.1. Study Characteristics
3.2. Use of Wearable GPS to Measure Mobility in NNDs
3.3. Use of Accelerometry to Measure PA in NDDs
4. Discussion
4.1. Summary of Overall Findings
4.2. Current Usage of GPS and Accelerometers and Their Potential
4.3. Strengths, Limitations, and Acceptability of Wearable GPS
4.4. Strength, Limitation and Acceptability of Wearable Accelerometers
4.5. Future Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Tiwari, S.; Venkata, A.; Kaushik, A.; Adriana, Y.; Nair, M. Alzheimer’ s Disease Diagnostics and Therapeutics Market. Int. J. Nanomed. 2019, 2019, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol. 2013, 47, 495–508. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G. The diagnosis of dementia due to Alzheimer’s disease. Alzheimers Dement. 2012, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, S.B.; Nilsson, M.H.; Lexell, J.; Carlsson, G. Experiences of fear of falling in persons with Parkinson’s disease—A qualitative study. BMC Geriatr. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Young, Y.; Papenkov, M.; Nakashima, T. Who Is Responsible? A Man with Dementia Wanders From Home, Is Hit by a Train, and Dies. J. Am. Med. Dir. Assoc. 2018, 19, 563–567. [Google Scholar] [CrossRef]
- Zajac, J.A.; Cavanaugh, J.T.; Baker, T.; Colón-Semenza, C.; DeAngelis, T.R.; Duncan, R.P.; Fulford, D.; LaValley, M.; Nordahl, T.; Rawson, K.S.; et al. Are mobile persons with Parkinson disease necessarily more active? J. Neurol. Phys. Ther. 2021, 45, 259–265. [Google Scholar] [CrossRef]
- Sakata, N.; Okumura, Y. Job Loss after Diagnosis of Early-Onset Dementia: A Matched Cohort Study. J. Alzheimer’s Dis. 2017, 60, 1231–1235. [Google Scholar] [CrossRef]
- Fillekes, M.P.; Giannouli, E.; Kim, E.-K.; Zijlstra, W.; Weibel, R. Towards a comprehensive set of GPS-based indicators reflecting the multidimensional nature of daily mobility for applications in health and aging research. Int. J. Health Geogr. 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Fillekes, M.P.; Röcke, C.; Katana, M.; Weibel, R. Self-reported versus GPS-derived indicators of daily mobility in a sample of healthy older adults. Soc. Sci. Med. 2019, 220, 193–202. [Google Scholar] [CrossRef]
- Tung, J.Y.; Rose, R.V.; Gammada, E.; Lam, I.; Roy, E.A.; Black, S.E.; Poupart, P. Measuring life space in older adults with mild-to-moderate Alzheimer’s disease using mobile phone GPS. Gerontology 2014, 60, 154–162. [Google Scholar] [CrossRef]
- Fillekes, M.P.; Kim, E.-K.; Trumpf, R.; Zijlstra, W.; Giannouli, E.; Weibel, R. Assessing Older Adults’ Daily Mobility: A Comparison of GPS-Derived and Self-Reported Mobility Indicators. Sensors 2019, 19, 4551. [Google Scholar] [CrossRef]
- Zhu, L.; Duval, C.; Boissy, P.; Montero-Odasso, M.; Zou, G.; Jog, M.; Speechley, M. Comparing GPS-Based Community Mobility Measures with Self-report Assessments in Older Adults with Parkinson’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Boissy, P.; Blamoutier, M.; Brière, S.; Duval, C. Quantification of Free-Living Community Mobility in Healthy Older Adults Using Wearable Sensors. Front. Public Health 2018, 6, 216. [Google Scholar] [CrossRef] [PubMed]
- Thorp, J.E.; Adamczyk, P.G.; Ploeg, H.-L.; Pickett, K.A. Monitoring Motor Symptoms During Activities of Daily Living in Individuals With Parkinson’s Disease. Front. Neurol. 2018, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.J.; Lu, Z.; Jareonsettasin, P.; Antoniades, C.A. Quantifying motor impairment in movement disorders. Front. Neurosci. 2018, 12, 202. [Google Scholar] [CrossRef]
- Lord, S.; Rochester, L.; Baker, K.; Nieuwboer, A. Concurrent validity of accelerometry to measure gait in Parkinsons Disease. Gait Posture 2008, 27, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Hassett, L.M.; Nguy, V.; Allen, N.E. A Comparison of Activity Monitor Data from Devices Worn on the Wrist and the Waist in People with Parkinson’s Disease. Mov. Disord. Clin. Pract. 2019, 6, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Tăuţan, A.M.; Ionescu, B.; Santarnecchi, E. Artificial intelligence in neurodegenerative diseases: A review of available tools with a focus on machine learning techniques. Artif. Intell. Med. 2021, 117, 102081. [Google Scholar] [CrossRef] [PubMed]
- Ravi, D.; Wong, C.; Lo, B.; Yang, G.-Z. A Deep Learning Approach to on-Node Sensor Data Analytics for Mobile or Wearable Devices. IEEE J. Biomed. Health Inform. 2017, 21, 56–64. [Google Scholar] [CrossRef]
- Qadri, Y.A.; Nauman, A.; Zikria, Y.B.; Vasilakos, A.V.; Kim, S.W. The Future of Healthcare Internet of Things: A Survey of Emerging Technologies. IEEE Commun. Surv. Tutorials 2020, 22, 1121–1167. [Google Scholar] [CrossRef]
