Calculating the Effect of Ribs on the Focus Quality of a Therapeutic Spherical Random Phased Array
Abstract
:1. Introduction
2. Materials and Methods
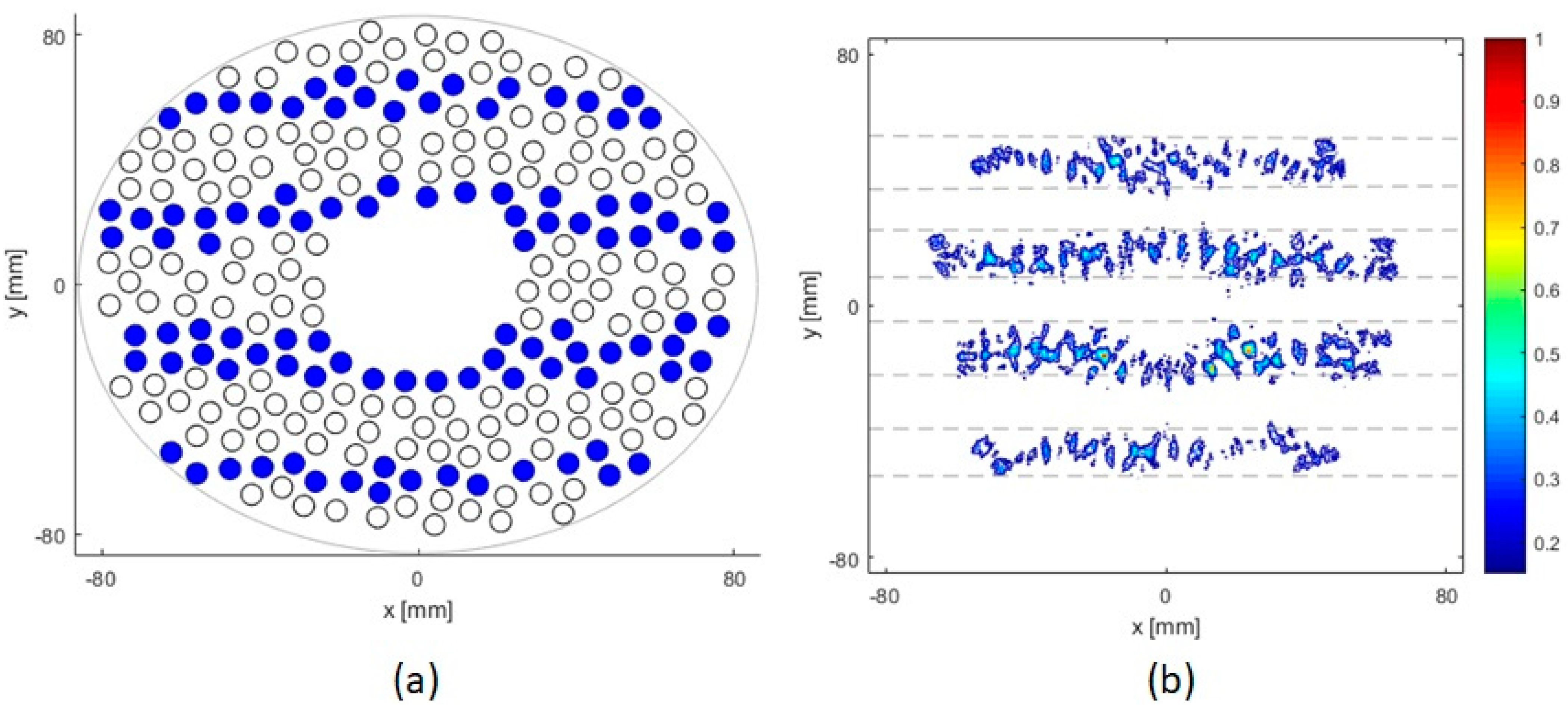
2.1. The Random Phased Array Transducer
2.2. Parametric Studies
2.3. Acoustic Field Modeling
2.4. Ribs Models
2.4.1. 2D Parallel Idealized Ribs Model
2.4.2. 3D Patient-Specific Ribs Model
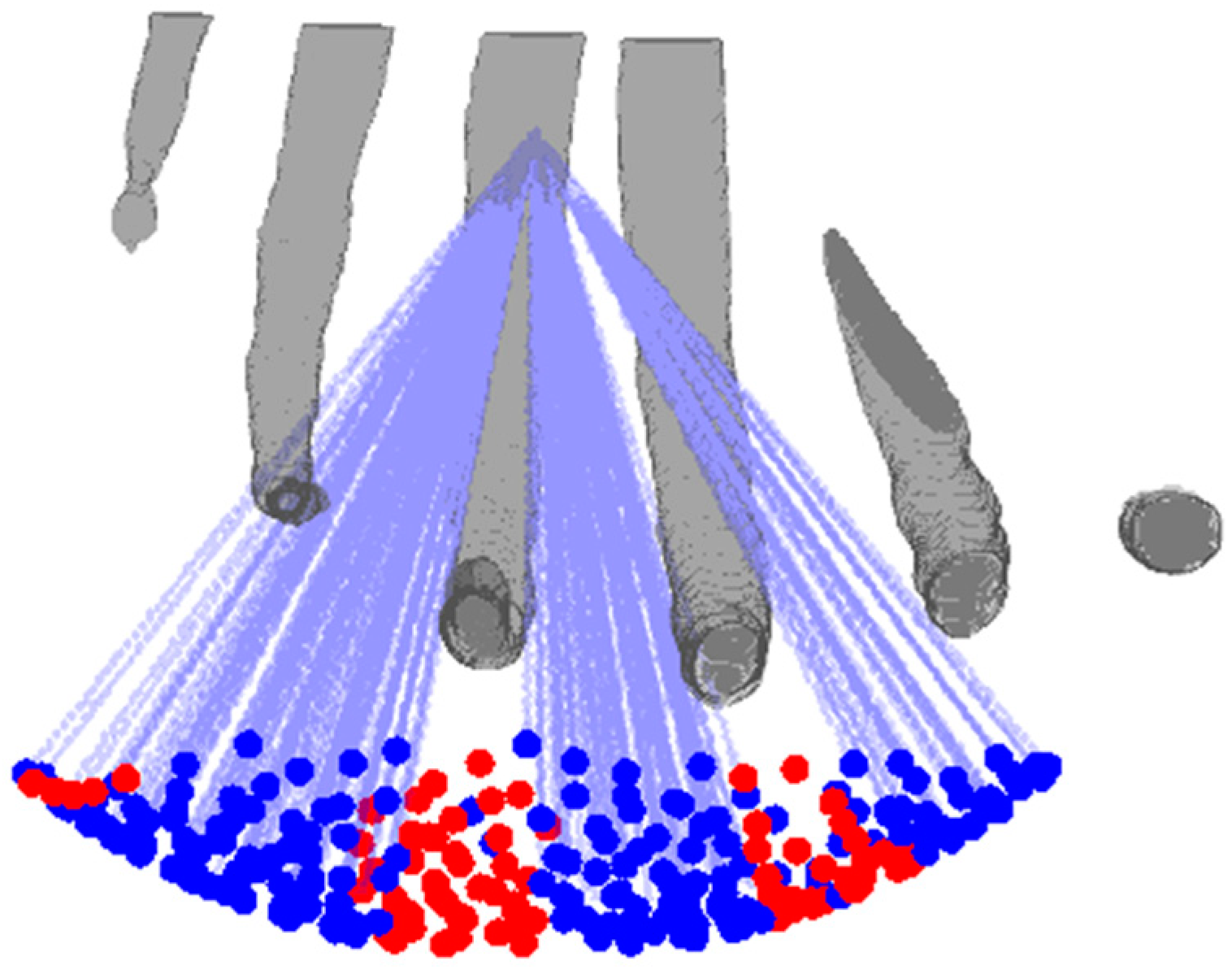
2.5. Ray Tracing
3. Results
3.1. Parallel Idealized 2D Ribs
3.2. Anatomically Correct 3D Ribs Model
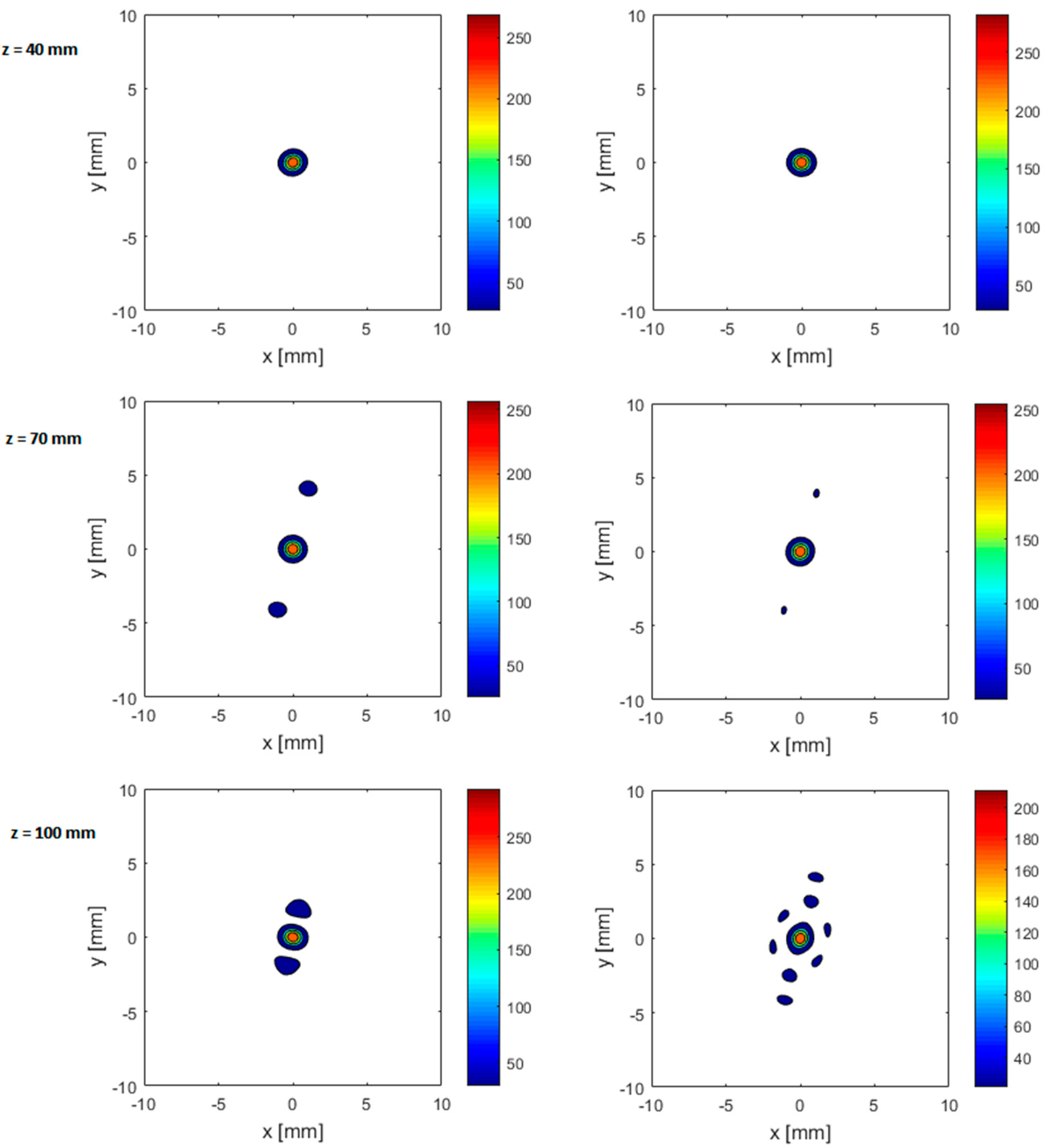
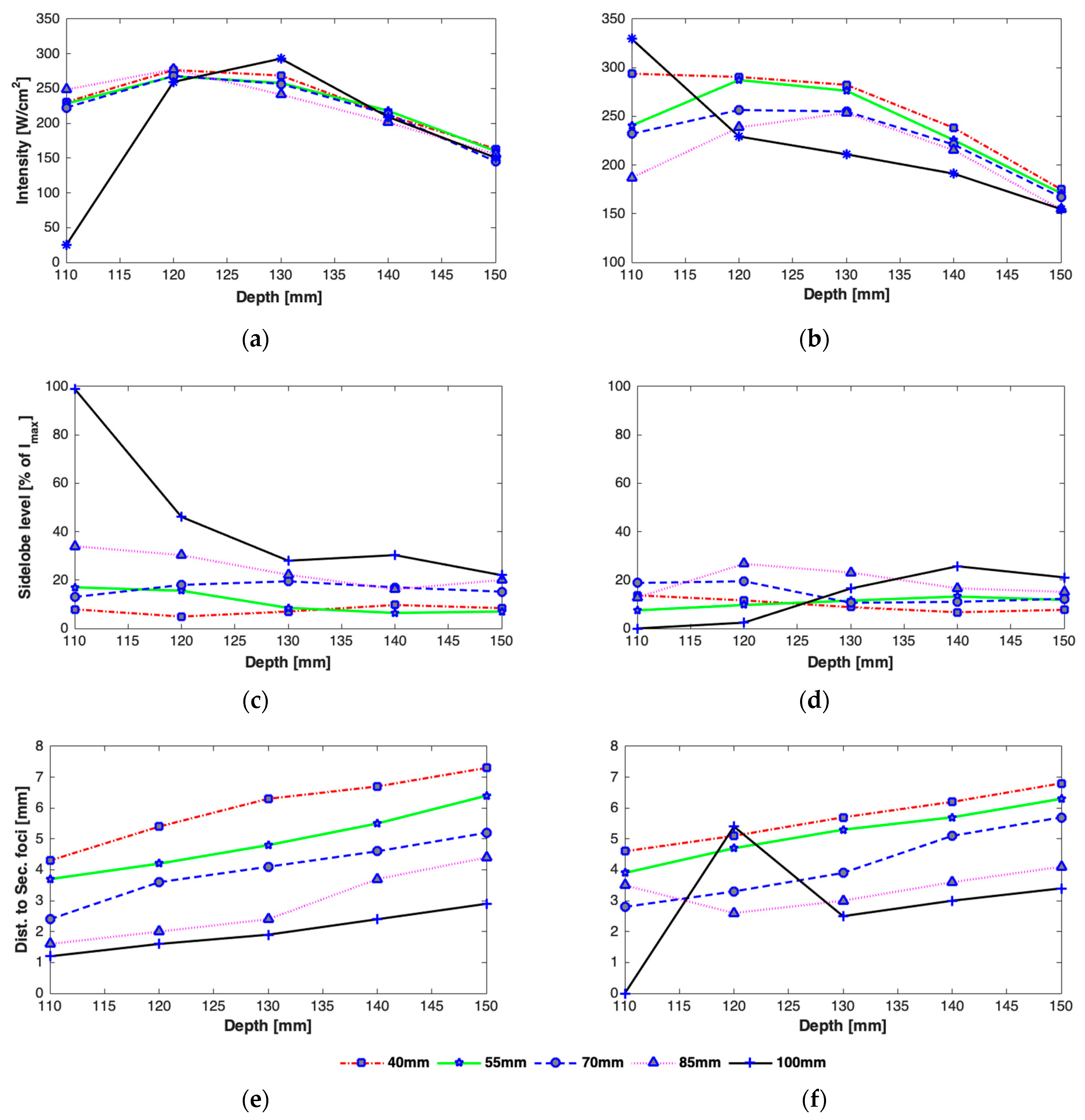
3.2.1. Effect of Axial Steering on Focus Quality
3.2.2. Effect of Transverse Steering on Focus Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. Ca A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Ter Haar, G.; Coussios, C. High Intensity Focused Ultrasound: Past, present and future. Int. J. Hyperth. 2007, 23, 85–87. [Google Scholar] [CrossRef] [Green Version]
- Ter Haar, G. HIFU Tissue Ablation: Concept and Devices; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 3–20. [Google Scholar]
- Ritchie, R.W.; Leslie, T.A.; Turner, G.D.H.; Roberts, I.S.D.; D’Urso, L.; Collura, D.; Demarchi, A.; Muto, G.; Sullivan, M.E. Laparoscopic high-intensity focused ultrasound for renal tumours: A proof of concept study. BJU Int. 2011, 107, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Chang, H.; Chen, W.-S.; Shih, T.-C.; Hsiao, J.-K.; Lin, W.