Linker-Free Magnetite-Decorated Gold Nanoparticles (Fe3O4-Au): Synthesis, Characterization, and Application for Electrochemical Detection of Arsenic (III)
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Instruments
2.2. Synthesis and Modification of Fe3O4-Au-IL Nanocomposite
2.3. Stripping Voltammetry Analysis of As(III)
2.4. Preparation of Water Samples
3. Results and Discussion
3.1. Physicochemical Characterization of Fe3O4-Au Nanocomposite
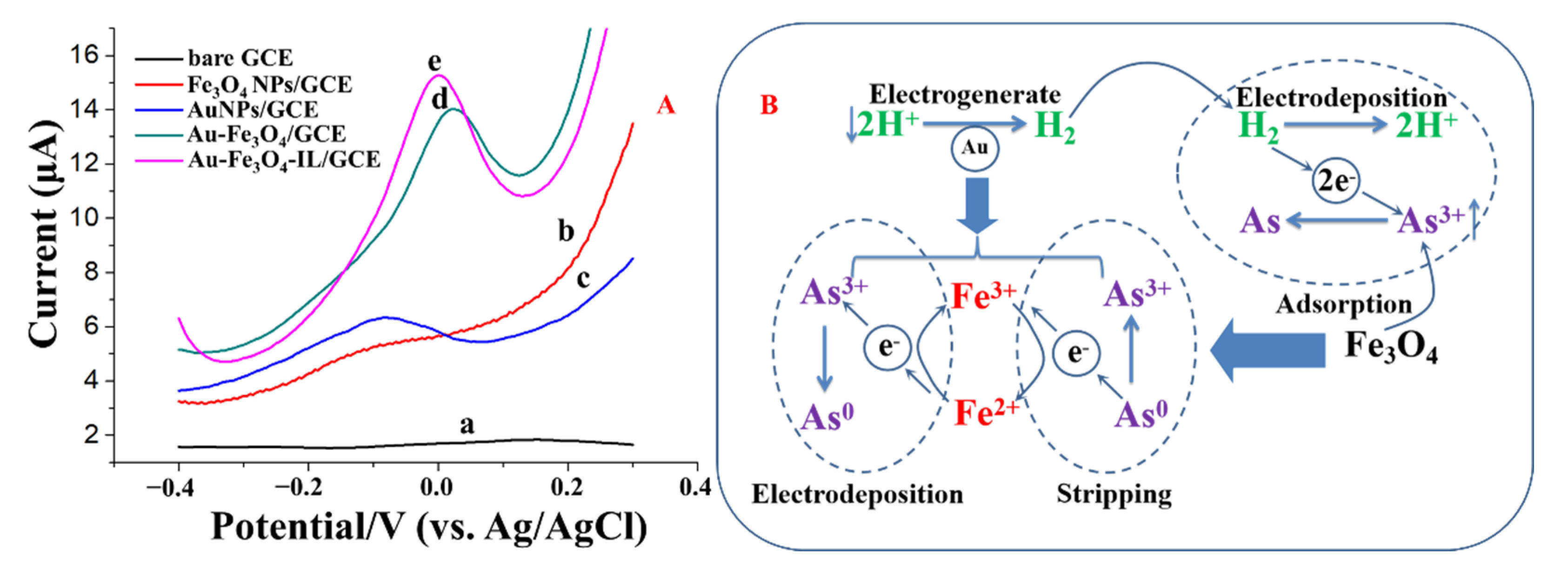
3.2. Electrochemical Characterizations of Modified Electrodes
3.3. Optimization of Analysis Parameters
3.4. Analytical Characteristics of Fe3O4-Au-IL/GCE for As(III)
3.4.1. Sensitivity, Limit of Detection, and Reproducibility
3.4.2. Selectivity
3.5. Analysis of Simulated Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater arsenic contamination throughout China. Science 2013, 341, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Lopipero, P.; Chung, J.; Haque, R.; Hernandez, A.; Moore, L.; Steinmaus, C. Arsenic in drinking water and cancer risks estimated from epidemiological studies in Argentina, Chile, Taiwan and Japan. Epidemiology 2000, 11, S93. [Google Scholar]
- Ning, Z.; Lobdell, D.T.; Kwok, R.K.; Liu, Z.; Zhang, S.; Ma, C.; Riediker, M.; Mumford, J.L. Residential exposure to drinking water arsenic in Inner Mongolia, China. Toxicol. Appl. Pharmacol. 2007, 222, 351–356. [Google Scholar] [CrossRef]
- Feeney, R.; Kounaves, S.P. On-site analysis of arsenic in groundwater using a microfabricated gold ultramicroelectrode array. Anal. Chem. 2000, 72, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Patlolla, A.K.; Centeno, J.A. Invited reviews: Carcinogenic and systemic health effects associated with arsenic exposure—A critical review. Toxicol. Pathol. 2003, 31, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, X.; Liu, K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem. 2004, 255, 67–78. [Google Scholar] [CrossRef]
- Melamed, D. Monitoring arsenic in the environment: A review of science and technologies with the potential for field measurements. Anal. Chim. Acta 2005, 532, 1–13. [Google Scholar] [CrossRef]
- Rahman, M.R.; Okajima, T.; Ohsaka, T. Selective detection of As (III) at the Au (111)-like polycrystalline gold electrode. Anal. Chem. 2010, 82, 9169–9176. [Google Scholar] [CrossRef]
- Mays, D.E.; Hussam, A. Voltammetric methods for determination and speciation of inorganic arsenic in the environment—A review. Anal. Chim. Acta 2009, 646, 6–16. [Google Scholar] [CrossRef]
- Sengupta, M.K.; Sawalha, M.F.; Ohira, S.-I.; Idowu, A.D.; Dasgupta, P.K. Green analyzer for the measurement of total arsenic in drinking water: Electrochemical reduction of arsenate to arsine and gas phase chemiluminescence with ozone. Anal. Chem. 2010, 82, 3467–3473. [Google Scholar] [CrossRef]
- Zaib, M.; Athar, M.M.; Saeed, A.; Farooq, U. Electrochemical determination of inorganic mercury and arsenic—A review. Biosens. Bioelectron. 2015, 74, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y. Speciation and analysis of mercury, arsenic, and selenium by atomic fluorescence spectrometry. TrAC Trends Anal. Chem. 2000, 19, 62–66. [Google Scholar] [CrossRef]
