Fiber-Optic Localized Surface Plasmon Resonance Sensors Based on Nanomaterials
Abstract
:1. Introduction
2. Fundamental Principles of Fiber-Optic Localized Surface Plasmon Resonance Sensors
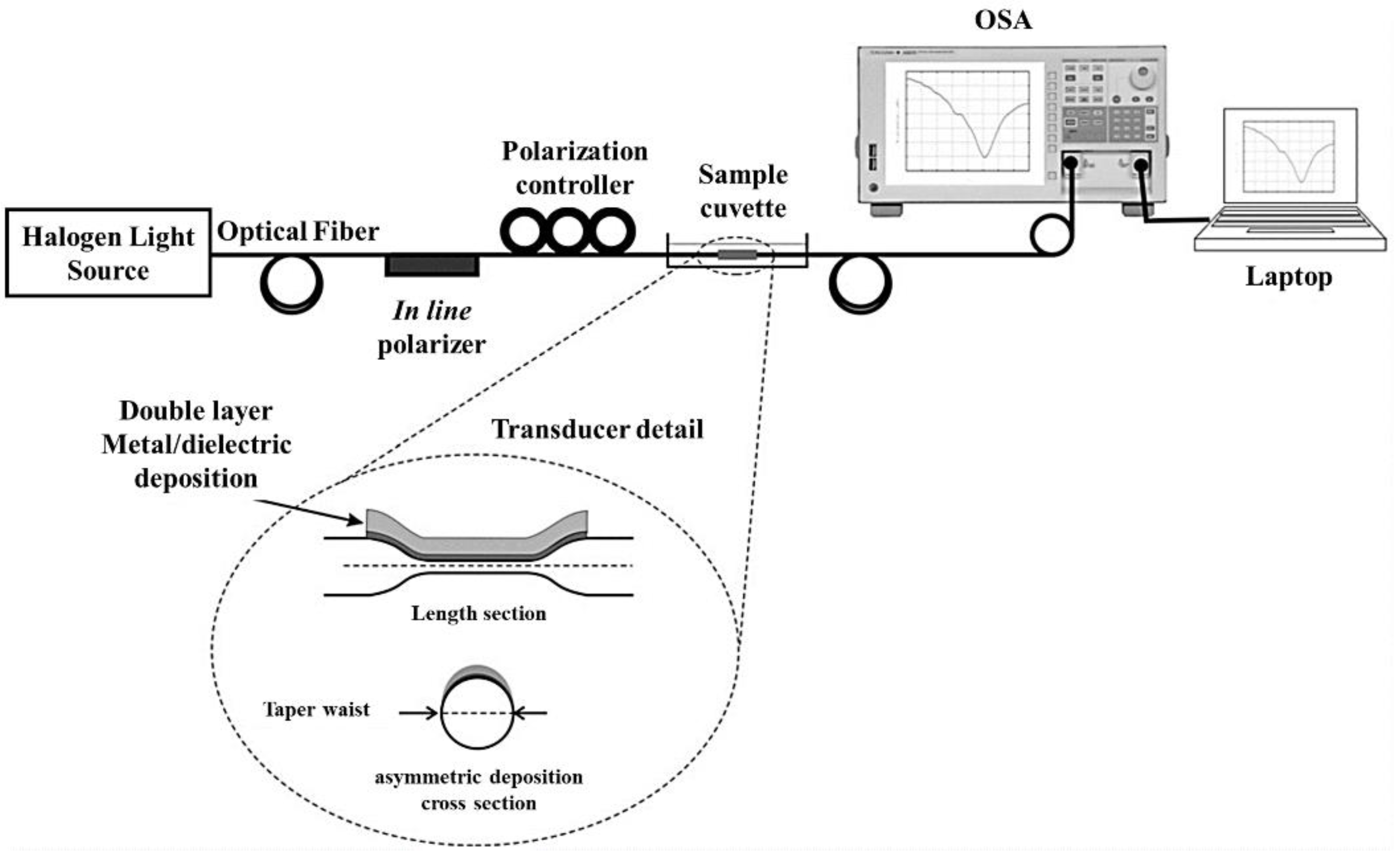
2.1. Fiber-Optic Plasmonic Sensors
2.2. Localized Surface Plasmon Resonance (LSPR)
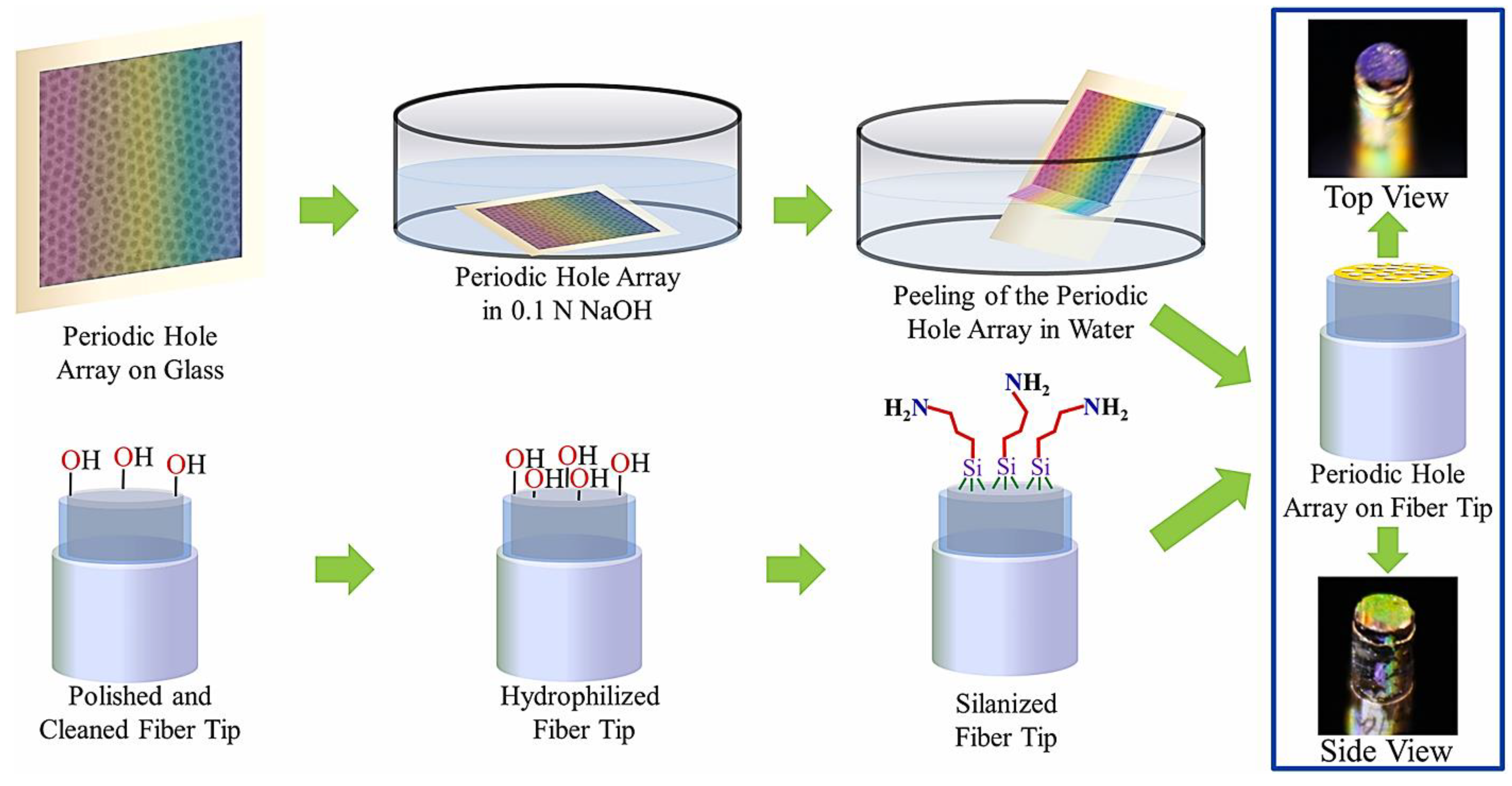
2.3. Integration of Localized Surface Plasmon Resonance to Fiber-Optic Plasmonic Sensors
3. Recent Studies of Fiber-Optic Localized Surface Plasmon Resonance Sensors Using Nanomaterials
3.1. Chemical Fiber-Optic Localized Surface Plasmon Resonance Sensors Based on Nanomaterials
3.2. Fiber-Optic Localized Surface Plasmon Resonance Sensors to Measure Physical Properties
3.3. Biological Applications of Fiber-Optic Localized Surface Plasmon Resonance Sensors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ng, S.M. Carbon dots as optical nanoprobes for biosensors. In Nanobiosensors for Biomolecular Targeting, 1st ed.; Gopinath, S.C.B., Lakshmipriya, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–300. [Google Scholar]
- Puyol, M.; Villuendas, F.; Dominguez, C.; Cadarso, V.; Llobera, A.; Salinas, I.; Garces, I.; Alonso, J. Absorbance-based integrated optical sensors. In Frontiers in Chemical Sensors, 1st ed.; Orellana, G., Moreno-Bondi, M.C., Eds.; Springer: Berlin, Germany, 2005; pp. 1–44. [Google Scholar]
- Xue, C.S.; Erika, G.; Homola, J. Surface plasmon resonance biosensor for the ultrasensitive detection of bisphenol A. Anal. Bioanal. Chem. 2019, 411, 5655–5658. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M.D.A. Development of a Graphene-Based Surface Plasmon Resonance Optical Sensor Chip for Potential Biomedical Application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.H.K.; Chan, K.K.; Zhang, G.; Tjin, S.C.; Yong, K.-T. Carbon Dot-functionalized Interferometric Optical Fiber Sensor for Detection of Ferric Ions in Biological Samples. ACS Appl. Mater. Interfaces 2019, 11, 28546–28553. [Google Scholar] [CrossRef]
- Aitkulov, A.; Tosi, D. Optical Fiber Sensor Based on Plastic Optical Fiber and Smartphone for Measurement of the Breathing Rate. IEEE Sens. J. 2019, 19, 3282–3287. [Google Scholar] [CrossRef]
- Elsherif, M.; Moreddu, R.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Real-time optical fiber sensors based on light diffusing microlens arrays. Lab Chip 2019, 19, 2060–2070. [Google Scholar] [CrossRef]
- Mattei, E.; Triventi, M.; Calcagnini, G.; Censi, F.; Kainz, W.; Bassen, H.I.; Bartolini, P. Temperature and SAR measurement errors in the evaluation of metallic linear structures heating during MRI using fluoroptic® probes. Phys. Med. Biol. 2007, 52, 1633–1646. [Google Scholar] [CrossRef]
- Hu, T.; Zhao, Y.; Song, A.-N. Fiber optic SPR sensor for refractive index and temperature measurement based on MMF-FBG-MMF structure. Sens. Actuators B Chem. 2016, 237, 521–525. [Google Scholar] [CrossRef]
- Wang, W.; Mai, Z.; Chen, Y.; Wang, J.; Li, L.; Su, Q.; Li, X.; Hong, X. A label-free fiber optic SPR biosensor for specific detection of C-reactive protein. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Vaiano, P.; Carotenuto, B.; Pisco, M.; Ricciardi, A.; Quero, G.; Consales, M.; Crescitelli, A.; Esposito, E.; Cusano, A. Lab on Fiber Technology for biological sensing applications. Laser Photonics Rev. 2016, 10, 922–961. [Google Scholar] [CrossRef]
- Paiva, J.S.; Jorge, P.A.; Rosa, C.C.; Cunha, J.P. Optical fiber tips for biological applications: From light confinement, biosensing to bioparticles manipulation. Biochim. Biophys. Acta BBA Gen. Subj. 2018, 1862, 1209–1246. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; González-Vila, Á.; Loyez, M.; Caucheteur, C. Plasmonic Optical Fiber-Grating Immunosensing: A Review. Sensors 2017, 17, 2732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socorro, A.B.; Santano, D.; Del Villar, I.; Matias, I. Trends in the design of wavelength-based optical fibre biosensors (2008–2018). Biosens. Bioelectron. X 2019, 1, 100015. [Google Scholar] [CrossRef]