-L. Feasibility of transrib focused ultrasound thermal ablation for liver tumors using a spherically curved 2D array: A numerical study. Med. Phys. 2007, 34, 3436–3448. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Chen, W.Z.; Wang, W.; Gui, Y.; Zhang, M.; Zheng, G.; Zhou, Y.; Xu, G.; Li, M.; et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: An overview. Ultrason. Sonochem. 2004, 11, 149–154. [Google Scholar] [CrossRef]
- Bobkova, S.; Gavrilov, L.; Khokhlova, V.; Shaw, A.; Hand, J. Focusing of High-Intensity Ultrasound Through the Rib Cage Using a Therapeutic Random Phased Array. Ultrasound. Med. Biol. 2010, 36, 888–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khokhlova, V.A.; Bobkova, S.M.; Gavrilov, L.R. Focus splitting associated with propagation of focused ultrasound through the rib cage. Acoust. Phys. 2010, 56, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Botros, Y.Y.; Ebbini, E.S.; Volakis, J.L. Two-step hybrid virtual array ray (VAR) technique for focusing through the rib cage. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998, 45, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Civale, J.; Clarke, R.; Rivens, I.; Ter Haar, G. The use of a segmented transducer for rib sparing in HIFU treatments. Ultrasound. Med. Biol. 2006, 32, 1753–1761. [Google Scholar] [CrossRef]
- Quesson, B.; Merle, M.; Köhler, M.O.; Mougenot, C.; Roujol, S.; De Senneville, B.D.; Moonen, C.T. A method for MRI guidance of intercostal high intensity focused ultrasound ablation in the liver. Med. Phys. 2010, 37, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Montaldo, G.; Tanter, M. Time-Reversal Acoustics in Biomedical Engineering. Annu. Rev. Biomed. Eng. 2003, 5, 465–497. [Google Scholar] [CrossRef] [Green Version]