- Álvarez-Llamas, G.; del Rosario Fernández de laCampa, M.; Sanz-Medel, A. ICP-MS for specific detection in capillary electrophoresis. TrAC Trends Anal. Chem. 2005, 24, 28–36. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, N.; Fang, Z.; Feng, Y.; Ke, L. Simultaneous multi-channel hydride generation atomic fluorescence spectrometry determination of arsenic, bismuth, tellurium and selenium in tea leaves. Food Chem. 2011, 124, 1185–1188. [Google Scholar] [CrossRef]
- Barek, J.; Peckova, K.; Vyskocil, V. Adsorptive stripping voltammetry of environmental carcinogens. Curr. Anal. Chem. 2008, 4, 242–249. [Google Scholar] [CrossRef]
- Alves, G.M.S.; Magalhães, J.M.C.S.; Salaün, P.; Van den Berg, C.M.G.; Soares, H.M.V.M. Simultaneous electrochemical determination of arsenic, copper, lead and mercury in unpolluted fresh waters using a vibrating gold microwire electrode. Anal. Chim. Acta 2011, 703, 1–7. [Google Scholar] [CrossRef]
- Castaneda, M.T.; Merkoçi, A.; Pumera, M.; Alegret, S. Electrochemical genosensors for biomedical applications based on gold nanoparticles. Biosens. Bioelectron. 2007, 22, 1961–1967. [Google Scholar] [CrossRef]
- Martínez-Paredes, G.; González-García, M.B.; Costa-García, A. Lead sensor using gold nanostructured screen-printed carbon electrodes as transducers. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 925–930. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nanoscience and technology: A collection of reviews from nature journals. World Sci. 2010, 308–319. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H. Heat-assisted electrodissolution of platinum in an ionic liquid. Angew. Chem. 2012, 124, 1716–1720. [Google Scholar] [CrossRef]
- Sasaki, K.; Naohara, H.; Cai, Y.; Choi, Y.M.; Liu, P.; Vukmirovic, M.B.; Wang, J.X.; Adzic, R.R. Core-protected platinum monolayer shell high-stability electrocatalysts for fuel-cell cathodes. Angew. Chem. 2010, 122, 8784–8789. [Google Scholar] [CrossRef]
- Guo, S.; Wang, E. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Bu, L.; Gu, T.; Ma, Y.; Chen, C.; Tan, Y.; Xie, Q.; Yao, S. Enhanced cathodic preconcentration of As (0) at Au and Pt electrodes for anodic stripping voltammetry analysis of As (III) and As (V). J. Phys. Chem. C 2015, 119, 11400–11409. [Google Scholar] [CrossRef]
- Majid, E.; Hrapovic, S.; Liu, Y.; Male, K.B.; Luong, J.H.T. Electrochemical determination of arsenite using a gold nanoparticle modified glassy carbon electrode and flow analysis. Anal. Chem. 2006, 78, 762–769. [Google Scholar] [CrossRef]
- Pungjunun, K.; Chaiyo, S.; Jantrahong, I.; Nantaphol, S.; Siangproh, W.; Chailapakul, O. Anodic stripping voltammetric determination of total arsenic using a gold nanoparticle-modified boron-doped diamond electrode on a paper-based device. Microchim. Acta 2018, 185, 324. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Lou, S.; Sillanpää, M. Removal of arsenic (V) by magnetic nanoparticle activated microfibrillated cellulose. Chem. Eng. J. 2015, 260, 886–894. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.-C.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, J.; Xie, F.; Xiong, S. Electrochemical detection of As (III) by a rGO/Fe3O4-modified screen-printed carbon electrode. Anal. Sci. 2016, 32, 1053–1058. [Google Scholar] [CrossRef]
- Devi, P.; Sharma, C.; Kumar, P.; Kumar, M.; Bansod, B.K.S.; Nayak, M.K.; Singla, M.L. Selective electrochemical sensing for arsenite using rGO/Fe3O4 nanocomposites. J. Hazard. Mater. 2017, 322, 85–94. [Google Scholar] [CrossRef]
