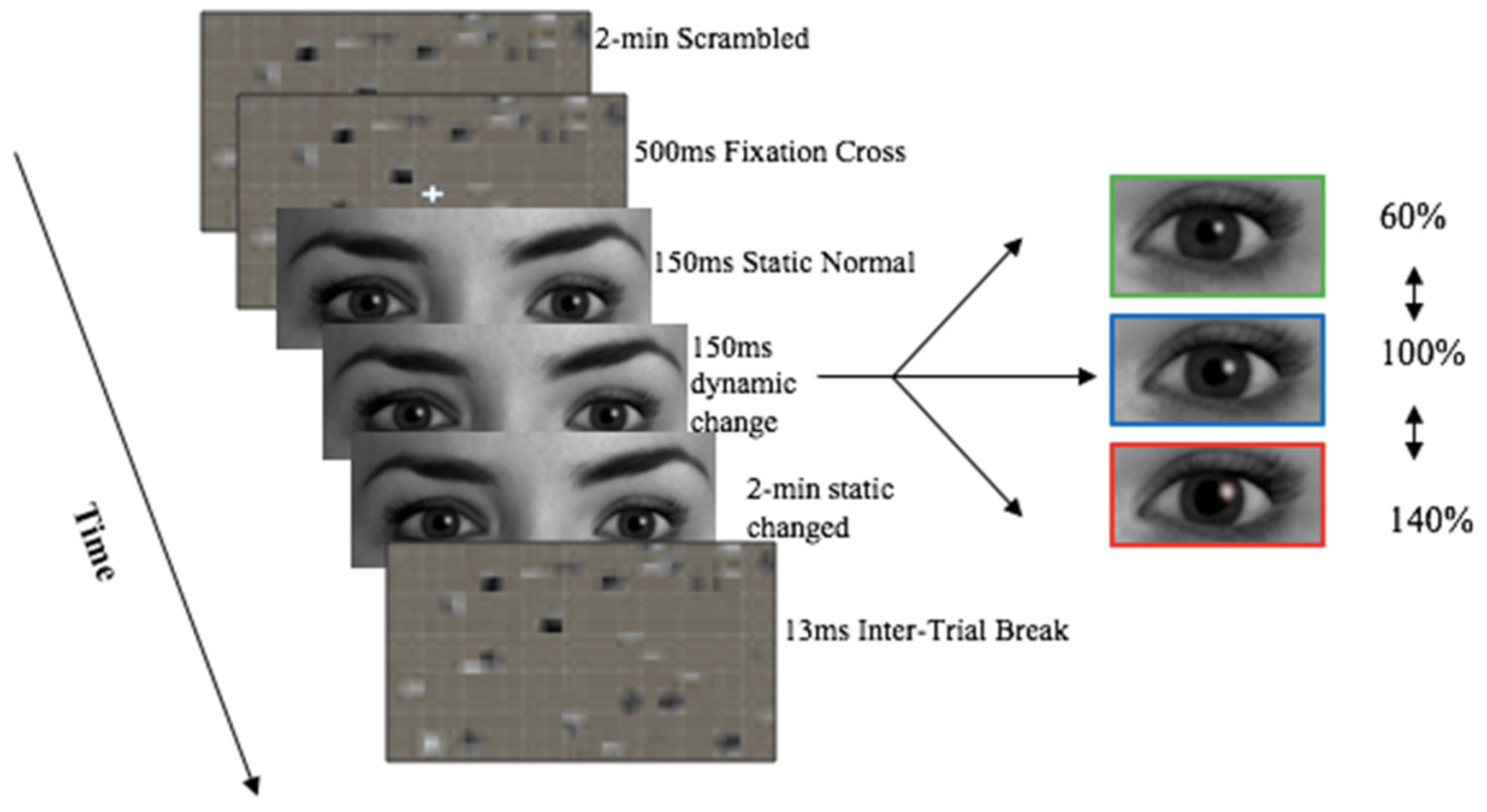
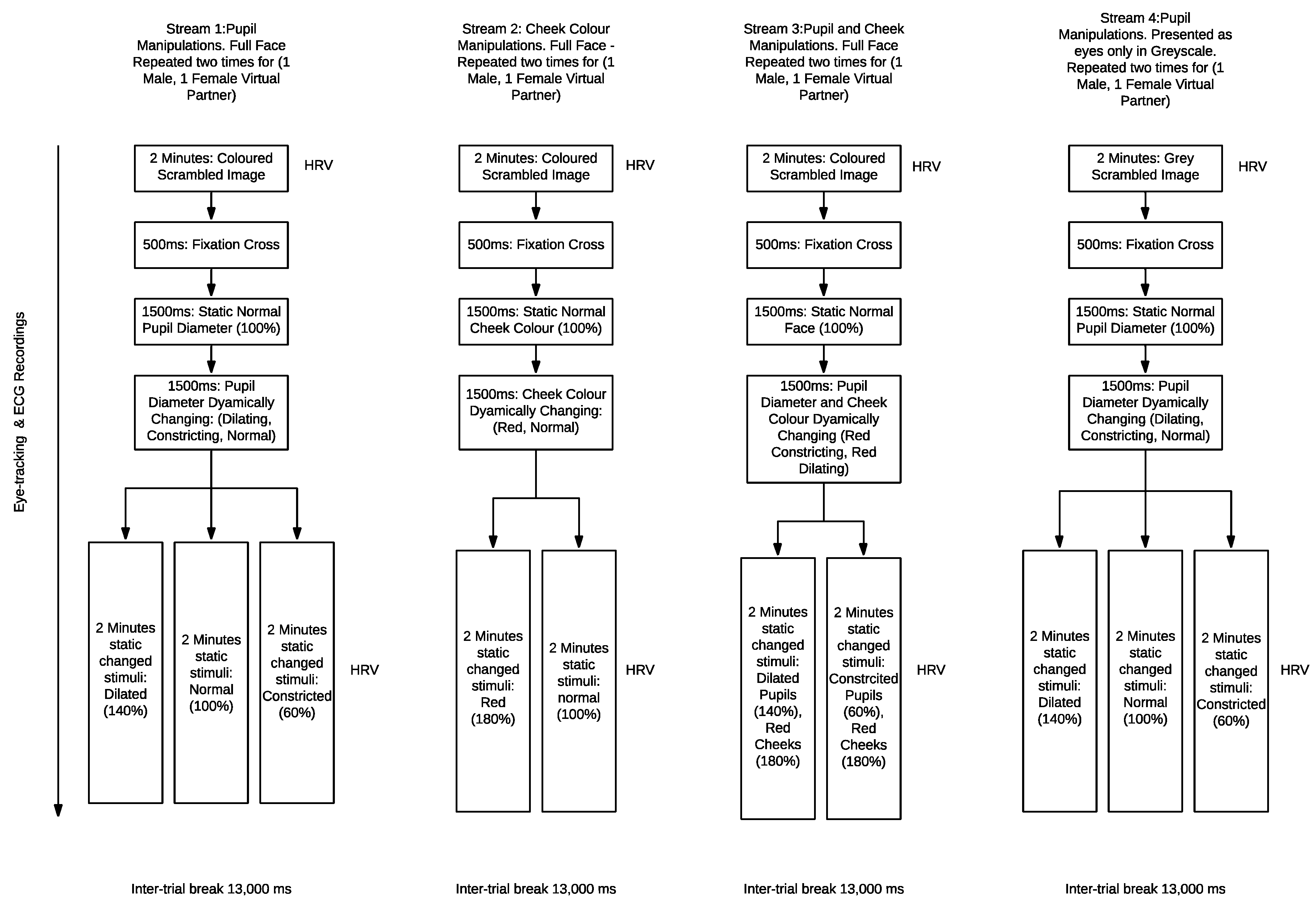
1. Introduction
Artificial intelligence (AI) describes a machine operating with human-like qualities [
1]. AI systems are rapidly advancing, from verbal based systems, such as Apple’s Siri, and Amazon’s Alexa, to the development of visual interaction partners with profoundly adapted interactive abilities [
1]. In 2018 a New-Zealand company ‘Soul Machines’ released a digital human called Nadia [
1]. Virtual human avatar interfaces such as Nadia have the ability to alter their physical parameters, such as pupil size [
1]. Pupil size and blush are salient visual expressions of sympathetic activity, and emotional arousal, controlled by the autonomic nervous system (ANS) [
2,
3]. These expressions aid human interactions by indicating how an interaction partner is feeling, therefore indicating how an observer should appropriately respond [
2]. Human-computer interaction studies found observers to mimic pupillary changes in a virtual partner, this enhanced trust, and affected subsequent decisions made by human observers [
4,
5]. Blush and pupil dilation are mediated by the sympathetic nervous system (SNS), and when presented in a virtual interaction partner enhance trust, and attractiveness ratings by both men and women [
6,
7,
8].