- Pisco, M.; Cusano, A. Lab-On-Fiber Technology: A Roadmap toward Multifunctional Plug and Play Platforms. Sensors 2020, 20, 4705. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Marques, C. Design and Performance Perspectives on Fiber Optic Sensors with Plasmonic Nanostructures and Gratings: A Review. IEEE Sens. J. 2019, 19, 7168–7178. [Google Scholar] [CrossRef]
- Li, Y.; Xin, H.; Zhang, Y.; Li, B. Optical Fiber Technologies for Nanomanipulation and Biodetection: A Review. J. Light. Technol. 2021, 39, 251–262. [Google Scholar] [CrossRef]
- Tabassum, S.; Kumar, R. Advances in Fiber-Optic Technology for Point-of-Care Diagnosis and In Vivo Biosensing. Adv. Mater. Technol. 2020, 5, 1900792. [Google Scholar] [CrossRef]
- Ricciardi, A.; Consales, M.; Pisco, M.; Cusano, A. Application of Nanotechnology to Optical Fibre Sensors: Recent Advancements and New Trends. In Optical Fibre Sensors: Fundamentals for Development of Optimized Devices; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 289–329. [Google Scholar]
- Xiong, Y.; Xu, F. Multifunctional integration on optical fiber tips: Challenges and opportunities. Adv. Photonics 2020, 2, 064001. [Google Scholar] [CrossRef]
- Tabassum, R.; Gupta, B.D. SPR based fiber-optic sensor with enhanced electric field intensity and figure of merit using different single and bimetallic configurations. Opt. Commun. 2016, 367, 23–34. [Google Scholar] [CrossRef]
- Gang, T.; Hu, M.; Rong, Q.; Qiao, X.; Liang, L.; Liu, N.; Tong, R.; Liu, X.; Bian, C. High-Frequency Fiber-Optic Ultrasonic Sensor Using Air Micro-Bubble for Imaging of Seismic Physical Models. Sensors 2016, 16, 2125. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Liu, Q.; He, Z. Phase-detection distributed fiber-optic vibration sensor without fading-noise based on time-gated digital OFDR. Opt. Express 2017, 25, 8315–8325. [Google Scholar] [CrossRef] [PubMed]
- Caucheteur, C.; Guo, T.; Albert, J. Polarization-Assisted Fiber Bragg Grating Sensors: Tutorial and Review. J. Light. Technol. 2017, 35, 3311–3322. [Google Scholar] [CrossRef]
- Yin, M.-J.; Gu, B.; An, Q.-F.; Yang, C.; Guan, Y.L.; Yong, K.-T. Recent development of fiber-optic chemical sensors and biosensors: Mechanisms, materials, micro/nano-fabrications and applications. Coord. Chem. Rev. 2018, 376, 348–392. [Google Scholar] [CrossRef]
- Lu, Y.; Li, H.; Qian, X.; Zheng, W.; Sun, Y.; Shi, B.; Zhang, Y.-N. Beta-cyclodextrin based reflective fiber-optic SPR sensor for highly-sensitive detection of cholesterol concentration. Opt. Fiber Technol. 2020, 56, 102187. [Google Scholar] [CrossRef]
- Lim, K.; Wong, L.; Chiu, W.K.; Kodikara, J. Distributed fiber optic sensors for monitoring pressure and stiffness changes in out-of-round pipes. Struct. Control. Heal. Monit. 2015, 23, 303–314. [Google Scholar] [CrossRef]
- Chu, S.; Nakkeeran, K.; Abobaker, A.M.; Aphale, S.S.; Babu, P.R.; Senthilnathan, K. Design and Analysis of Surface-Plasmon-Resonance-Based Photonic Quasi-Crystal Fiber Biosensor for High-Refractive-Index Liquid Analytes. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Cai, W.; Caswell, A.W.; Kraetschmer, T.; Sanders, S.T.; Roy, S.; Gord, J.R. Tomographic imaging of temperature and chemical species based on hyperspectral absorption spectroscopy. Opt. Express 2009, 17, 8602–8613. [Google Scholar] [CrossRef]
- Dwivedi, Y.S.; Sharma, A.K.; Gupta, B.D. Influence of Design Parameters on the Performance of a Surface Plasmon Sensor Based Fiber Optic Sensor. Plasmonics 2008, 3, 79–86. [Google Scholar] [CrossRef]
