Extending Porous Silicone Capacitive Pressure Sensor Applications into Athletic and Physiological Monitoring
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Fabrication Apparatus
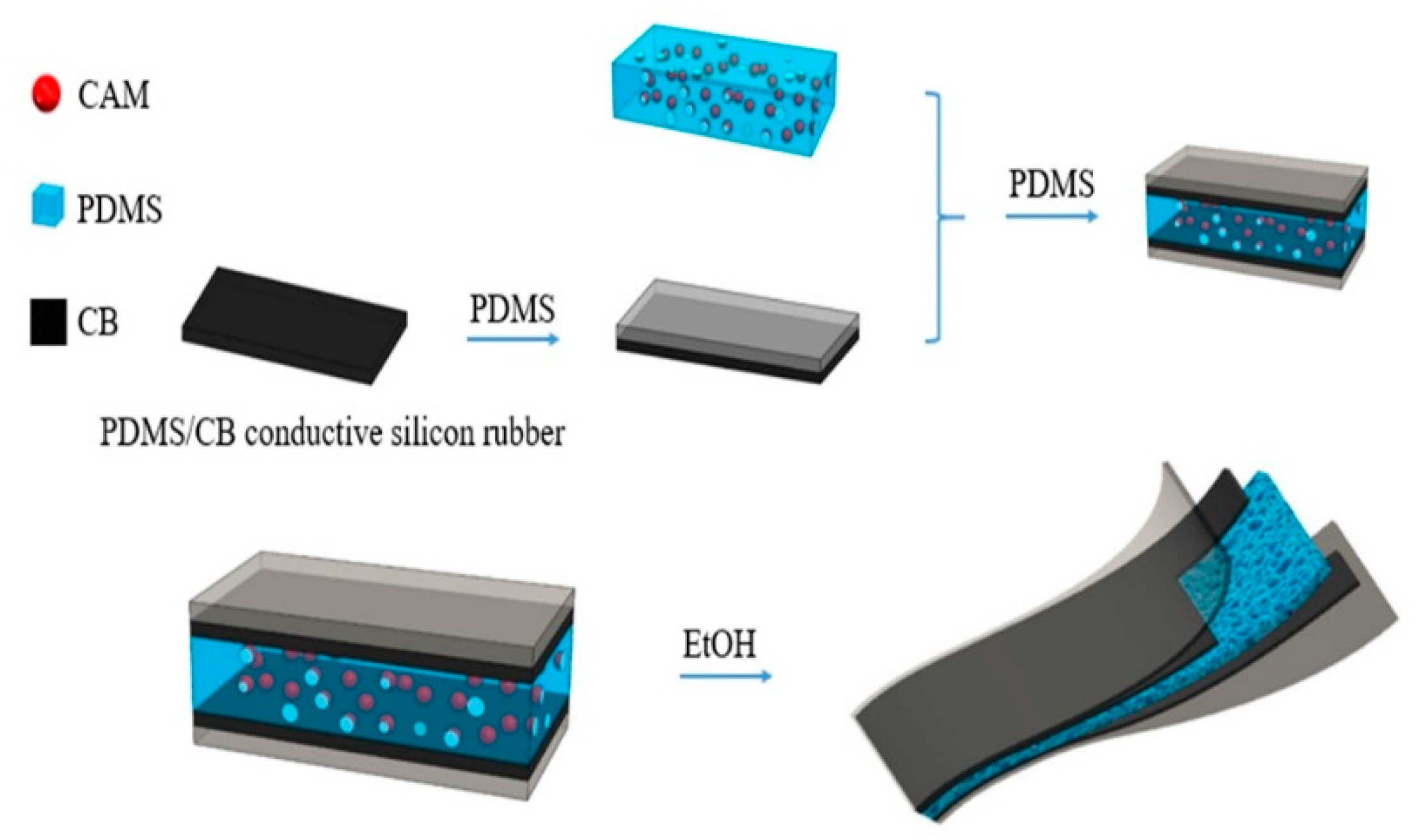
2.2. Fabrication of Sensor
2.3. Characterization
2.4. Device Integration
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample | mPDMS:mCAM | a Volume Reduction/% | b Force/N | Initial Volume/mm3 | Volume after Ethanol Soaking/mm3 |
|---|---|---|---|---|---|
| S1 | 3:1 | 22.3% | 0.3 | 8800 | 6840 |
| S2 | 5:1 | 22.3% | 0.113 | 8800 | 6840 |
| S3 | 7:1 | 47.5% | 0.112 | 8800 | 4624 |
| S4 | 9:1 | 56.5% | 0.103 | 8800 | 3825 |









References
- Trung, T.Q.; Lee, N.-E. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoringand Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.L.; Xia, Y.; Wu, X.; Kirk, T.V.; Chen, X.D. A low-cost and highly integrated sensing insole for plantar pressure measurement. Sens. Bio-Sens. Res. 2019, 26, 100298. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, H.; Seo, J.; Shin, S.; Koo, J.H.; Pang, C.; Son, S.; Kim, J.H.; Jang, Y.H.; Kim, D.E.; et al. Conductive fiber-based ultrasensitive textile pressure sensor for wearable electronics. Adv. Mater. 2015, 27, 2433–2439. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.L.; Xia, Y.; Zhang, P.F.; Kirk, T.V.; Chen, X.D. Textile-Only Capacitive Sensors for Facile Fabric Integration without Compromise of Wearability. Adv. Mater. Technol. 2019, 4, 1900485. [Google Scholar] [CrossRef]
- Choi, T.Y.; Hwang, B.U.; Kim, B.Y.; Trung, T.Q.; Nam, Y.H.; Kim, D.N.; Eom, K.; Lee, N.E. Stretchable, Transparent, and Stretch-Unresponsive Capacitive Touch Sensor Array with Selectively Patterned Silver Nanowires/Reduced Graphene Oxide Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 18022–18030. [Google Scholar] [CrossRef] [PubMed]
- Jcm, S.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS ONE 2017, 12, e0183989. [Google Scholar]
- Kwasnicki, R.M.; Ali, R.; Jordan, S.J.; Atallah, L.; Leong, J.J.; Jones, G.G.; Cobb, J.; Yang, G.Z.; Darzi, A. A wearable mobility assessment device for total knee replacement: A longitudinal feasibility study. Int. J. Surg. 2015, 18, 14–20. [Google Scholar] [CrossRef]
- Min, S.D.; Yun, Y.; Shin, H. Simplified Structural Textile Respiration Sensor Based on Capacitive Pressure Sensing Method. IEEE Sens. J. 2014, 14, 3245–3251. [Google Scholar]
- Yao, S.; Zhu, Y. Wearable multifunctional sensors using printed stretchable conductors made of silver nanowires. Nanoscale 2014, 6, 2345–2352. [Google Scholar] [CrossRef]
- You, B.; Han, C.J.; Kim, Y.; Ju, B.-K.; Kim, J.-W. A wearable piezocapacitive pressure sensor with a single layer of silver nanowire-based elastomeric composite electrodes. J. Mater. Chem. A 2016, 4, 10435–10443. [Google Scholar] [CrossRef]