Literature has further found manipulations of pupil size in virtual partners to alter human heart rate in an observer [
8]. A gap in the current literature is knowledge of how these manipulations affect heart rate variability. Pupil size, cheek colour, and heart rate variability (HRV) are mediated by the ANS, a non-conscious system regulated by parasympathetic (PNS) and SNS branches [
9]. Heart rate variability indicates variations in cardiac contractions timing, to suggest which branch of the ANS is dominating at a given point in time [
10]. Trust is promoted by salient visual cues, such as increase in pupil size, and cheek colour [
6,
7,
8]. If such cues elicit changes in HRV, this enables virtual partners to non-consciously manipulate human physiological functioning. It is important to determine whether virtual interaction partners possess the ability to modulate human pupil size and HRV, which regulates human emotion [
3,
5]. If changes in pupil size and cheek colour presented in a virtual partner induce subsequent changes in human HRV, this indicates that virtual interaction partners have the ability to non-consciously modulate human autonomic functioning; and emotional regulatory of how calm, or excited an individual is. This pertains to their subsequent trust and decisions as a result.
The present study investigated the effects of manipulations in virtual partners on human observers autonomic functioning. This research is the first study to investigate communicative effects of virtual humans on autonomic functioning in humans through the incorporation of heart rate variability measures, additionally being the first to incorporate stimuli consisting of full faces in colour, and a blush effect. The aim of the present research was to determine the effects of pupil size, and cheek colour manipulations of a virtual partner, on human observer autonomic functioning, measurable in pupil diameter and heart rate variability stimuli responses. Our primary hypothesis proposed manipulations of pupil size and cheek colour in a virtual human interface to modulate pupil size and heart rate variability in an observer. With a secondary hypothesis predicting gender of the virtual partner to differentially affect the male participants’ response.
Overall this research aims to communicate the importance of understanding the physiological modulating abilities of virtual humans, during interactions. This research may also be applicable in clinical practice in inducing ANS changes to individuals with short term autonomic dysfunction, such as those who experience panic attacks, or to assist anxious patients in hospitals.
Presented below are the methods and analysis utilized for this study, along with our novel findings and discussion. Further discussion into the applications/impacts of our study and future work are also provided.
4. Discussion
Evolving technology has resulted in realistic, and dynamic, virtual interaction partners, which possess the ability to manipulate their own salient visual communication features such as pupil diameter. Implementation, of such technology into areas such as banking [
1], and potential implementations in clinical settings raises ethical concerns regarding their potential influence on human autonomic functioning. Previous research presented virtual human stimuli consisting of the eye region in grey scale derived from a human face bank. This holds little ecological validity regarding human interactions [
2,
5,
11]. Such research found virtual partners modulate emotions, and trust in human observers [
29]. An area which required further investigation were the effects of this technology on human HRV, which is the gold standard for measuring human autonomic functioning and has also been associated with stress and emotion [
9].
The present study explored potential modulation of human autonomic function by dynamically manipulating salient visual features in virtual human partners; pupil size, and cheek colour. Measurements of these effects on participants’ autonomic pupil diameter and heart rate variability responses were achieved using eye tracking and electrocardiogram recording and analysis. This provided novel insights on the effects of pupil mimicry of virtual humans on HRV. Additionally, the incorporation of blush, and pupil manipulations in virtual human stimuli, created by ‘Soul Machines,’ a company which design virtual humans, enhanced the ecological validity of our experimental stimuli, comparative to previous research. Our investigations provide first evidence of female virtual partners’ ability to modulate mimicry in male observers’ and also is the first to observe a pupillary lateralization effect.