- Esteban, Ó.; Naranjo, F.B.; Díaz-Herrera, N.; Valdueza-Felip, S.; Navarrete, M.-C.; González-Cano, A. High-sensitive SPR sensing with Indium Nitride as a dielectric overlay of optical fibers. Sens. Actuators B Chem. 2011, 158, 372–376. [Google Scholar] [CrossRef]
- Lin, Y.; Zou, Y.; Mo, Y.; Guo, J.; Lindquist, R.G. E-Beam Patterned Gold Nanodot Arrays on Optical Fiber Tips for Localized Surface Plasmon Resonance Biochemical Sensing. Sensors 2010, 10, 9397–9406. [Google Scholar] [CrossRef]
- Kim, S.A.; Byun, K.M.; Kim, K.; Jang, S.M.; Ma, K.; Oh, Y.; Kim, D.; Kim, S.G.; Shuler, M.L.; Kim, S.J. Surface-enhanced localized surface plasmon resonance biosensing of avian influenza DNA hybridization using subwavelength metallic nanoarrays. Nanotechnology 2010, 21, 355503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.; Lee, W.; Kim, Y.; Kim, D. Self-aligned colocalization of 3D plasmonic nanogap arrays for ultra-sensitive surface plasmon resonance detection. Biosens. Bioelectron. 2014, 51, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cuesta, I.; West, M.M.; Montinaro, E.; Schwartzberg, A.; Cabrini, S. A nanochannel through a plasmonic antenna gap: An integrated device for single particle counting. Lab Chip 2019, 19, 2394–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tittl, A.; Giessen, H.; Liu, N. Plasmonic gas and chemical sensing. Nanophotonics 2014, 3, 157–180. [Google Scholar] [CrossRef] [Green Version]
- Kaniber, M.; Schraml, K.; Regler, A.; Bartl, J.; Glashagen, G.; Flassig, F.; Wierzbowski, J.; Finley, J.J. Surface plasmon resonance spectroscopy of single bowtie nano-antennas using a differential reflectivity method. Sci. Rep. 2016, 6, 23203. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Chau, L.-K. Colloidal Gold-Modified Optical Fiber for Chemical and Biochemical Sensing. Anal. Chem. 2002, 75, 16–21. [Google Scholar] [CrossRef]
- Chau, L.-K.; Lin, Y.-F.; Cheng, S.-F.; Lin, T.-J. Fiber-optic chemical and biochemical probes based on localized surface plasmon resonance. Sens. Actuators B Chem. 2006, 113, 100–105. [Google Scholar] [CrossRef]
- Chau, L.-K.; Kuo, C.-W.; Chu, Y.-W.; Liao, S.-H.; Lin, Y.-T.; Wang, C.R.C. A Fiber Optic Particle Plasmon Resonance Biosensing Platform Based on Detection of Light Scattering Intensity from the Proximal End. J. Chin. Chem. Soc. 2011, 58, 786–792. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Huang, C.-H.; Chau, L.-K. Fiber optic particle plasmon resonance sensor based on plasmonic light scattering interrogation. Ann. Phys. 2012, 524, 705–712. [Google Scholar] [CrossRef]
- Tang, J.-L.; Cheng, S.-F.; Hsu, W.-T.; Chiang, T.-Y.; Chau, L.-K. Fiber-optic biochemical sensing with a colloidal gold-modified long period fiber grating. Sens. Actuators B Chem. 2006, 119, 105–109. [Google Scholar] [CrossRef]
- Lam, C.C.C.; Mandamparambil, R.; Sun, T.; Grattan, K.T.V.; Nanukuttan, S.; Taylor, S.E.; Basheer, P.M. Optical Fiber Refractive Index Sensor for Chloride Ion Monitoring. IEEE Sens. J. 2009, 9, 525–532. [Google Scholar] [CrossRef]
- Csáki, A.; Jahn, F.; Latka, I.; Henkel, T.; Malsch, D.; Schneider, T.; Schröder, K.; Schuster, K.; Schwuchow, A.; Spittel, R.; et al. Nanoparticle Layer Deposition for Plasmonic Tuning of Microstructured Optical Fibers. Small 2010, 6, 2584–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C. Localized surface plasmonic resonance study of silver nanocubes for photonic crystal fiber sensor. Opt. Lasers Eng. 2012, 50, 1592–1595. [Google Scholar] [CrossRef]