- Moyle, W.; Jones, C.; Murfield, J.; Draper, B.; Beattie, E.; Shum, D.; Thalib, L.; O’Dwyer, S.; Mervin, C.M. Levels of physical activity and sleep patterns among older people with dementia living in long-term care facilities: A 24-h snapshot. Maturitas 2017, 102, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 1–9. [Google Scholar]
- van Uem, J.M.T.; Cerff, B.; Kampmeyer, M.; Prinzen, J.; Zuidema, M.; Hobert, M.A.; Graber, S.; Berg, D.; Maetzler, W.; Liepelt-Scarfone, I. The association between objectively measured physical activity, depression, cognition, and health-related quality of life in Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 48, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Kelly, V.E. Quantifying physical activity in early Parkinson disease using a commercial activity monitor. Parkinsonism Relat. Disord. 2019, 66, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Mantri, S.; Wood, S.; Duda, J.E.; Morley, J.F. Comparing self-reported and objective monitoring of physical activity in Parkinson disease. Parkinsonism Relat. Disord. 2019, 67, 56–59. [Google Scholar] [CrossRef]
- Nero, H.; Benka Wallén, M.; Franzén, E.; Ståhle, A.; Hagströmer, M. Accelerometer Cut Points for Physical Activity Assessment of Older Adults with Parkinson’s Disease. PLoS ONE 2015, 10, e0135899. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, P.; Gräber, S.; Schaeffer, E.; van Lummel, R.; Berg, D.; Maetzler, W.; Liepelt-Scarfone, I. Cognitive impairment and sedentary behavior predict health-related attrition in a prospective longitudinal Parkinson’s disease study. Park. Relat. Disord. 2021, 82, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; Watts, A. Daily Physical Activity Patterns during the Early Stage of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 55, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Nero, H.; Benka Wallén, M.; Franzén, E.; Conradsson, D.; Ståhle, A.; Hagströmer, M. Objectively Assessed Physical Activity and its Association with Balance, Physical Function and Dyskinesia in Parkinson’s Disease. J. Parkinsons. Dis. 2016, 6, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Klenk, J.; Srulijes, K.; Schatton, C.; Schwickert, L.; Maetzler, W.; Becker, C.; Synofzik, M. Ambulatory Activity Components Deteriorate Differently across Neurodegenerative Diseases: A Cross-Sectional Sensor-Based Study. Neurodegener. Dis. 2016, 16, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Pilloni, G.; Pau, M.; Pili, R.; Casula, C.; Cossu, G.; Murgia, M. Association between objectively measured physical activity and gait patterns in people with Parkinson’s disease: Results from a 3-month monitoring. Parkinsons. Dis. 2018, 2018, 7806574. [Google Scholar] [CrossRef]
- Hildebrand, M.; Van Hees, V.T.; Hansen, B.H.; Ekelund, U. Age group comparability of raw accelerometer output from wrist-and hip-worn monitors. Med. Sci. Sports Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef]
- Benka Wallén, M.; Franzén, E.; Nero, H.; Hagströmer, M. Levels and Patterns of Physical Activity and Sedentary Behavior in Elderly People With Mild to Moderate Parkinson Disease. Phys. Ther. 2015, 95, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, R.P.A.; Bakers, J.N.E.; Bunte, T.M.; de Fockert, A.J.; Eijkemans, M.J.C.; van den Berg, L.H. Accelerometry for remote monitoring of physical activity in amyotrophic lateral sclerosis: A longitudinal cohort study. J. Neurol. 2019, 266, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Cerff, B.; Maetzler, W.; Sulzer, P.; Kampmeyer, M.; Prinzen, J.; Hobert, M.A.; Blum, D.; van Lummel, R.; Del Din, S.; Gräber, S.; et al. Home-Based Physical Behavior in Late Stage Parkinson Disease Dementia: Differences between Cognitive Subtypes. Neurodegener. Dis. 2017, 17, 135–144. [Google Scholar] [CrossRef]
- Paul, S.S.; Ellis, T.D.; Dibble, L.E.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Cavanaugh, J.T. Obtaining reliable estimates of ambulatory physical activity in people with Parkinson’s disease. J. Parkinsons. Dis. 2016, 6, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Bernad-Elazari, H.; Herman, T.; Mirelman, A.; Gazit, E.; Giladi, N.; Hausdorff, J.M. Objective characterization of daily living transitions in patients with Parkinson’s disease using a single body-fixed sensor. J. Neurol. 2016, 263, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Finnanger Garshol, B.; Ellingsen-Dalskau, L.H.; Pedersen, I. Physical activity in people with dementia attending farm-based dementia day care—a comparative actigraphy study. BMC Geriatr. 2020, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Farías, N.; Brown, W.J.; Peeters, G.M.E.E.G. ActiGraph GT3X+ cut-points for identifying sedentary behaviour in older adults in free-living environments. J. Sci. Med. Sport 2014, 17, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Danzl, M.M.; Ulanowski, E.; Paydo, C. A pilot study evaluating the association between physical activity and cognition among individuals with Parkinson’s disease. Disabil. Health J. 2018, 11, 165–168. [Google Scholar] [CrossRef]