4.1. Right vs. Left Pupil Response
Previous literature analysed mimicry as a mean pupil diameter change of both eyes [
5], reporting mimicry to occur in both [
30]. The present study is the first to consider pupil mimicry using a distinct analysis specific to each eye. Pupil manipulations in virtual partners presented as an eye region in grey scale, and a full face in colour elicited a right dominant pupillary lateralization response, with pupil manipulations in both male and female virtual partners inducing a significant pupillary response in participants’ right eye only (
Figure 5 and
Figure 7,
Supplementary Tables S1 and S4). This lateralization response may be attributed to the pupillary response axis; the Locus Coeruleus norepinephrine system which regulates differences in pupil diameter between both eyes [
31], inducing lateralization in response to emotional images [
32]. Differential activation of this system is a potential mechanism inducing pupillary emotional responses, and mimicry which stimulate the Locus Corelus to induce a lateralisation effect. Thereby causing differentiation in the diameter between left and right pupils. Our finding of lateralization indicates the right eye to adhere greater responsiveness to activation initiated by emotional stimuli. No previous pupil mimicry studies having reported lateralization in humans, or primates’. Future mimicry studies investigating this effect are needed for greater understanding.
4.2. Pupil Constriction vs. Dilation
To determine effects of virtual human pupil size on human autonomic functioning, virtual partners’ pupils were made to dynamically constrict, and dilate by 40% comparative to normal pupil diameter. Differences in pupil diameter between constriction and dilation conditions were 80%, adhering to the physiological range for pupillary changes [
30]. Human observer pupillary and HRV responses, were measured. Previous mimicry studies reported a 40% difference between constriction and dilation of the virtual partners’ pupils sufficient to induce pupil mimicry [
30], this conflicts with our findings of an 80% difference only to be sufficient. This may be attributable to previous studies analysing mimicry effects on participants as a mean of both pupils, rather than individually [
30].
Virtual partners presented as a full face in color, induced a mimicry response to the female virtual partner only with a statistically significant difference in participants’ mean pupil size over two minutes in response to pupil dilation and constriction (
Figure 5), (see
Supplementary Table S1). This mimicry response was reflected in the participants PNS cardiac activity (
Figure 6,
Table 1). Pupil constriction of the female virtual partner induced a significant increase in the participants parasympathetic pupillary and heart rate variability PNS response, in comparison to dilation which induced a decrease (
Figure 5 and
Figure 6). Pupil constriction in the female virtual partner induced a reduction in participants’ right eye pupil diameter, indicating parasympathetic dominance, in combination with an increase in the PNS output of participants’ heart rate variability. This was expected for pupil constriction is parasympathetically controlled and communicates this activity to an observer [
33]. Female Virtual partner pupil dilation induced a decrease in parasympathetic activity, which also was expected due to being sympathetically modulated [
34].
Differences between dilated and constricted pupil conditions compared to the normal over two minutes was not statistically significant. However, there was a trend with pupil dilation of the female virtual partner inducing an increase in participants’ pupil diameter compared to normal, and constriction inducing a decrease compared to normal (
Supplementary Table S1). This result is likely due to pupil dilation, induced by an increase in sympathetic activity and constriction caused by greater parasympathetic activity compared to the normal [
33,
34]. This trend in mimicry over two minutes was likely in response to the participants’ pupil dynamically changing over 1.5 s to mirror that of the virtual partner, as previous literature found pupil manipulations to affect participants trust, which was quantified by a decision they made subsequent to viewing dynamically changing pupils, indicating pupillary stimuli to induce effects which continue even after cessation of stimuli presentation [
30].