- Kim, H.-M.; Park, J.-H.; Lee, S.-K. Fiber optic sensor based on ZnO nanowires decorated by Au nanoparticles for improved plasmonic biosensor. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Wang, J.; Wang, F.; Su, W.; Yang, L.; Lv, J.; Fu, G.; Li, X.; Liu, Q.; Sun, T.; et al. Surface plasmon resonance (SPR) infrared sensor based on D-shape photonic crystal fibers with ITO coatings. Opt. Commun. 2020, 464, 125496. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Huang, T.-T.; Wang, C.-H.; Huang, C.-J.; Tsai, T.-H.; Yu, S.-N.; Chen, Y.-T.; Hong, S.-W.; Hsu, C.-W.; Chang, T.-C.; et al. Fiber optic nanogold-linked immunosorbent assay for rapid detection of procalcitonin at femtomolar concentration level. Biosens. Bioelectron. 2020, 151, 111871. [Google Scholar] [CrossRef]
- Meriaudeau, F.; Wig, A.; Passian, A.; Downey, T.; Buncick, M.; Ferrell, T. Gold island fiber optic sensor for refractive index sensing. Sens. Actuators B Chem. 2000, 69, 51–57. [Google Scholar] [CrossRef]
- Mitsui, K.; Handa, Y.; Kajikawa, K. Optical fiber affinity biosensor based on localized surface plasmon resonance. Appl. Phys. Lett. 2004, 85, 4231–4233. [Google Scholar] [CrossRef]
- Scherino, L.; Giaquinto, M.; Micco, A.; Aliberti, A.; Bobeico, E.; La Ferrara, V.; Ruvo, M.; Ricciardi, A.; Cusano, A. A Time-Efficient Dip Coating Technique for the Deposition of Microgels onto the Optical Fiber Tip. Fibers 2018, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Polley, N.; Basak, S.; Hass, R.; Pacholski, C. Fiber optic plasmonic sensors: Providing sensitive biosensor platforms with minimal lab equipment. Biosens. Bioelectron. 2019, 132, 368–374. [Google Scholar] [CrossRef]
- Pathak, A.; Gupta, B.D. Fiber optic plasmonic sensor utilizing carbon nanotubes based surface imprinted matrix for the sensing of dopamine. In Proceedings of the 2018 Conference on Lasers and Electro-Optics Pacific Rim (CLEO-PR), Hong Kong, 29 July–3 August 2018; pp. 1–2. [Google Scholar]
- Yu, C.; Wu, Y.; Liu, X.; Fu, F.; Gong, Y.; Rao, Y.-J.; Chen, Y. Miniature fiber-optic NH3 gas sensor based on Pt nanoparticle-incorporated graphene oxide. Sens. Actuators B Chem. 2017, 244, 107–113. [Google Scholar] [CrossRef]
- Lin, Y.; Zou, Y.; Lindquist, R.G. A reflection-based localized surface plasmon resonance fiber-optic probe for biochemical sensing. Biomed. Opt. Express 2011, 2, 478–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nugroho, F.A.A.; Eklund, R.; Nilsson, S.; Langhammer, C. A fiber-optic nanoplasmonic hydrogen sensor via pattern-transfer of nanofabricated PdAu alloy nanostructures. Nanoscale 2018, 10, 20533–20539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Zhou, J.; Liu, T.; Tao, Y.; Jiang, R.; Liu, M.; Xiao, G.; Zhu, J.; Zhou, Z.-K.; Wang, X.; et al. Plasmonic gold mushroom arrays with refractive index sensing figures of merit approaching the theoretical limit. Nat. Commun. 2013, 4, 2381. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Huang, C.-H.; Lu, S.-H.; Kuo, I.-T.; Chau, L.-K. Direct detection of orchid viruses using nanorod-based fiber optic particle plasmon resonance immunosensor. Biosens. Bioelectron. 2014, 51, 371–378. [Google Scholar] [CrossRef]
- Paul, D.; Dutta, S.; Saha, D.; Biswas, R. LSPR based Ultra-sensitive low cost U-bent optical fiber for volatile liquid sensing. Sens. Actuators B Chem. 2017, 250, 198–207. [Google Scholar] [CrossRef]
- Saikia, R.; Buragohain, M.; Datta, P.; Nath, P.; Barua, K. Fiber-Optic pH Sensor Based on SPR of Silver Nanostructured Film. AIP 2009, 1147, 249–255. [Google Scholar]
- Giaquinto, M.; Micco, A.; Aliberti, A.; Bobeico, E.; La Ferrara, V.; Ruvo, M.; Ricciardi, A.; Cusano, A. Optimization Strategies for Responsivity Control of Microgel Assisted Lab-On-Fiber Optrodes. Sensors 2018, 18, 1119. [Google Scholar] [CrossRef] [Green Version]
- Hocker, G.B. Fiber-optic sensing of pressure and temperature. Appl. Opt. 1979, 18, 1445–1448. [Google Scholar] [CrossRef]
- Choi, H.Y.; Park, K.S.; Park, S.J.; Paek, U.-C.; Lee, B.H.; Choi, E.S. Miniature fiber-optic high temperature sensor based on a hybrid structured Fabry–Perot interferometer. Opt. Lett. 2008, 33, 2455–2457. [Google Scholar] [CrossRef]
