Simultaneous Measurements of Dose and Microdosimetric Spectra in a Clinical Proton Beam Using a scCVD Diamond Membrane Microdosimeter
Abstract
:1. Introduction
2. Materials and Methods
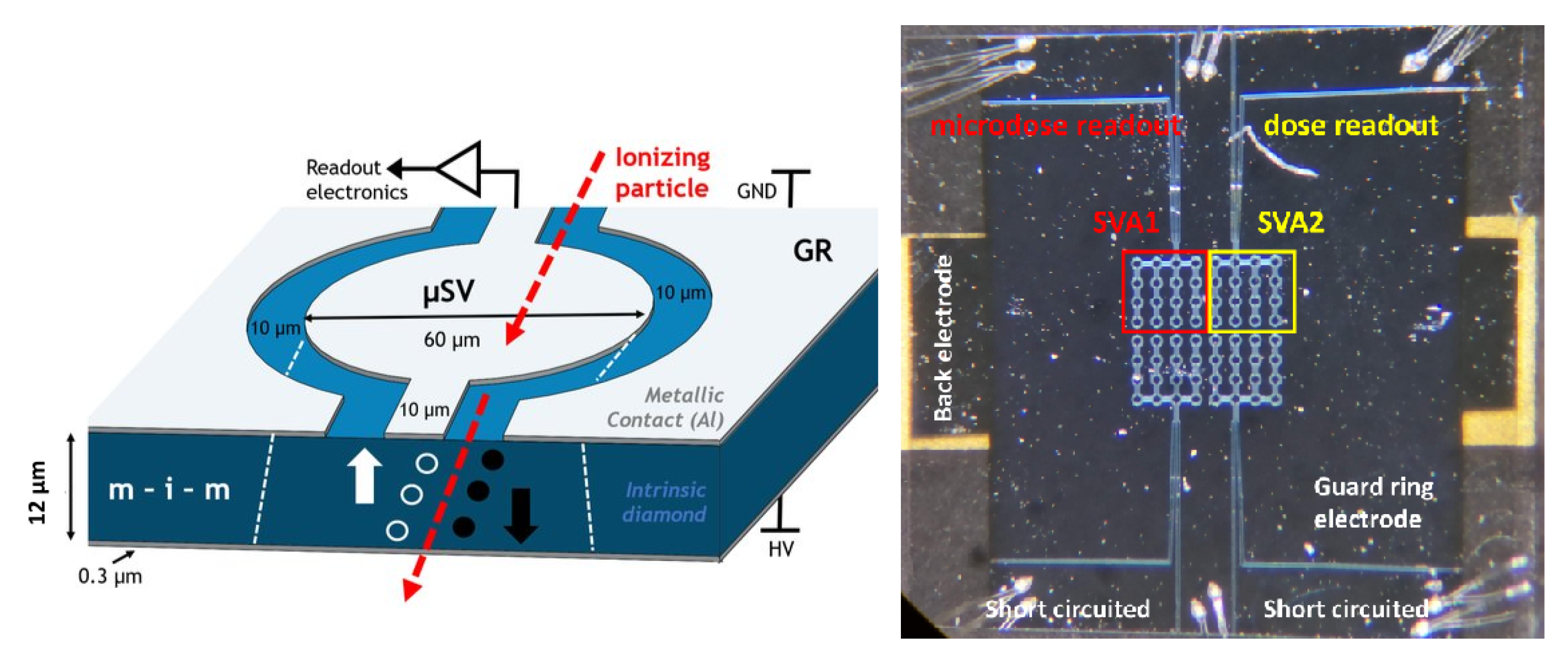
2.1. scCVD Diamond Membrane Guard Ring (GR) Prototype
2.2. Measurement Set-Up
2.3. Passive Scattering Beamline at the Proton Therapy Centre, Orsay
2.4. Energy Calibration Procedure for Microdosimetric Spectra Measurements
3. Results
3.1. Dosimetric Performance of the System
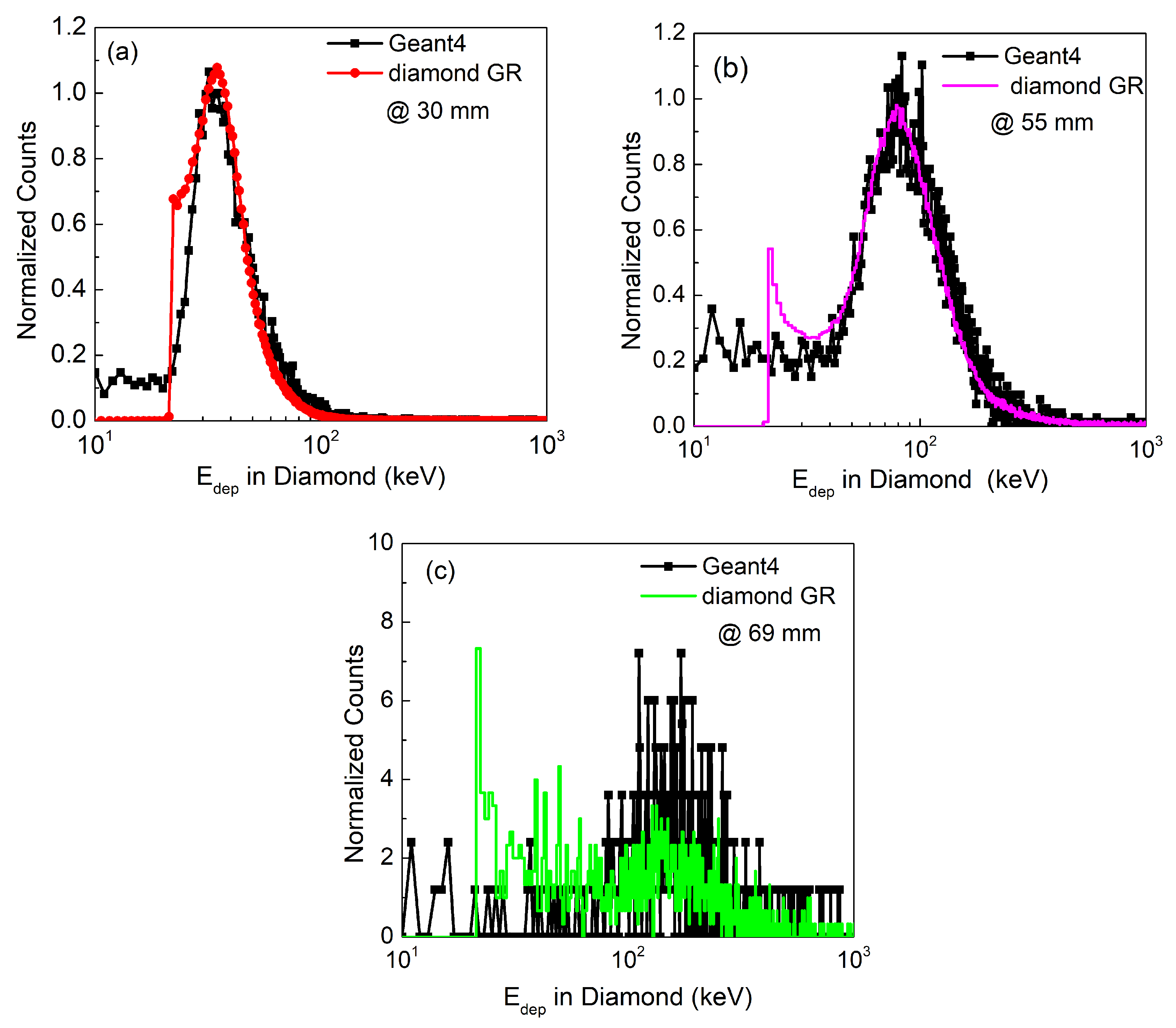
3.2. Energy Loss Spectra
3.3. Water-Equivalent Microdosimetric Spectra
4. Discussion
Comparison of Calculated and Literature Results
5. Conclusions and Current Perspectives
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grau, C.; Durante, M.; Georg, D.; Langendijk, J.A.; Weber, D.C. Particle therapy in Europe. Molecular Oncol. 2020, 10, 142–149. [Google Scholar]
- Ten Haken, R.K.; Marks, L.B.; Bentzen, S.M.; Constine, L.S.; Deasy, J.O.; Eisbruch, A.; Jackson, A. Quantitative analyses of normal tissue effects in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 10, 1–160. [Google Scholar]