- Aubry, J.-F.; Pernot, M.; Marquet, F.; Tanter, M.; Fink, M. Transcostal high-intensity-focused ultrasound: Ex vivo adaptive focusing feasibility study. Phys. Med. Biol. 2008, 53, 2937–2951. [Google Scholar] [CrossRef] [Green Version]
- Gélat, P.; Ter Haar, G.; Saffari, N. Modelling of the acoustic field of a multi-element HIFU array scattered by human ribs. Phys. Med. Biol. 2011, 56, 5553–5581. [Google Scholar] [CrossRef]
- Cochard, E.; Prada, C.; Aubry, J.F.; Fink, M. Ultrasonic focusing through the ribs using the DORT method. Med. Phys. 2009, 36, 3495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochard, E.; Aubry, J.F.; Tanter, M.; Prada, C. Adaptive projection method applied to three-dimensional ultrasonic focusing and steering through the ribs. J. Acoust. Soc. Am. 2011, 130, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Marquet, F.; Aubry, J.F.; Pernot, M.; Fink, M.; Tanter, M. Optimal transcostal high-intensity focused ultrasound with combined real-time 3D movement tracking and correction. Phys. Med. Biol. 2011, 56, 7061–7080. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, P.; De Greef, M.; Moonen, C.T.W.; Ries, M.G. Cavitation-Enhanced Back Projection for Acoustic Rib Detection and Attenuation Mapping. Ultrasound. Med. Biol. 2015, 41, 1726–1736. [Google Scholar] [CrossRef]