- Totsu, K.; Haga, Y.; Esashi, M. Ultra-miniature fiber-optic pressure sensor using white light interferometry. J. Micromechan. Microeng. 2004, 15, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, A. Miniature fiber-optic pressure sensor. IEEE Photonics Technol. Lett. 2005, 17, 447–449. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Gupta, B.D. Simulation of a localized surface-plasmon-resonance-based fiber optic temperature sensor. J. Opt. Soc. Am. A 2010, 27, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; Garcia-Camara, B.; Garcia-Garcia, A.; Urruchi, V.; Sánchez-Pena, J.M. Fiber Optic Temperature Sensor Based on Amplitude Modulation of Metallic and Semiconductor Nanoparticles in a Liquid Crystal Mixture. J. Light. Technol. 2015, 33, 2451–2455. [Google Scholar] [CrossRef]
- Ohodnicki, P.R.; Buric, M.P.; Brown, T.D.; Matranga, C.; Wang, C.; Baltrus, J.; Andio, M. Plasmonic nanocomposite thin film enabled fiber optic sensors for simultaneous gas and temperature sensing at extreme temperatures. Nanoscale 2013, 5, 9030–9039. [Google Scholar] [CrossRef]
- Lechuga, L.M. Optical biosensors. Compr. Anal. Chem. 2005, 44, 209–250. [Google Scholar]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar]
- Yang, C.-Y.; Brooks, E.; Li, Y.; Denny, P.; Ho, C.-M.; Qi, F.; Shi, W.; Wolinsky, L.; Wu, B.; Wong, D.T.W.; et al. Detection of picomolar levels of interleukin-8 in human saliva by SPR. Lab Chip 2005, 5, 1017–1023. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Cheng, W.; Xu, Y.; Zhou, X.; Li, X.; Ding, X.; Ding, S. A simple surface plasmon resonance biosensor for detection of PML/RARα based on heterogeneous fusion gene-triggered nonlinear hybridization chain reaction. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Firdous, S.; Anwar, S.; Rafya, R. Development of surface plasmon resonance (SPR) biosensors for use in the diagnostics of malignant and infectious diseases. Laser Phys. Lett. 2018, 15, 065602. [Google Scholar] [CrossRef]
- Rich, R.L.; Day, Y.S.; Morton, T.A.; Myszka, D.G. High-Resolution and High-Throughput Protocols for Measuring Drug/Human Serum Albumin Interactions Using BIACORE. Anal. Biochem. 2001, 296, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Jason-Moller, L.; Murphy, M.; Bruno, J. Overview of Biacore Systems and Their Applications. Curr. Protoc. Protein Sci. 2006, 45, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, Y. Effect of shape of silver nanoplates on the enhancement of surface plasmon resonance (SPR) signals. J. Nanosci. Nanotechnol. 2008, 8, 5026–5029. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.M.; Yoon, S.J.; Kim, D.; Kim, S.J. Experimental study of sensitivity enhancement in surface plasmon resonance biosensors by use of periodic metallic nanowires. Opt. Lett. 2007, 32, 1902–1904. [Google Scholar] [CrossRef]
- Yoo, S.M.; Kim, D.-K.; Lee, S.Y. Aptamer-functionalized localized surface plasmon resonance sensor for the multiplexed detection of different bacterial species. Talanta 2015, 132, 112–117. [Google Scholar] [CrossRef]
- Jackman, J.A.; Avsar, S.Y.; Ferhan, A.R.; Li, D.; Park, J.H.; Zhdanov, V.P.; Cho, N.-J. Quantitative Profiling of Nanoscale Liposome Deformation by a Localized Surface Plasmon Resonance Sensor. Anal. Chem. 2016, 89, 1102–1109. [Google Scholar] [CrossRef]
- Guner, H.; Ozgur, E.; Kokturk, G.; Celik, M.; Esen, E.; Topal, A.E.; Ayas, S.; Uludag, Y.; Elbuken, C.; Dana, A. A smartphone based surface plasmon resonance imaging (SPRi) platform for on-site biodetection. Sens. Actuators B Chem. 2017, 239, 571–577. [Google Scholar] [CrossRef]