- Buckley, C.; Cavadino, A.; Del Din, S.; Lord, S.; Taylor, L.; Rochester, L.; Kerse, N. Quantifying reliable walking activity with a wearable device in aged residential care: How many days are enough? Sensors 2020, 20, 6314. [Google Scholar] [CrossRef] [PubMed]
- Del Din, S.; Godfrey, A.; Galna, B.; Lord, S.; Rochester, L. Free-living gait characteristics in ageing and Parkinson’s disease: Impact of environment and ambulatory bout length. J. Neuroeng. Rehabil. 2016, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- van Alphen, H.J.M.; Volkers, K.M.; Blankevoort, C.G.; Scherder, E.J.A.; Hortobagyi, T.; van Heuvelen, M.J.G. Older Adults with Dementia Are Sedentary for Most of the Day. PLoS ONE 2016, 11, e0152457. [Google Scholar] [CrossRef] [PubMed]
- von Rosen, P.; Hagströmer, M.; Franzén, E.; Leavy, B. Physical activity profiles in Parkinson’s disease. BMC Neurol. 2021, 21, 71. [Google Scholar] [CrossRef]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Moyle, W.; Jones, C.; Murfield, J.; Thalib, L.; Beattie, E.; Shum, D.; O’Dwyer, S.; Mervin, M.C.; Draper, B. Effect of a robotic seal on the motor activity and sleep patterns of older people with dementia, as measured by wearable technology: A cluster-randomised controlled trial. Maturitas 2018, 110, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Holmes, J.; Brooks, C. Measurements of Daily Energy Intake and Total Energy Expenditure in People with Dementia in Care Homes: The Use of Wearable Technology. J. Nutr. Health Aging 2017, 21, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Leavy, B.; Hagströmer, M.; Conradsson, D.M.; Franzén, E. Physical activity and perceived health in people with Parkinson disease during the first wave of COVID-19 pandemic: A cross-sectional study from Sweden. J. Neurol. Phys. Ther. 2021, 45, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.L.; Esliger, D.W. Accelerometer assessment of physical activity in active, healthy older adults. J. Aging Phys. Act. 2009, 17, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kok, J.S.; Berg, I.J.; Blankevoort, G.C.G.; Scherder, E.J.A. Rest-activity rhythms in small scale homelike care and traditional care for residents with dementia. BMC Geriatr. 2017, 17, 137. [Google Scholar] [CrossRef]
- Hartman, Y.A.W.; Karssemeijer, E.G.A.; van Diepen, L.A.M.; Olde Rikkert, M.G.M.; Thijssen, D.H.J. Dementia Patients Are More Sedentary and Less Physically Active than Age- and Sex-Matched Cognitively Healthy Older Adults. Dement. Geriatr. Cogn. Disord. 2018, 46, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Fleiner, T.; Haussermann, P.; Mellone, S.; Zijlstra, W. Sensor-based assessment of mobility-related behavior in dementia: Feasibility and relevance in a hospital context. Int. Psychogeriatrics 2016, 28, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.L.; Xiong, M.; Tarolli, C.G.; Sharma, S.; Zhu, W.; Goldenthal, S.; Biglan, K.M.; Dorsey, E.R.; Dinesh, K.; Sheth, N.; et al. Multiple wearable sensors in parkinson and huntington disease individuals: A pilot study in clinic and at home. Digit. Biomarkers 2017, 1, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, K.; Xiong, M.; Adams, J.; Dorsey, R.; Sharma, G. Signal analysis for detecting motor symptoms in Parkinson’s and Huntington’s disease using multiple body-Affixed sensors: A pilot study. In Proceedings of the 2016 IEEE Western New York Image Signal Process Work (WNYISPW), Rochester, NY, USA, 17–18 November 2016. [Google Scholar] [CrossRef]
- Feng, H.; Chen, L.; Liu, Y.; Chen, X.; Wang, J.; Yu, M.W.M.; Huang, B.; Li, S.X.; Chau, S.W.H.; Chan, J.W.Y.; et al. Rest-Activity Pattern Alterations in Idiopathic REM Sleep Behavior Disorder. Ann. Neurol. 2020, 88, 817–829. [Google Scholar] [CrossRef]
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 2019, 142, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.A.; Winters, M.; Ashe, M.C.; Clarke, P.J.; McKay, H.A. Destinations That Older Adults Experience Within Their GPS Activity Spaces: Relation to Objectively Measured Physical Activity. Environ. Behav. 2016, 48, 55–77. [Google Scholar] [CrossRef]