- Chhetry, A.; Yoon, H.; Park, J.Y. A flexible and highly sensitive capacitive pressure sensor based on conductive fibers with a microporous dielectric for wearable electronics. J. Mater. Chem. C 2017, 5, 10068–10076. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.-U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, P.; Chou, J.B.; Xu, R.; Zhao, R.; Hart, A.J.; Kim, S.G. Extremely Elastic Wearable Carbon Nanotube Fiber Strain Sensor for Monitoring of Human Motion. ACS Nano 2015, 9, 5929–5936. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Zhong, W.; Zhao, Q.; Wang, D. Stretchable conductive polypyrrole/polyurethane (PPy/PU) strain sensor with netlike microcracks for human breath detection. ACS Appl. Mater. Interfaces 2014, 6, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Fahad, H.M.; Chen, K.; Emaminejad, S.; Gao, Y.; Tai, L.-C.; Ota, H.; Wu, E.; et al. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Ma, J.; Lv, J.; Ma, H.; Xia, H.; Wang, J.; Yang, C.; Xue, M.; Li, G.; Zhu, N. Facile Wearable Vapor/Liquid Amphibious Methanol Sensor. ACS Sens. 2019, 4, 152–160. [Google Scholar] [CrossRef]
- Ma, S.; Ye, T.; Zhang, T.; Wang, Z.; Li, K.; Chen, M.; Zhang, J.; Wang, Z.; Ramakrishna, S.; Wei, L. Highly Oriented Electrospun P(VDF-TrFE) Fibers via Mechanical Stretching for Wearable Motion Sensing. Adv. Mater. Technol. 2018, 3, 1800033. [Google Scholar] [CrossRef]
- Amit, M.; Mishra, R.K.; Hoang, Q.; Galan, A.M.; Wang, J.; Ng, T.N. Point-of-use robotic sensors for simultaneous pressure detection and chemical analysis. Mater. Horiz. 2019, 6, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Fan, S.; Qiu, H.; Su, D.; Li, L.; Su, J. CB Nanoparticles Optimized 3D Wearable Graphene Multifunctional Piezoresistive Sensor Framed by Loofah Sponge. ACS Appl. Mater. Interfaces 2020, 12, 36540–36547. [Google Scholar] [CrossRef]
- Chen, S.; Wu, R.; Li, P.; Li, Q.; Gao, Y.; Qian, B.; Xuan, F. Acid-Interface Engineering of Carbon Nanotube/Elastomers with Enhanced Sensitivity for Stretchable Strain Sensors. ACS Appl. Mater. Interfaces 2018, 10, 37760–37766. [Google Scholar] [CrossRef]
- Luo, N.; Huang, Y.; Liu, J.; Chen, S.-C.; Wong, C.P.; Zhao, N. Hollow-Structured Graphene–Silicone-Composite-Based Piezoresistive Sensors: Decoupled Property Tuning and Bending Reliability. Adv. Mater. 2017, 29, 1702675. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Mitra, D.; Peterson, K.; Maharbiz, M.M. A Highly Elastic, Capacitive Strain Gauge Based on Percolating Nanotube Networks. Nano Lett. 2012, 12, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Atalay, A.; Sanchez, V.; Atalay, O.; Vogt, D.M.; Haufe, F.; Wood, R.J.; Walsh, C.J. Batch Fabrication of Customizable Silicone-Textile Composite Capacitive Strain Sensors for Human Motion Tracking. Adv. Mater. Technol. 2017, 2, 1700136. [Google Scholar] [CrossRef] [Green Version]