- Suthanthiraraj, P.P.A.; Sen, A.K. Localized surface plasmon resonance (LSPR) biosensor based on thermally annealed silver nanostructures with on-chip blood-plasma separation for the detection of dengue non-structural protein NS1 antigen. Biosens. Bioelectron. 2019, 132, 38–46. [Google Scholar] [CrossRef]
- Goldberg, R.J. A Theory of Antibody—Antigen Reactions. I. Theory for Reactions of Multivalent Antigen with Bivalent and Univalent Antibody2. J. Am. Chem. Soc. 1952, 74, 5715–5725. [Google Scholar] [CrossRef]
- Sela-Culang, I.; Kunik, V.; Ofran, Y. The structural basis of antibody-antigen recognition. Front. Immunol. 2013, 4, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Xu, S.; Zheng, X.; Wang, Y.; Xu, W. Optical Fiber LSPR Biosensor Prepared by Gold Nanoparticle Assembly on Polyelectrolyte Multilayer. Sensors 2010, 10, 3585–3596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.-H.; Erdene, N.; Park, J.-H.; Jeong, D.H.; Lee, H.-Y.; Lee, S.-K. Real-time label-free immunoassay of interferon-gamma and prostate-specific antigen using a Fiber-Optic Localized Surface Plasmon Resonance sensor. Biosens. Bioelectron. 2013, 39, 346–351. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Huang, C.-H.; Cheng, G.-L.; Chen, N.-K.; Chui, H.-C. Tapered optical fiber sensor based on localized surface plasmon resonance. Opt. Express 2012, 20, 21693–21701. [Google Scholar] [CrossRef] [PubMed]
- Chiavaioli, F.; Zubiate, P.; Del Villar, I.; Zamarreño, C.R.; Giannetti, A.; Tombelli, S.; Baldini, F. Femtomolar detection by nanocoated fiber label-free biosensors. ACS Sens. 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Satija, J.; Tharion, J.; Mukherji, S. Facile synthesis of size and wavelength tunable hollow gold nanostructures for the development of a LSPR based label-free fiber-optic biosensor. RSC Adv. 2015, 5, 69970–69979. [Google Scholar] [CrossRef]
- Chang, T.-C.; Wu, C.-C.; Wang, S.-C.; Chau, L.-K.; Hsieh, W.-H. Using a Fiber Optic Particle Plasmon Resonance Biosensor to Determine Kinetic Constants of Antigen–Antibody Binding Reaction. Anal. Chem. 2012, 85, 245–250. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2014; pp. 175–236. [Google Scholar]
- McKendry, R.; Zhang, J.; Arntz, Y.; Strunz, T.; Hegner, M.; Lang, H.P.; Baller, M.K.; Certa, U.; Meyer, E.; Güntherodt, H.-J.; et al. Multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array. Proc. Natl. Acad. Sci. USA 2002, 99, 9783–9788. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Sheng, Y.; Zhou, K.; Liu, Q.; Liu, L.; Wu, H.-C. Analyte-Triggered DNA-Probe Release from a Triplex Molecular Beacon for Nanopore Sensing. Angew. Chem. Int. Ed. 2018, 57, 3602–3606. [Google Scholar] [CrossRef]
- Kaye, S.; Zeng, Z.; Sanders, M.; Chittur, K.; Koelle, P.M.; Lindquist, R.; Manne, U.; Lin, Y.; Wei, J. Label-free detection of DNA hybridization with a compact LSPR-based fiber-optic sensor. Analyst 2017, 142, 1974–1981. [Google Scholar] [CrossRef]
- Roether, J.; Chu, K.-Y.; Willenbacher, N.; Shen, A.Q.; Bhalla, N. Real-time monitoring of DNA immobilization and detection of DNA polymerase activity by a microfluidic nanoplasmonic platform. Biosens. Bioelectron. 2019, 142, 111528. [Google Scholar] [CrossRef]
- Hernández, J.; Thompson, I.M. Prostate-specific antigen: A review of the validation of the most commonly used cancer biomarker. Cancer 2004, 101, 894–904. [Google Scholar] [CrossRef]
- Bryant, A.K.; D’Amico, A.V.; Nguyen, P.L.; Einck, J.; Kane, C.J.; McKay, R.R.; Simpson, D.R.; Mundt, A.J.; Murphy, J.D.; Rose, B.S. Three-month posttreatment prostate-specific antigen level as a biomarker of treatment response in patients with intermediate-risk or high-risk prostate cancer treated with androgen deprivation therapy and radiotherapy. Cancer 2018, 124, 2939–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Li, X.; Yan, T.; Li, Y.; Zhang, Y.; Wu, D.; Wei, Q.; Du, B. Electrochemiluminescent immunosensing of prostate-specific antigen based on silver nanoparticles-doped Pb (II) metal-organic framework. Biosens. Bioelectron. 2016, 79, 379–385. [Google Scholar] [CrossRef]