- Waterbury, T. Trip Rate Analysis in GPS-Enhaced Personal Travel Surveys. Eletronic Libr. 2018, 34, 1–5. [Google Scholar]
- Sturge, J.; Klaassens, M.; Lager, D.; Weitkamp, G.; Vegter, D.; Meijering, L. Using the concept of activity space to understand the social health of older adults living with memory problems and dementia at home. Soc. Sci. Med. 2021, 288, 113208. [Google Scholar] [CrossRef]
- Bayat, S.; Babulal, G.M.; Schindler, S.E.; Fagan, A.M.; Morris, J.C.; Mihailidis, A.; Roe, C.M. GPS driving: A digital biomarker for preclinical Alzheimer disease. Alzheimer’s Res. Ther. 2021, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.R.; Forchhammer, B.H.; Maier, A.M. Development of a Sensor-Based Behavioral Monitoring Solution to Support Dementia Care. JMIR mHealth uHealth 2019, 7, e12013. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, M.; Wahl, H.W.; Shoval, N.; Auslander, G.; Oswald, F.; Heinik, J. Identifying Mobility Types in Cognitively Heterogeneous Older Adults Based on GPS-Tracking: What Discriminates Best? J. Appl. Gerontol. 2015, 34, 1001–1027. [Google Scholar] [CrossRef] [PubMed]
- Megges, H.; Freiesleben, S.D.; Rosch, C.; Peters, O.; Knoll, N.; Wessel, L. User experience and clinical effectiveness with two wearable global positioning system devices in home dementia care. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Freiesleben, S.D.; Megges, H.; Herrmann, C.; Wessel, L.; Peters, O. Overcoming barriers to the adoption of locating technologies in dementia care: A multi-stakeholder focus group study. BMC Geriatr. 2021, 21, 378. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; Beck, C.; Liu, Z.; Moore, R.; Matthews, C.E.; Buchowski, M.S. PhysicalActivity: Process Accelerometer Data for Physical Activity Measurement. R Package Version 0.2-4. 2021. Available online: https://cran.r-project.org/package=PhysicalActivity (accessed on 6 December 2021).
- Lord, S.; Galna, B.; Rochester, L. Moving forward on gait measurement: Toward a more refined approach. Mov. Disord. 2013, 28, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, P.X.; Gravina, R.; Fortino, G. An Emerging Wearable World: New Gadgetry Produces a Rising Tide of Changes and Challenges. IEEE Syst. Man Cybern. Mag. 2018, 4, 6–14. [Google Scholar] [CrossRef]
- Onnela, J.P. Opportunities and challenges in the collection and analysis of digital phenotyping data. Neuropsychopharmacology 2021, 46, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Pantelopoulos, A.; Bourbakis, N.G. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 2010, 40, 1–12. [Google Scholar] [CrossRef]
- Darwish, A.; Hassanien, A.E. Wearable and implantable wireless sensor network solutions for healthcare monitoring. Sensors 2011, 11, 5561–5595. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.B.; Lee, J. Smart Devices for Older Adults Managing Chronic Disease: A Scoping Review. JMIR mHealth uHealth 2017, 5, e7141. [Google Scholar] [CrossRef]
| Author (Year) | Study Population | Device; Anatomical Site | Duration | Study Design | Reported Sensor Derived Outcome Measures | Data Processing Details | Key Findings |
|---|---|---|---|---|---|---|---|
| Wearable GPS based studies | |||||||
| Zhu et al. (2020) [15] | PD, n = 54, age 67.5 (6.3) yr | WIMU-GPS; upper back | 14 days (waking hours) | Cross-sectional | Trip frequency, duration outside, life space size | Displacement and LSM data reported using a geospatial statistical approach based on computation of minimum span ellipse fitting all data points for each individual. Surface area of minimum span ellipse used to quantify life space size. Excluded if <6 days (>10 h/d) wear. | Reasonable agreement between GPS data, LSA, and mobility diaries for trip frequency and duration, but not life space size. GPS may help overcome the floor and ceiling effects of the LSA. |
| Accelerometer based studies | |||||||
| van Uem et al. (2018) [26] | PD, n = 39; PDD n = 8, age 69 yr (mean of both groups) | DynaPort Minimod; lower back | 3 days | Cross-sectional | Time spent in sedentary and active episodes, TEE, number of ”bouts”, mean bout length | Data collected at 100 Hz. Self-report of non-wear and special activities (i.e., sports). Raw data analysed with manufacturer’s proprietary algorithms. | Total energy expenditure predicts PDQ-SI and PDQ-Mobility scores. Sedentary activity predicts PDQ-Mobility. |