Our findings show a female virtual partner, presented as a full face in colour, to modulate parasympathetic functioning of male humans, by dynamically manipulating pupil diameter. The autonomic response of participants’ pupils, and heart rate variability showed synchrony in their PNS response, indicating the manipulations in virtual interaction partners can modulate mimicry effects in humans (
Table 1). There was no significant sympathetic (SNS), response in HRV of participants (
Table 1). Heart rate variability enables insight into the contribution of parasympathetic and sympathetic activity relative to one another [
30,
35]. The sympathetic, and parasympathetic nervous system is part of one intricate system; the autonomic nervous system, which operates in a dynamic balance. Despite suppressing one another to maintain balance, these systems may act independently as well due to comprising distinct neural pathways [
19]. This may explain this lack of sympathetic response. Therefore, when virtual partners’ pupils constricted, this induced an increase in participant PNS activity via mimicry, indicating the participant was in a more relaxed state due to the PNS, which is a resting system being dominant [
35].
4.3. Grey Scale Cropped Region
Virtual human stimuli presented as a cropped eye region in grey scale was included, using our own unique stimuli to replicate methods used in previous research [
2,
5,
11]. This was to confirm the reproducibility of prior research, and have a comparison model for responses to manipulations in a virtual partner when presented as a more ecologically valid model; full face in colour. Previous relevant literature reported pupil mimicry [
2,
5,
11]. The present study found no significant mimicry effects (
p > 0.05) when pupillary changes were presented in a virtual partner as a cropped eye region in greyscale on human observer heart rate variability and pupillary parameters (see
Supplementary Table S8).
Our findings oppose the mimicry response reported in previous literature [
2,
5,
11], where dilation of the virtual partner in fact reduced the pupil diameter (
Figure 7). When presented as an eye region in greyscale the male virtual human induced a participant response which opposed mimicry, and the female virtual partner induced no significant participant response. When presented as a full face colour the male virtual partner induced no significant response, comparative to the female virtual partner which induced a mimicry response. This result may be attributable to the full face of the female being viewed as more pleasing, and trustworthy, when presented in colour inducing a significant mimicry response, as opposed to when the female was presented in greyscale which reduced ecological validity, likely reducing participant relatability to her and resulting in this presentation of the female partner being unable to induce a significant mimicry response [
4].
Comparatively the lack of mimicry in response to the male virtual partner when presented in the full face in colour, and the induction of a significant response opposing mimicry when the male was presented as the cropped eye region in grey scale may have been due to the male being seen as competition or potentially threatening and this perception being enhanced when the male was presented as eyes only [
5]. These ideas are further discussed under gender effects.
4.4. Blush
Blush is a sympathetically modulated emotional response; observation of an emotional response evokes such emotion in an observer [
36]. Presentation of blush in a virtual partner, was hypothesised to modulate sympathetic activity in humans, inducing pupil dilation, and a decreased in heart rate variability. Furthermore, blush combined with dynamic pupil dilation was hypothesized to increase sympathetic modulatory responses comparable to that induced by blush alone due to displaying two visual stimuli, with sympathetic activity [
33,
37]. Blush presented in conjunction with pupil constriction enabled understanding of human responses to stimuli regulated by different branches of the autonomic nervous system with opposing functions. Pupil constriction is under parasympathetic mediation, blush; sympathetic. This additionally enabled insight of whether pupils or cheeks are more salient communications factors depending on participant’s response being parasympathetic, or sympathetically dominated.
Blush was presented in the virtual humans gradually over 1.5 s, as an 80% increase from the normal cheek colour and faded after 1–2 min to be consistent with dynamic pupillary changes presented. Blush induced no significant effects on participants autonomic functioning, as determined by pupil diameter, and heart rate variability responses (
Supplementary Tables S2 and S6). This may be due to blush distracting from the pupils of the virtual partners, when presented with pupil manipulations, or may have made the virtual partner less relatable and therefore associated as an out group partner, which defines an individual that the participants’ associates as different from them self, humans do not mimic outgroup partners [
4]. Alternatively, the lack of response to the blush may indicate blush to not be key a communicative cue. The timing of human blush presentation, in addition to the percentage of blush colouration has not been reported in literature, such research would benefit further understanding.