- Almekkawy, M.; Ebbini, E.S. The Optimization of Transcostal Phased Array Refocusing Using the Semidefinite Relaxation Method. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Harput, S.; Dickinson, R. 3D Ultrasound Image Guidance and Therapy Through the Rib Cage with a Therapeutic Random Phased Array. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–9. [Google Scholar]
- Muhammad, Z.; Robert, J.D. 3D synthetic aperture imaging with a therapeutic spherical random phased array for transcostal applications. Phys. Med. Biol. 2020. [Google Scholar] [CrossRef]
- Hand, J.W.; Shaw, A.; Sadhoo, N.; Rajagopal, S.; Dickinson, R.J.; Gavrilov, L.R. A random phased array device for delivery of high intensity focused ultrasound. Phys. Med. Biol. 2009, 54, 5675–5693. [Google Scholar] [CrossRef]
- Ilyin, S.A.; Yuldashev, P.; Khokhlova, V.; Gavrilov, L.; Rosnitskiy, P.; Sapozhnikov, O. Analytical method for evaluating the quality of acoustic fields radiated by a multielement therapeutic array with electronic focus steering. Acoust. Phys. 2015, 61, 52–59. [Google Scholar] [CrossRef]
- Zubair, M.; Dickinson, R.J. Simulation of a Modified Multielement Random Phased Array for Image Guidance and Therapy. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 1137–1140. [Google Scholar]
- Ebbini, E.S.; Cain, C.A. A spherical-section ultrasound phased array applicator for deep localized hyperthermia. IEEE Trans. Biomed. Eng. 1991, 38, 634–643. [Google Scholar] [CrossRef]