| Pradhan & Kelly (2019) [27] | PD, n = 30, age 68.6 (5.9) y; healthy older adults n = 30, age 67.4 (4.7) yr | Fitbit Charge HR; wrist | 14 days | Cross-sectional | Step count, number of bouts, timing of bouts of activity | PA intensity determined by proprietary algorithms that define MET values based on steps/minute and heart rate activity. PA intensity defined as: very active (≥6 METs); fairly active (3–6 METs), lightly active (1–3 METs), and sedentary. | Individuals with PD spent fewer minutes per day in moderate/vigorous activity, more minutes sedentary vs. healthy controls. |
| Mantri et al. (2019) [28] | PD, n = 30 #, median (IQR) age 70 (69–74) yr | Actigraph GT3X; waist | 7 days | Cross-sectional | Step count, MVPA | Raw activity data was analysed in 30 s epochs. PD-specific algorithm [29] used to define steps and activity intensity. MVPA defined as >3.0 METs, or the equivalent of >1.31 m/s brisk walk. Valid wear time ≥ 10 h, excessively high counts excluded. | Median step count correlates with PASE. |
| Sulzer et al. (2021) [30] | PD, n = 45 #, median (range) age 71 (44–80) yr | DynaPort Minimod; lower back | 3 days, data only collected at baseline | Longitudinal | Time spent in physical behaviour, number of behaviour bouts, timing of bouts of activity, step count, TEE | Manufacturer’s proprietary algorithms used to define posture/activity. Lying and sitting combined as SB. Standing, shuffling, and walking periods combined as active behaviour. For valid days (24 h registration, wear time > 80%), behaviours further categorised by time spent in behaviour, no. of behaviour bouts (i.e., >1 s, walking > 3 consecutive steps), mean bout length. TEE and step count calculated. | Longer sedentary bouts predict illness and death. |
| Moyle et al. (2017) [24] | Dementia, n = 192, age 85.5 (7.7) yr | SenseWear activity-armbands; arm | 24 h | Cross-sectional | Step count, TEE, MET, Time spent physically active, lying down, awake, asleep | All data recorded in 60 s epochs. Analysed with manufacturer’s proprietary algorithms based on AI. Valid wear-time defined as ≥21 h. | Individuals in care home engaged in light physical activity, varying dependent on mobility factors, age, and sex. |
| Varma & Watts (2017) [31] | Alzheimer’s disease, n = 39, age 73.5 (7.9) yr; controls, n = 53, age 73.2 (6.5) yr | Actigraph GT3X+; hip | 7 days | Cross-sectional | Intensity, activity type | Aggregation of VM data into 60 s epochs, average VM calculated for minute. Excluded days with <10 h of wear time. Wear/non-wear time classification algorithm used [32]. Intensity defined as 0–149 CPM for SB, 150–2689 CPM for light-intensity, and ≥2690 CPM for MVPA. | Mild AD is associated with lower intensity and complexity of the activity. Not associated with longer sedentary activity. |
| Kim et al. (2019) [20] | PD, n = 46, age 68 (7.9) yr | ActiGraph GT3X+; wrist and waist | 7 days | Cross-sectional | Step count, activity count, time spent in activity type | Activity levels defined as SB (≤100 CPM), light (101–1951 CPM), and moderate (1952–5737 CPM) to vigorous PA (≥5738 CPM). PA intensity determined using cut-off points [33]. Walking speed classed as <1.04 m/s, 1.05 to 1.3 m/s, and > 1.31 m/s. PD-specific algorithm applied to percent time spent in different walking speeds [34]. | Higher numbers of steps recorded with wrist accelerometer versus waist accelerometer, however, remained proportionate. Variation in recordings linked to increased tremor and bradykinesia. |
| Klenk et al. (2016) [35] | NDD (n = 103; n = 34 ataxia, age 58(11.3) yr, n = 15 PSP, age 66.2 (5.5) yr, n = 16 PD, age 72.2 (6.5) yr); n = 18 healthy young adults, age 28.3 (9.6) yr; n = 38 healthy older adults age 70.6 (3.9) yr | ActivPAL3; thigh | 7 days | Cross-sectional | Walking duration, step count, cadence, walking bout length, absolute and relative number of walking bouts, number of daily sit-to-stand transfers | Manufacturer’s proprietary algorithms detect upright posture and classified activities as lying or sitting, standing, and walking. Days < 24 h data considered invalid. | Daily walking duration decreased for PD, ataxia and PSP groups versus health controls. Daily walking duration was lower for PD, ataxia and PSP groups compared to healthy controls; walking duration diminished as the diseases progressed. |
| Porta et al. (2018) [36] | PD, n = 18, age 68 (10.8) yr | ActiGraph GT3X; wrist | 3 months | Cross-sectional | Step count, intensity of activity by time period | Data collected using 60 s epochs at 30 Hz, processed in manufacturer’s software. Valid wear-time >16 h/day. Step counts and PA classification based on the cut-off points for wrist-worn accelerometry in older adults [37], and (hip-worn) PD specific algorithms [29,38]. Nero algorithm provides walking speeds cut-points. | Peaks of physical activity in early morning and early evening. Physical activity intensity correlates with quantitative spatiotemporal and kinematic gait parameters. |