- Khan, M.S.; Ameer, H.; Ali, A.; Zhu, W.; Li, X.; Yang, L.; Wang, H.; Feng, R.; Wei, Q. Label-free electrochemiluminescent immunosensor for prostate specific antigen ultrasensitive detection based on novel luminophore Ag3PO4 decorated GO. J. Electroanal. Chem. 2019, 847, 113266. [Google Scholar] [CrossRef]
- Sanders, M.; Lin, Y.; Wei, J.; Bono, T.; Lindquist, R.G. An enhanced LSPR fiber-optic nanoprobe for ultrasensitive detection of protein biomarkers. Biosens. Bioelectron. 2014, 61, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Park, J.-H.; Jeong, D.H.; Lee, H.-Y.; Lee, S.-K. Real-time detection of prostate-specific antigens using a highly reliable fiber-optic localized surface plasmon resonance sensor combined with micro fluidic channel. Sens. Actuators B Chem. 2018, 273, 891–898. [Google Scholar] [CrossRef]
- Kim, H.-M.; Uh, M.; Jeong, D.H.; Lee, H.-Y.; Park, J.-H.; Lee, S.-K. Localized surface plasmon resonance biosensor using nanopatterned gold particles on the surface of an optical fiber. Sens. Actuators B Chem. 2019, 280, 183–191. [Google Scholar] [CrossRef]
- Sciacca, B.; Monro, T.M. Dip Biosensor Based on Localized Surface Plasmon Resonance at the Tip of an Optical Fiber. Langmuir 2014, 30, 946–954. [Google Scholar] [CrossRef]
- Camara, A.D.R.; Gouvêa, P.M.P.; Dias, A.C.M.S.; Braga, A.M.B.; Dutra, R.A.F.; De Araujo, R.E.; Carvalho, I.C.S. Dengue immunoassay with an LSPR fiber optic sensor. Opt. Express 2013, 21, 27023–27031. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. LSPR- and SPR-Based Fiber-Optic Cholesterol Sensor Using Immobilization of Cholesterol Oxidase Over Silver Nanoparticles Coated Graphene Oxide Nanosheets. IEEE Sens. J. 2018, 18, 1039–1046. [Google Scholar] [CrossRef]
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic dopamine sensor using green synthesized silver nanoparticles. Sens. Actuators B Chem. 2016, 224, 600–606. [Google Scholar] [CrossRef]
- Khan, R.R.; Watekar, A.V.; Kang, S.-W. Fiber-Optic Biosensor to Detect pH and Glucose. IEEE Sens. J. 2017, 18, 1528–1538. [Google Scholar] [CrossRef]
- Trienekens, J.; Zuurbier, P. Quality and safety standards in the food industry, developments and challenges. Int. J. Prod. Econ. 2008, 113, 107–122. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Alocilja, E.C.; Radke, S.M. Market analysis of biosensors for food safety. Biosens. Bioelectron. 2003, 18, 841–846. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. A localized and propagating SPR, and molecular imprinting based fiber-optic ascorbic acid sensor using an in situ polymerized polyaniline–Ag nanocomposite. Nanotechnology 2016, 27, 345501. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Punjabi, N.S.; Sharma, D.K.; Mukherji, S. A silicon nitride coated LSPR based fiber-optic probe for possible continuous monitoring of sucrose content in fruit juices. Sens. Actuators B Chem. 2016, 222, 1240–1250. [Google Scholar] [CrossRef]
- Chang, K.; Wang, S.; Zhang, H.; Guo, Q.; Hu, X.; Lin, Z.; Sun, H.; Jiang, M.; Hu, J. Colorimetric detection of melamine in milk by using gold nanoparticles-based LSPR via optical fibers. PLoS ONE 2017, 12, e0177131. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.-H.; Byun, J.-Y.; Kim, J.H.; Kim, M.-G. An optical fiber-based LSPR aptasensor for simple and rapid in-situ detection of ochratoxin A. Biosens. Bioelectron. 2018, 102, 504–509. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, B.D. Fiber optic surface plasmon resonance based hexachlorobenzene sensor exploiting layer-by-layer coatings of GNP/SnO2 dendrites nanocomposite. Mater. Res. Express 2017, 4, 115022. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Mishra, S.K.; Gupta, B.D. Localized and propagating surface plasmon resonance based fiber optic sensor for the detection of tetracycline using molecular imprinting. Mater. Res. Express 2015, 2, 035007. [Google Scholar] [CrossRef]