- Wei, J.; Li, S.-S.; Guo, Z.; Chen, X.; Liu, J.-H.; Huang, X.-J. Adsorbent assisted in situ electrocatalysis: An ultra-sensitive detection of As (III) in water at Fe3O4 nanosphere densely decorated with Au nanoparticles. Anal. Chem. 2016, 88, 1154–1161. [Google Scholar] [CrossRef]
- Li, S.-S.; Zhou, W.-Y.; Jiang, M.; Guo, Z.; Liu, J.-H.; Zhang, L.; Huang, X.-J. Surface Fe (II)/Fe (III) cycle promoted ultra-highly sensitive electrochemical sensing of arsenic (III) with dumbbell-like Au/Fe3O4 nanoparticles. Anal. Chem. 2018, 90, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.; Chuang, C.-H.; Kobayashi, Y.; Coombs, N.; Gorantla, S.; Botton, G.A.; Winnik, M.A.; Burda, C.; Scholes, G.D. Synthesis and optical properties of linker-free TiO2/CdSe nanorods. J. Phys. Chem. C 2014, 118, 3347–3358. [Google Scholar] [CrossRef]
- Sedki, M.; Khalil, I.A.; El-Sherbiny, I.M. Hybrid nanocarrier system for guiding and augmenting simvastatin cytotoxic activity against prostate cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, S641–S650. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Ahn, K.-H.; Lee, H.-J.; Lee, K.-H.; Kwak, Y.-J.; Song, K.-G. Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res. 1999, 33, 145–154. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Analytical applications of room-temperature ionic liquids: A review of recent efforts. Anal. Chim. Acta 2006, 556, 38–45. [Google Scholar] [CrossRef]
- Rozniecka, E.; Shul, G.; Sirieix-Plenet, J.; Gaillon, L.; Opallo, M. Electroactive ceramic carbon electrode modified with ionic liquid. Electrochem. Commun. 2005, 7, 299–304. [Google Scholar] [CrossRef]
- Khani, H.; Rofouei, M.K.; Arab, P.; Gupta, V.K.; Vafaei, Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion (II). J. Hazard. Mater. 2010, 183, 402–409. [Google Scholar] [CrossRef]
- Xiong, S.-Q.; Wei, Y.; Guo, Z.; Chen, X.; Wang, J.; Liu, J.-H.; Huang, X.-J. Toward membrane-free amperometric gas sensors: An ionic liquid–nanoparticle composite approach. J. Phys. Chem. C 2011, 115, 17471–17478. [Google Scholar] [CrossRef]
- Huang, X.-J.; Aldous, L.; O’Mahony, A.M.; del Campo, F.J.; Compton, R.G. Toward membrane-free amperometric gas sensors: A microelectrode array approach. Anal. Chem. 2010, 82, 5238–5245. [Google Scholar] [CrossRef]
- Das, R.S.; Singh, B.; Mukhopadhyay, S.; Banerjee, R. Gold nano particles catalyzed oxidation of hydrazine by a metallo-superoxide complex: Experimental evidences for surface activity of gold nano particles. Dalt. Trans. 2012, 41, 4641–4648. [Google Scholar] [CrossRef] [PubMed]
- Olesiak-Banska, J.; Gordel, M.; Kolkowski, R.; Matczyszyn, K.; Samoc, M. Third-order nonlinear optical properties of colloidal gold nanorods. J. Phys. Chem. C 2012, 116, 13731–13737. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Lu, X.; Duan, W.; Moriga, T. Synthesis and evaluation of the SERS effect of Fe3O4–Ag Janus composite materials for separable, highly sensitive substrates. RSC Adv. 2019, 9, 2877–2884. [Google Scholar] [CrossRef]