| van Eijk et al. (2019) [39] | Amyotrophic lateral sclerosis, n = 42, age 60 (12) yr | ActiGraph GT9X Link; hip | 7 days every 2–3 months | Longitudinal | Activity count, MET | Data captured at 30 Hz, processed using manufacturer’s software in 10 s epochs using low-frequency extension (LFE) algorithm. Non-wear time algorithm applied [32], no activity defined as 150 min in advanced disease. Days < 8 h wear-time excluded. Percentage of time active estimated as the proportion of VM counts > 100 CPM. VM counts translated to METs to summarize average daily MET score. | Decline in physical activity of 0.64% per month. Correlation in decline of activity with ALSFRS daily function score. |
| Cerff et al. (2017) [40] | PD, n = 48: n = 17 PD-NC age 71 yr, n = 22 PD-MCI age 68 yr, n = 9 PDD age 72 yr | DynaPort Minimod; lower back | 3 days | Cross-sectional | Step count, posture, Type, duration, intensity and volume of activity MET | Data collected at 100 Hz and a resolution of 1 milli g -force, manufacturer’s algorithms defined posture/activities. Lying and sitting combined as SB. Standing, shuffling, and walking combined as active behaviour. Days with <24 h, or wear time <80% excluded. | Increased number and longer sedentary bouts for individuals with PD dementia versus PD MCI and PD with cognitive impairment. |
| Paul et al. (2016) [41] | PD, n = 92, median (IQR) age 67.3 (7.8) yr | StepWatch 3 Step Activity Monitor; ankle | 7 days | Cross-sectional | Step count, intensity | Stride counts recorded in 1-min intervals, manufacturer’s software used to transform stride counts into step counts (i.e., step count = stride count × 2) and to calculate amount of step activity, and minutes/day of MVPA (i.e., minutes > 100 steps/minute). | Two days of monitoring determined as adequate period to obtain sufficient activity level estimates. |
| Elazari et al. (2016) [42] | PD, n = 99 age 64.8 (9.5) yr; healthy older adults, n = 38 age 78.7 (4.4) yr | Dynaport Hybrid; lower back | 3 days | Cross-sectional | Type, duration, posture | Data recorded at 100 Hz, analysed using MATLAB. Activities detected automatically based on the local mean of acceleration signals. Transitions between activity segments automatically determined. ML algorithm applied to assess the ability of the entire feature set to discriminate between PD and non-PDs. The algorithm identified the features of PD using SVM in MATLAB. | Machine learning algorithm is able to differentiate walk-to-sit and sit-to-walk transitions which vary according to the severity of PD and between patients with PD and healthy older adults. |
| Nero et al. (2016) [34] | PD, n = 91, age 73 (6) yr | Actigraph GT3X; hip | 7 days | Cross-sectional | Duration, type | Manufacturer’s software used to process raw data in 15 s epochs, episodes of ≥90 min of consecutive 0 s considered non-wear and excluded. Data excluded <4 days data. Normal data band-pass filter option utilized. PA expressed per day in total activity counts (TAC). Brisk walking (>1.0 m/s) in minutes/day calculated [43]. Sedentary time calculated using cut-off points [44]. | Motor impairment is negatively associated with PA. Reported an association between PA and both physical function and balance. Motor impairment, body mass index and dyskinesia contribute to the variation in PA levels. PA and brisk walking had principally different associated factors. |
| Loprinzi et al. (2018) [45] | PD, n = 25, 68.7 yr | ActiGraph GT1M; hip | 1–2 weeks | Cross-sectional | Type, intensity | Minimum of 4 days with ≥10 h per day data were included in the analyses. Non-wear defined by a period of a minimum of 60 consecutive minutes of zero activity counts, with the allowance of 1–2 min of activity counts between 0 and 100. Activity CPM of ≥1952 denoted MVPA intensity. | Regardless of motor impairment, age and gender. MVPA improves cognition quantified with MoCA. |
| Buckley et al. (2020) [46] | Aged residential care residents, n = 257, age 84.5 (7.2) yr, Dementia, n = 34, age 81.53 (7.07) yr | Axivity AX3; lower back | 7 days | Cross-sectional | Steps, volume, walking time, bouts per day, pattern or variability of activity behaviours | Programmed to sample acceleration at a frequency of 100 Hz (range ±8 g). Only participants who had at least 3 full days of analysis. Custom automated MATLAB algorithm calculated PA walking outcomes according to a previously established gait model [19,47] | Optimal number of days recording for reliable estimate of activity determined to be between 2–5 days, dependent on required level of dementia care. |