- Atalay, O. Textile-Based, Interdigital, Capacitive, Soft-Strain Sensor for Wearable Applications. Materials 2018, 11, 768. [Google Scholar] [CrossRef] [Green Version]
- White, E.L.; Yuen, M.C.; Case, J.C.; Kramer, R.K. Low-Cost, Facile, and Scalable Manufacturing of Capacitive Sensors for Soft Systems. Adv. Mater. Technol. 2017, 2, 1700072. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Q.; Wu, X.E.; Kirk, T.V.; Chen, X.D. Practical and Durable Flexible Strain Sensors Based on Conductive Carbon Black and Silicone Blends for Large Scale Motion Monitoring Applications. Sensors 2019, 19, 4553. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.-Y.; Kim, J.; Kim, H.; Kim, C.; Lee, S.-D. Low-cost flexible pressure sensor based on dielectric elastomer film with micro-pores. Sens. Actuators A Phys. 2016, 240, 103–109. [Google Scholar] [CrossRef]
- Kang, S.; Lee, J.; Lee, S.; Kim, S.; Kim, J.-K.; Algadi, H.; Al-Sayari, S.; Kim, D.-E.; Kim, D.; Lee, T. Highly Sensitive Pressure Sensor Based on Bioinspired Porous Structure for Real-Time Tactile Sensing. Adv. Electron. Mater. 2016, 2, 1600356. [Google Scholar] [CrossRef]
- Li, J.; Bao, R.; Tao, J.; Peng, Y.; Pan, C. Recent progress in flexible pressure sensor arrays: From design to applications. J. Mater. Chem. C 2018, 6, 11878–11892. [Google Scholar] [CrossRef]
- Aqueveque, P.; Osorio, R.; Pastene, F.; Saavedra, F.; Pino, E. Capacitive Sensors Array for Plantar Pressure Measurement Insole fabricated with Flexible PCB. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4393–4396. [Google Scholar]
- Tolvanen, J.; Hannu, J.; Jantunen, H. Hybrid Foam Pressure Sensor Utilizing Piezoresistive and Capacitive Sensing Mechanisms. IEEE Sens. J. 2017, 17, 4735–4746. [Google Scholar] [CrossRef]
- Chang, W.Y.; Huang, C.C.; Chen, C.C.; Chang, C.C.; Yang, C.L. Design of a novel flexible capacitive sensing mattress for monitoring sleeping respiratory. Sensors 2014, 14, 22021–22038. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Zhao, X.; Matsuo, K.; Liu, J.; Mukai, T. Unconstrained Detection of the Respiratory Motions of Chest and Abdomen in Different Lying Positions Using a Flexible Tactile Sensor Array. IEEE Sens. J. 2019, 19, 10067–10076. [Google Scholar] [CrossRef]
- Laurino, M.; Arcarisi, L.; Carbonaro, N.; Gemignani, A.; Menicucci, D.; Tognetti, A. A Smart Bed for Non-Obtrusive Sleep Analysis in Real World Context. IEEE Access 2020, 8, 45664–45673. [Google Scholar] [CrossRef]