4.5. Male vs. Female/Gender Effects
A male and female virtual partner were incorporated to determine the effects of virtual partner gender on the quality of interactions with participants’, as quantified by participants’ autonomic responses as a secondary outcome we were investigating. The gender of the virtual partner elicited differential responses. Only the female virtual partner modulated a mimicry effect on male participants parasympathetic functioning, in both pupillary and HRV responses (
Figure 5 and
Figure 6), (see
Table 1,
Supplementary Table S1). Comparatively, the virtual male partner evoked an opposing significant autonomic response in the cropped grey scale images. These findings indicate the presence of a gender effect in response to the virtual partners. The greater mimicry response to the female virtual partner, may be attributable to male participants associating more with her [
4]. Majority of participants verbalized preference for the female virtual interaction partner, describing her as less intimidating and easier to fixate on.
Pupil mimicry is absent in a competitive context, when competition is removed pupil mimicry is observed [
5]. The absence of significant mimicry between the male participants, and male virtual partner in the present study may be due to such competition. These effects were likely in response to the gender of the virtual partner, opposed to virtual partner’s facial features, due to facial features being controlled for by presenting the same face for both male and female and overlaying it with a gender face filter using Faceapp (Face App version 3.4.12); manufactured by Wireless Lab, (Skolkovo, Russia). Previous literature presented cropped regions of male and female virtual partners face in grey scale, and found gender effects with a stronger mimicry response to pupil constriction in response to a partner of opposite sex, however, found no gender effects on pupil dilation mimicry [
30].
Gender effects were not statistically significant; however, a trend was apparent with mimicry responses indicating male participant preference for female virtual interaction partners. Further research deducing reason for observed gender effects through inclusion of female, and homosexual participants, may further expand understanding of virtual human interactions and gender implications.
4.6. Limitations & Future Research
The present study included young healthy male participants to control for differential autonomic nervous system activation. Females and those of an older age demographic were excluded due to variations in HRV. HRV decreases with age, with older individuals exhibiting reductions in heart rate deceleration, attributed to a reduction in parasympathetic cardiac control [
12,
13]. Sex specific differences in females are due to their menstrual cycle which causes an increased HRV compared to males [
10]. Research incorporating females, and an older age demographic would enable comparisons to responses measured in young male adults. Previous research has shown females to exhibit increased arousal responses to stimuli [
32]. The effects of virtual partners on females would be of interest due to pupillary responses in humans affected by arousal [
13]. Therefore, females may adhere larger responses to manipulations in virtual humans. Incorporation of an older age demographic would enable comparisons between generations, with individuals who grew up using technology and those who did not. This would determine whether these groups are differentially affected. Influences of an interaction partner depends on the susceptibility of the individual they are associating with [
38]. Therefore, older generations who use technology less may be less susceptible to such effects.
Previous mimicry research included experimental trials seconds in length, those in the present study were 5 min, due to incorporating heart rate variability, which has not previously been incorporated in this field of research. Heart rate variability requires two minutes of baseline and two minutes of stimuli presentation to attain accurate RMSSD HRV measurements [
17]. Stimuli presentation in the present study was randomized to control for fatigue affecting participants’ response. Participants varied in their ability to focus on the virtual partner, comparing autonomic responses of these participants would enable understanding of how attention to an interaction partner influences consequent responses.
Previous research presented virtual partners as a cropped eye region in grey scale. This made it implausible for the present research to present highly dynamic virtual partners. We presented stimuli as faces which dynamically changed, however, virtual humans presented in this context cannot completely replace face to face human interactions due to limitations in their comparable relatability. Replication of the present research in the context of virtual partners, who move and blink, or are presented in 3D would likely increase adherence to real life human interactions, enhancing ecological validity and potential relatability.
Overall our findings provide insights into the potential benefits, regarding both health, and communication. These findings are significant, as they can influence the development of future virtual humans to aid clinical applications. With further investigation, we envisage applications in clinical settings, which could potentially aid short, and long term autonomic nervous system health. Further investigations could lead to the development of short term applications which could induce an immediate calming effect, and long term changes to aid individuals affected with disorders in autonomic nervous system functioning. Additionally, these findings highlight the ongoing diversity in the development and application of virtual humans into everyday life making future human interactions with such virtual humans inevitable.