| van Alphen et al. (2016) [48] | Institutionalised dementia, n = 83, age 83 (7.6) yr Community dwelling dementia, n = 37, age 77.3 (5.6) yr Healthy older adults, n = 26, age 79.5 (5.6) yr | Actiwatch AW-4; wrist | 6 days | Cross-sectional data from a longitudinal study | Sedentary behaviour, type, Intensity, amount, duration, patterns | Data collected at 32 Hz using 60 s epochs. Raw data, evaluated without cut-off points. 0 assigned to epochs without acceleration. PA quantified as time spent in specific zones of activity counts with ranges of 100 CPM, <100 CPM classed as SB. | Institutionalised individuals with dementia are more sedentary than community-dwelling individuals with dementia who in turn are sedentary for longer than healthy controls. |
| von Rosen et al. (2021) [49] | PD, n = 301, age 71.4 (6.4) yr | Actigraph GT3X+; hip | 7 days | Cross-sectional | Duration, type, intensity, sedentary behaviour | Manufacturer’s software used to process raw data, collected using 60 s epochs. Non-wear defined as ≥60 min of 0 counts, allowing for 2 min of counts between 0 and 100. Data included from participants with at least 1 day with ≥10 h wear time. Epochs classified as intensity levels using cut-off points for older adults: SB (<100 CPM), LIPA (100–1040 CPM), and MVPA (≥1041 CPM) [50]. | The group categorised as being ”sedentary” as opposed to “light-mover” or “steady-mover” performed worse on tests of balance, mobility, and had an increased likelihood of falling. |
| Moyle et al. (2018) [51] | Dementia, n = 455, age 85.3 (1.0) yr | SenseWear Professional 8 armband; arm | 24 h at baseline, during two single days in weeks 5, 10 and 15 | Longitudinal/RCT | Step count, duration, MET Time spent lying down, awake, and asleep | Manufacturer’s proprietary software and algorithms used. Data recorded in 60 s epochs. Valid wear time for analysis defined as ≥21 h. | A robotic seal intervention given to individuals cared for in a dementia care home led to a reduction in step count both during the daytime and at night and a reduction in daytime PA. |
| Murphy et al. (2017) [52] | Dementia, n = 20, age 78.7 (11.8) yr | Sensewear Armband; arm | 7 days | Cross-sectional | TEE, step count, sedentary behaviour, duration | Limited data processing information provided. | TEE positively correlated with weight and negatively correlated with duration of sleep. |
| Leavy et al. (2021) [53] | PD, n = 89, age 71.0 (6.0) yr | Actigraph GT3x; hip | 7 days | Cross-sectional | Intensity, step count, duration, sedentary behaviour | Manufacturer’s software used to process raw data, collected in 60 s epochs. Non-wear defined as ≥60 min of 0 counts, allowing for 2 min of counts between 0 and 100. Data included if ≥4 days with ≥9 h wear-time. Epochs classified as intensity levels using cut-off points: SB (<100 CPM), LIPA (100–1040 CPM), and MVPA (≥1041 CPM) [54,55]. | Data collected during COVID-19 pandemic. 67% reported a pandemic-related reduction in exercise habits. Being female, aged over 70 years and older, and mobility problems associated with being less physically active. |
| Kok et al. (2017) [56] | Dementia, regular special care unit n = 48, age 82.9 (8.3) yr Small-scale homelike SCU, n = 67, age 83.3 (6.3) yr | Actiwatch; wrist | 7 days | Longitudinal study, data collected at 3-month intervals | Duration, sleep activity | Limited data processing information provided. Incomplete recordings were excluded from analysis. Valid data was collected 7 × 24 h for each measurement. In case missing or invalid data device worn for one more week. | Patients with dementia moved from regular Special Care Unit (SCU) to a small scaled homelike SCU. No effect on activity levels between groups following intervention. |
| Hartman et al. (2018) [57] | Dementia n = 45, age 79.6 (5.9) yr Control, n = 49, age 80.0 (7.7) yr | Phillips Acti-watch 2; wrist | 7 days | Cross-sectional | Sedentary behaviour, intensity, duration | Manufacturer’s software used to export data, sleep intervals manually set and excluded by custom software in MATLAB. Measures obtained every 30 s, wrist accelerations translated to counts to estimate PA/SB. Data included if ≥6 valid days (>10 h). Counts per epoch converted to CPM. Cut-off points of 145, 145–274, 274–597, and >597 CPM used for SB and very light, light-to-moderate, and MVPA. Prolonged SB defined as 30 min with <145 CPM without 1 min of >145 CPM. | People with dementia demonstrated increased sedentary periods and decreased time in light-moderate and moderate-vigorous activity as compared with healthy controls. |