- Filonenko, E.A.; Gavrilov, L.R.; Khokhlova, V.A.; Hand, J.W. Heating of biological tissues by two-dimensional phased arrays with random and regular element distributions. Acoust. Phys. 2004, 50, 222–231. [Google Scholar] [CrossRef]
- Goss, S.A.; Frizzell, L.A.; Kouzmanoff, J.T.; Barich, J.M.; Yang, J.M. Sparse random ultrasound phased array for focal surgery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1996, 43, 1111–1121. [Google Scholar] [CrossRef]
- Hand, J.; Gavrilov, L. Arrays of quasi-randomly distributed ultrasound transducers. J. Acoust. Soc. Am. 2003, 113, 2968–2969. [Google Scholar] [CrossRef]
- Gavrilov, L.R.; Hand, J.W. A theoretical assessment of the relative performance of spherical phased arrays for ultrasound surgery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 125–139. [Google Scholar] [CrossRef]








| Case | Depth [mm] | Ribs Width [mm] | Ipeak [W/cm2] | Number of Foci | Distance to Secondary Foci [mm] | Secondary Foci Level [% of Ipeak] | Number of Active Elements |
|---|---|---|---|---|---|---|---|
| I | 40 | ½ d | 226.20 | 3 | 5 | 20 | 149 |
| d | 195.83 | 3 | 4.5 | 38 | 129 | ||
| 2d | 92.6 | 5 | 5 and 10 | 67 and 18 | 61 | ||
| 70 | ½ d | 247.45 | 3 | 3.5 | 20 | 149 | |
| d | 179.13 | 3 | 2.7 | 38 | 129 | ||
| 2d | 113.86 | 7 | 3.4 and 5.7 and 9 | 67 and 18 | 61 | ||
| 100 | ½ d | 274.78 | 3 | 1.8 | 38.5 | 181 | |
| d | 192.80 | 5 | 1.7 and 3.3 | 64 and 13.2 | 127 | ||
| 2d | 113.86 | 7 | 1.7 and 3.3 and 5 | 97.3 and 60 and 25 | 75 | ||
| II | 40 | ½ d | 236.82 | 3 | 5.3 | 20.5 | 156 |
| d | 176.10 | 3 | 4.6 | 33.7 | 116 | ||