- Márquez, F.; Herrera, G.M.; Campo, T.; Cotto, M.; Ducongé, J.; Sanz, J.M.; Elizalde, E.; Perales, Ó.; Morant, C. Preparation of hollow magnetite microspheres and their applications as drugs carriers. Nanoscale Res. Lett. 2012, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Esterle, A.; Sharma, N.C.; Sahi, S. V Yucca-derived synthesis of gold nanomaterial and their catalytic potential. Nanoscale Res. Lett. 2014, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Van Quy, D.; Hieu, N.M.; Tra, P.T.; Nam, N.H.; Hai, N.H.; Thai Son, N.; Nghia, P.T.; Van Anh, N.T.; Hong, T.T.; Luong, N.H. Synthesis of silica-coated magnetic nanoparticles and application in the detection of pathogenic viruses. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Adams, D.M.; Brus, L.; Chidsey, C.E.D.; Creager, S.; Creutz, C.; Kagan, C.R.; Kamat, P.V.; Lieberman, M.; Lindsay, S.; Marcus, R.A. Charge transfer on the nanoscale: Current status. J. Phys. Chem. B 2003, 107, 6668–6697. [Google Scholar] [CrossRef]
- Sedki, M.; Chen, X.; Chen, C.; Ge, X.; Mulchandani, A. Non-lytic M13 phage-based highly sensitive impedimetric cytosensor for detection of coliforms. Biosens. Bioelectron. 2020, 148, 111794. [Google Scholar] [CrossRef]
- Zhao, G.; Sedki, M.; Ma, S.; Villarreal, C.; Mulchandani, A.; Jassby, D. Bismuth subcarbonate decorated reduced graphene oxide nanocomposite for the sensitive stripping voltammetry analysis of Pb(II) and Cd(II) in water. Sensors 2020, 20, 6085. [Google Scholar] [CrossRef]
- Renock, D.; Voorhis, J. Electrochemical investigation of arsenic redox processes on pyrite. Environ. Sci. Technol. 2017, 51, 3733–3741. [Google Scholar] [CrossRef]
- Misra, A.K. A river about to die: Yamuna. J. Water Resour. Prot. 2010, 2, 489. [Google Scholar] [CrossRef]
- Malik, D.; Singh, S.; Thakur, J.; Singh, R.K.; Kaur, A.; Nijhawan, S. Heavy metal pollution of the Yamuna River: An introspection. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 856–863. [Google Scholar]







| Sample No. | Found a (μg/L) | Added (μg/L) | Detected after Adding a (μg/L) | Mean Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 3.12 ± 1.08 | 5 | 7.98 ± 1.23 | 97.2 | 4.8 |
| 2 | 4.89 ± 0.89 | 10 | 15.02 ± 1.15 | 101.3 | 3.3 |
| 3 | 9.95 ± 0.96 | 15 | 24.75 ± 0.90 | 98.67 | 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedki, M.; Zhao, G.; Ma, S.; Jassby, D.; Mulchandani, A. Linker-Free Magnetite-Decorated Gold Nanoparticles (Fe3O4-Au): Synthesis, Characterization, and Application for Electrochemical Detection of Arsenic (III). Sensors 2021, 21, 883. https://doi.org/10.3390/s21030883
Sedki M, Zhao G, Ma S, Jassby D, Mulchandani A. Linker-Free Magnetite-Decorated Gold Nanoparticles (Fe3O4-Au): Synthesis, Characterization, and Application for Electrochemical Detection of Arsenic (III). Sensors. 2021; 21(3):883. https://doi.org/10.3390/s21030883
Chicago/Turabian StyleSedki, Mohammed, Guo Zhao, Shengcun Ma, David Jassby, and Ashok Mulchandani. 2021. "Linker-Free Magnetite-Decorated Gold Nanoparticles (Fe3O4-Au): Synthesis, Characterization, and Application for Electrochemical Detection of Arsenic (III)" Sensors 21, no. 3: 883. https://doi.org/10.3390/s21030883
APA StyleSedki, M., Zhao, G., Ma, S., Jassby, D., & Mulchandani, A. (2021). Linker-Free Magnetite-Decorated Gold Nanoparticles (Fe3O4-Au): Synthesis, Characterization, and Application for Electrochemical Detection of Arsenic (III). Sensors, 21(3), 883. https://doi.org/10.3390/s21030883