- Harada, T.; Sakata, A.; Mori, T.; Sato, T. Sensor pillow system: Monitoring respiration and body movement in sleep. In Proceedings of the 2000 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2000), Takamatsu, Japan, 31 October–5 November 2000; pp. 351–356. [Google Scholar]
- Tian, M.; Lu, Y.; Qu, L.; Zhu, S.; Zhang, X.; Chen, S. A Pillow-Shaped 3D Hierarchical Piezoresistive Pressure Sensor Based on Conductive Silver Components-Coated Fabric and Random Fibers Assembly. Ind. Eng. Chem. Res. 2019, 58, 5737–5742. [Google Scholar] [CrossRef]
- Lokavee, S.; Puntheeranurak, T.; Kerdcharoen, T.; Watthanwisuth, N.; Tuantranont, A. Sensor pillow and bed sheet system: Unconstrained monitoring of respiration rate and posture movements during sleep. In Proceedings of the 2012 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Seoul, Korea, 14–17 October 2012; pp. 1564–1568. [Google Scholar]
- Yu, Y.; Luo, Y.; Guo, A.; Yan, L.; Wu, Y.; Jiang, K.; Li, Q.; Fan, S.; Wang, J. Flexible and transparent strain sensors based on super-aligned carbon nanotube films. Nanoscale 2017, 9, 6716–6723. [Google Scholar] [CrossRef]
- Wan, S.; Bi, H.; Zhou, Y.; Xie, X.; Su, S.; Yin, K.; Sun, L. Graphene oxide as high-performance dielectric materials for capacitive pressure sensors. Carbon 2017, 114, 209–216. [Google Scholar] [CrossRef]
- Sahatiya, P.; Badhulika, S. Eraser-based eco-friendly fabrication of a skin-like large-area matrix of flexible carbon nanotube strain and pressure sensors. Nanotechnology 2017, 28, 095501. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, S.; Park, H.W.; Park, D.H.; Jeong, Y.; Kim, D.H. Highly Sensitive and Multimodal All-Carbon Skin Sensors Capable of Simultaneously Detecting Tactile and Biological Stimuli. Adv. Mater. 2015, 27, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.H.; Sun, Q.; Kim, S.Y.; Han, J.T.; Kim, D.H.; Cho, J.H. Stretchable and Multimodal All Graphene Electronic Skin. Adv. Mater. 2016, 28, 2601–2608. [Google Scholar] [CrossRef]
- He, Z.; Chen, W.; Liang, B.; Liu, C.; Yang, L.; Lu, D.; Mo, Z.; Zhu, H.; Tang, Z.; Gui, X. Capacitive Pressure Sensor with High Sensitivity and Fast Response to Dynamic Interaction Based on Graphene and Porous Nylon Networks. ACS Appl. Mater. Interfaces 2018, 10, 12816–12823. [Google Scholar] [CrossRef]
- Guo, X.; Huang, Y.; Cai, X.; Liu, C.; Liu, P. Capacitive wearable tactile sensor based on smart textile substrate with carbon black/silicone rubber composite dielectric. Meas. Sci. Technol. 2016, 27, 045105. [Google Scholar] [CrossRef]
- Tsouti, V.; Mitrakos, V.; Broutas, P.; Chatzandroulis, S. Modeling and Development of a Flexible Carbon Black-Based Capacitive Strain Sensor. IEEE Sens. J. 2016, 16, 3059–3067. [Google Scholar] [CrossRef]