- Dikovska, A.; Atanasova, G.; Nedyalkov, N.; Stefanov, P.; Atanasov, P.; Karakoleva, E.; Andreev, A. Optical sensing of ammonia using ZnO nanostructure grown on a side-polished optical-fiber. Sens. Actuators B Chem. 2010, 146, 331–336. [Google Scholar] [CrossRef]
- Shabaneh, A.; Girei, S.; Thirunavakkarasu, P.M.; Mahdi, M.; Rashid, S.A.; Paiman, S.; Yaacob, M. Dynamic Response of Tapered Optical Multimode Fiber Coated with Carbon Nanotubes for Ethanol Sensing Application. Sensors 2015, 15, 10452–10464. [Google Scholar] [CrossRef] [PubMed]
- Bremer, K.; Roth, B. Fibre optic surface plasmon resonance sensor system designed for smartphones. Opt. Express 2015, 23, 17179–17184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Calis, A.; Luo, Y.; Chen, C.; Lutton, M.; Rivenson, Y.; Lin, X.; Koydemir, H.C.; Zhang, Y.; Wang, H.; et al. Label-free bioaerosol sensing using mobile microscopy and deep learning. ACS Photon. 2018, 5, 4617–4627. [Google Scholar] [CrossRef]
- Öktem, B.; Pavlov, I.; Ilday, S.; Kalaycıoğlu, H.; Rybak, A.; Yavaş, S.; Erdoğan, M.; Ilday, F.Ö. Nonlinear laser lithography for indefinitely large-area nanostructuring with femtosecond pulses. Nat. Photon. 2013, 7, 891–901. [Google Scholar] [CrossRef]
- Xu, Q.; Bao, J.; Rioux, R.M.; Perez-Castillejos, R.; Capasso, F.; Whitesides, G.M. Fabrication of large-area patterned nanostrcutures for optical applications by nanoskiving. Nano Lett. 2007, 9, 280–2805. [Google Scholar]
- Mishra, A.K.; Mishra, S.K.; Verma, R.K. Doped Single-Wall Carbon Nanotubes in Propagating Surface Plasmon Resonance-Based Fiber Optic Refractive Index Sensing. Plasmonics 2016, 12, 1657–1663. [Google Scholar] [CrossRef]
- Nayak, J.K.; Parhi, P.; Jha, R. Graphene oxide encapsulated gold nanoparticle based stable fibre optic sucrose sensor. Sens. Actuators B Chem. 2015, 221, 835–841. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Z.; Zhang, C.; Gao, S.; Qiu, H.; Li, C.; Yang, C.; Liu, M.; Liu, Y. A novel U-bent plastic optical fibre local surface plasmon resonance sensor based on a graphene and silver nanoparticle hybrid structure. J. Phys. D Appl. Phys. 2017, 50, 165105. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yuan, H.; Wang, F.; Peng, W. A Smartphone-Based Red–Green Dual Color Fiber Optic Surface Plasmon Resonance Sensor. IEEE Photon. Technol. Lett. 2018, 30, 927–930. [Google Scholar] [CrossRef]
- Moon, G.; Son, T.; Lee, H.; Kim, D. Deep Learning Approach for Enhanced Detection of Surface Plasmon Scattering. Anal. Chem. 2019, 91, 9538–9545. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ray, A.; Wei, Q.; Feizi, A.; Tong, X.; Chen, E.; Luo, Y.; Ozcan, A. Deep Learning Enables High-Throughput Analysis of Particle-Aggregation-Based Biosensors Imaged Using Holography. ACS Photon. 2018, 6, 294–301. [Google Scholar] [CrossRef] [Green Version]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Song, H.; Ahn, H.; Kim, S.; Choi, J.-r.; Kim, K. Fiber-Optic Localized Surface Plasmon Resonance Sensors Based on Nanomaterials. Sensors 2021, 21, 819. https://doi.org/10.3390/s21030819
Lee S, Song H, Ahn H, Kim S, Choi J-r, Kim K. Fiber-Optic Localized Surface Plasmon Resonance Sensors Based on Nanomaterials. Sensors. 2021; 21(3):819. https://doi.org/10.3390/s21030819
Chicago/Turabian StyleLee, Seunghun, Hyerin Song, Heesang Ahn, Seungchul Kim, Jong-ryul Choi, and Kyujung Kim. 2021. "Fiber-Optic Localized Surface Plasmon Resonance Sensors Based on Nanomaterials" Sensors 21, no. 3: 819. https://doi.org/10.3390/s21030819