| 2d | 110.82 | 3 | 5.1 | 50 | 74 | ||
| 70 | ½ d | 236.82 | 3 | 3.5 | 10 | 137 | |
| d | 186.73 | 3 | 3.1 | 43 | 123 | ||
| 2d | 112.34 | 5 | 3.4 and 6.8 | 70.5 and 22.2 | 74 | ||
| 100 | ½ d | 208 | 3 | 3.5 | 10 | 137 | |
| d | 171.54 | 3 | 4 | 8 | 113 | ||
| 2d | 88.05 | 1 | 0 | 0 | 58 |
| Case | Depth [mm] | Ipeak [W/cm2] | Number of Foci | Distance to Secondary Foci [mm] | Secondary Foci Level [% of Ipeak] | Number of Active Elements |
|---|---|---|---|---|---|---|
| I | 40 | 268.70 | 3 | 6.3 | 7 | 177 |
| 55 | 258 | 3 | 4.8 | 8.5 | 167 | |
| 70 | 256.56 | 3 | 4.1 | 19.45 | 169 | |
| 85 | 241.3 | 3 | 2.4 | 22.2 | 160 | |
| 100 | 293 | 3 | 1.9 | 28.18 | 193 | |
| II | 40 | 282.37 | 3 | 5.7 | 8.8 | 186 |
| 55 | 276.3 | 3 | 5.3 | 11.5 | 180 | |
| 70 | 255.04 | 3 | 4 | 10.6 | 168 | |
| 85 | 253.5 | 5 | 3 | 23.1 | 164 | |
| 100 | 211.02 | 5 | 2.5 and 4.1 | 16 and 3.5 | 139 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubair, M.; Dickinson, R. Calculating the Effect of Ribs on the Focus Quality of a Therapeutic Spherical Random Phased Array. Sensors 2021, 21, 1211. https://doi.org/10.3390/s21041211
Zubair M, Dickinson R. Calculating the Effect of Ribs on the Focus Quality of a Therapeutic Spherical Random Phased Array. Sensors. 2021; 21(4):1211. https://doi.org/10.3390/s21041211
Chicago/Turabian StyleZubair, Muhammad, and Robert Dickinson. 2021. "Calculating the Effect of Ribs on the Focus Quality of a Therapeutic Spherical Random Phased Array" Sensors 21, no. 4: 1211. https://doi.org/10.3390/s21041211