- Hu, W.; Niu, X.; Zhao, R.; Pei, Q. Elastomeric transparent capacitive sensors based on an interpenetrating composite of silver nanowires and polyurethane. Appl. Phys. Lett. 2013, 102, 083303. [Google Scholar] [CrossRef]
- Ma, L.; Shuai, X.; Hu, Y.; Liang, X.; Zhu, P.; Sun, R.; Wong, C.-P. A highly sensitive and flexible capacitive pressure sensor based on a micro-arrayed polydimethylsiloxane dielectric layer. J. Mater. Chem. C 2018, 6, 13232–13240. [Google Scholar] [CrossRef]
- Qiu, J.; Guo, X.; Chu, R.; Wang, S.; Zeng, W.; Qu, L.; Zhao, Y.; Yan, F.; Xing, G. Rapid-Response, Low Detection Limit, and High-Sensitivity Capacitive Flexible Tactile Sensor Based on Three-Dimensional Porous Dielectric Layer for Wearable Electronic Skin. ACS Appl. Mater. Interfaces 2019, 11, 40716–40725. [Google Scholar] [CrossRef]
- Cai, L.; Song, L.; Luan, P.; Zhang, Q.; Zhang, N.; Gao, Q.; Zhao, D.; Zhang, X.; Tu, M.; Yang, F.; et al. Super-stretchable, transparent carbon nanotube-based capacitive strain sensors for human motion detection. Sci. Rep. 2013, 3, 3048. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.P.; Zhao, Z.G.; Liu, X.W.; Zhang, Z.X.; Suo, C.G. A Capacitive Humidity Sensor Based on Multi-Wall Carbon Nanotubes (MWCNTs). Sensors 2009, 9, 7431–7444. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [Green Version]
- Biot, M.A. General Theory of Three-Dimensional Consolidation. J. Appl. Phys. 1941, 12, 155–164. [Google Scholar] [CrossRef]










| Flexible Conductive Material | Ref. | Main Structure and Material | Sensor Type | G Factor | Sensitivity | Wearable Experiment | Durability Testing |
|---|---|---|---|---|---|---|---|
| CNT | [42] | MWCNT as conductive layer, Eraser as dielectric layer | Capacitive pressure | - | 0.135 MPa−1 | √ | × |
| [3] | CNT as conductive layer, PDMS layers fixed with silicone adhesive as dielectric layer | Capacitive Pressure and Strain | 0.41 | 0.23 MPa−1 | × | × | |
| [43] | CNT microyarn as conductive wire, Ecoflex as dielectric layer | Capacitive pressure | - | 0.5 MPa−1 | √ | 2000 cycles pressure | |
| Graphene | [44] | graphene as conductive layer, PDMS as dielectric layer | Capacitive pressure | - | 2 MPa−1 | × | × |
| [45] | CVD deposited graphene transferred to PDMS, nylon mesh dieletric and silver electrode sandwiched between two graphene-PDMS layers | Capacitive pressure | - | 7 MPa−1 | √ | 1050 cycles pressure | |
| [41] | Graphene oxide (GO) foam between reduced GO (rGO) patterned PET substrates | Capacitive pressure | - | 150 MPa−1 | × | 1000 bending cycles | |
| Carbon Black | [46] | Organo-silicone conductive silver adhesive serves as a flexible electrodes carbon black (CB)/silicone rubber (SR) composite dielectric | Capacitive pressure | - | 0.2536 MPa−1 | √ | × |
| [47] | Curing and gluing together successive blended PDMS/CB electrode layers and PDMS layers (dielectric, packaging) | Capacitive Strain | 1 | - | × | × | |