| Fleiner et al. (2016) [58] | Dementia, n = 45, age 45 (7) yr | uSense sensor device; lower back | 3 days | Cross-sectional as part of an RCT | Posture, intensity, type, sedentary behaviour, duration | Data sampled at 100 Hz, limited data processing information provided. | Overall low level of activity and wide variety of activity patterns observed. Patients spent 45% of their time lying, 41% sedentary while sitting or standing, 7% active while sitting, and 7% walking. |
| Garshol et al. (2020) [43] | Dementia, farm-based n = 29, age 74.0 (7.2) yr Regular day care, n = 39, age 83.4 (8.1) yr | Actisleep+ and Actigraph; wrist | 7 days | Cross-sectional | Intensity, sedentary activity, duration, step count | Manufacturer’s software used to report PA, limited data processing information provided. | Higher levels of moderate activity were observed in individuals attending farm-based day care activities versus standard care, whilst similar time spent on sedentary or light activity for both groups. |
| Adams et al. (2017) [59] | PD, n = 16, age 68 (8.7) yr HD (n = 15), age 55.0 (10.7) yr Prodromal HD, n = 5, age 38 (8.6) yr Controls without a movement disorder n = 20, age 58 (16.2) yr | BioStampRC; placed on chest, limb, thigh and forearm | 2 days | Cross-sectional | Posture, duration, type | Data collected at 31.25 Hz. Chest and thigh sensors identified posture. Further analysis differentiated walking and standing. Walking durations identified with normalized autocorrelation-based analysis [60]. Walking split into epochs within upright posture intervals, lags of 0.3–1.2 s considered as plausible range of step durations. | Individuals with PD spend less time lying down than those with Huntington’s disease but a similar amount of time to healthy controls. |
| Feng et al. (2020) [61] | Case control study: Idiopathic REM sleep behaviour disorder (iRBD), n = 88, age 69.8 (7.7) yr Non-RBD controls, n = 44, age 70.1 (10) yr Clinically diagnosed alpha-synucleinopathies *, n = 44, age 70.7 (8.8) yr Prospective nested case-control study: non-convertors **, n = 66, age 70.9 (7.5) yr; convertors, n = 22, age 72.1 (7.6) yr | Actiwatch Spectrum Plus, wrist | 7 days | Case-control study and a prospective nested case-control study | Pattern, duration, sleep-activity, type (rest-activity patterns) | Manufacturer’s software used to report data by 60 s epochs. Data excluded as invalid/missing data if exceeded 33% of total for day/interval. | Actigraphy-derived napping (percentage of time, episodes, and duration) occurred more in those with PD and DLB, than iRBD and, in turn, controls with a similar pattern of decreasing activity across the three groups. More napping and lower activity levels were seen in the iRBD group who had a clinically diagnosed alpha-synucleinopathies (at ~2 years) as compared with those that had not “converted”. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ó Breasail, M.; Biswas, B.; Smith, M.D.; Mazhar, M.K.A.; Tenison, E.; Cullen, A.; Lithander, F.E.; Roudaut, A.; Henderson, E.J. Wearable GPS and Accelerometer Technologies for Monitoring Mobility and Physical Activity in Neurodegenerative Disorders: A Systematic Review. Sensors 2021, 21, 8261. https://doi.org/10.3390/s21248261
Ó Breasail M, Biswas B, Smith MD, Mazhar MKA, Tenison E, Cullen A, Lithander FE, Roudaut A, Henderson EJ. Wearable GPS and Accelerometer Technologies for Monitoring Mobility and Physical Activity in Neurodegenerative Disorders: A Systematic Review. Sensors. 2021; 21(24):8261. https://doi.org/10.3390/s21248261
Chicago/Turabian StyleÓ Breasail, Mícheál, Bijetri Biswas, Matthew D. Smith, Md Khadimul A. Mazhar, Emma Tenison, Anisha Cullen, Fiona E. Lithander, Anne Roudaut, and Emily J. Henderson. 2021. "Wearable GPS and Accelerometer Technologies for Monitoring Mobility and Physical Activity in Neurodegenerative Disorders: A Systematic Review" Sensors 21, no. 24: 8261. https://doi.org/10.3390/s21248261
APA StyleÓ Breasail, M., Biswas, B., Smith, M. D., Mazhar, M. K. A., Tenison, E., Cullen, A., Lithander, F. E., Roudaut, A., & Henderson, E. J. (2021). Wearable GPS and Accelerometer Technologies for Monitoring Mobility and Physical Activity in Neurodegenerative Disorders: A Systematic Review. Sensors, 21(24), 8261. https://doi.org/10.3390/s21248261