| This work | Carbon black conductive material PDMS sponge as dielectric layer | Capacitive Pressure and Strain | 0.51 | 100 MPa−1 < 0.4 kPa, 49 MPa−1 > 0.4 kPa | √ | 10,000 cycles both strain and pressure | |
| Nanowires | [4] | SBS/AgNP composite coated on the fiber as conductive fiber, PDMS as dielectric layer | Capacitive Pressure | - | 64 MPa−1 | √ | 10,000 bending cycles |
| [48] | Transparent AgNW-PU composite as conductive layer, acrylic elastomer layer as the dielectric layer | Capacitive Pressure and Strain | 0.5 | 3.3 MPa−1 | × | 10 cycles stretch | |
| [10] | AgNWs blend with PDMS as conductive layer, PDMS as dielectric layer | Capacitive Pressure and Strain | 0.7 | 1.62 MPa−1 | √ | 100 cycles stretch |
| Ref. | Dielectric | Electrodes | Sensitivity (kPa−1) | Durability Cycles | Applications |
|---|---|---|---|---|---|
| [20] | Porous PDMS | AgNW + PDMS | 0.057 | 50 | Glove thumb tip pressure |
| [33] | PU foam + graphite | Silver coated nylon textile | 0.05 | - | Insoles |
| [49] | Plasma etched porous PDMS surface | AgNW + PDMS | 0.57 | 3000 | Glove finger tips, pressure mat array |
| [50] | GNPs/MWCNTs/SR coated PS sponge | silicone + Ag paste | 0.033 | 2000 | band: swallowing, breathing, pulse, muscle movement pad: air pressure, air flow, water drop or sand detection |
| [30] | Porous PDMS | ITO films | 0.125 | 10,000 | ant on band, pressure mat array |
| [29] | Porous PDMS | ITO/PET films | low range only | - | Pressure mat array |
| [12] | Porous PDMS coating of conductive fibers | Ag NP coated SBS coated Twaron fibers | 0.104 | 10,000 | Single sensor woven into mat |
| This Work | Porous PDMS | PDMS/CB blend | 0.049 to 0.100 | 10,000 | Pressure mat array, loading of band, insole, Pillow head position detection, and mattress insert for respiration |
| Test Conditions | Gauge Factor |
|---|---|
| 20 °C, 50 RH% | 0.51 |
| 20 °C, 60 RH% | 0.52 |
| 20 °C, 70 RH% | 0.53 |
| 25 °C, 60 RH% | 0.51 |
| 30 °C, 60 RH% | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Gu, H.; Xu, L.; Chen, X.D.; Kirk, T.V. Extending Porous Silicone Capacitive Pressure Sensor Applications into Athletic and Physiological Monitoring. Sensors 2021, 21, 1119. https://doi.org/10.3390/s21041119
Xia Y, Gu H, Xu L, Chen XD, Kirk TV. Extending Porous Silicone Capacitive Pressure Sensor Applications into Athletic and Physiological Monitoring. Sensors. 2021; 21(4):1119. https://doi.org/10.3390/s21041119
Chicago/Turabian StyleXia, Yun, Hao Gu, Lei Xu, Xiao Dong Chen, and Tim V. Kirk. 2021. "Extending Porous Silicone Capacitive Pressure Sensor Applications into Athletic and Physiological Monitoring" Sensors 21, no. 4: 1119. https://doi.org/10.3390/s21041119