- Freund, D.; Zhang, R.; Sanders, M.; Newhauser, W. Predictive risk of radiation induced cerebral necrosis in pediatric brain cancer patients after VMAT versus proton therapy. Cancers 2015, 7, 617–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuemann, J.; Giantsoudi, D.; Niemierko, A.; Maquilan, G.; Shih, H.; Busse, P.; Niyazi, M.; Paganetti, H. Brain Necrosis in Adult Proton Therapy Patients. Do Necrotic Regions Have Elevated Linear Energy Transfer? Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, S230. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Laack, N.N.; Terezakis, S. Humbling advances in technology: Protons, brainstem necrosis, and the self-driving car. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Zaider, M.; Rossi, B.H.H.; Zaider, M. Microdosimetry and Its Applications, 1st ed.; Springer: Berlin, Germany, 1996; p. 11. [Google Scholar]
- Paganetti, H. Relating proton treatments to photon treatments via the relative biological effectiveness—Should we revise current clinical practice? Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Guatelli, S.; Reinhard, M.I.; Mascialino, B.; Prokopovich, D.A.; Dzurak, A.S.; Zaider, M.; Rosenfeld, A.B. Tissue Equivalence Correction in Silicon Microdosimetry for Protons Characteristic of the LEO Space Environment. IEEE Trans. Nuclear Sci. 2008, 55, 3407–3413. [Google Scholar] [CrossRef] [Green Version]
- Guardiola, C.; Quirion, D.; Pellegrini, G.; Fleta, C.; Esteban, S.; Cortés-Giraldo, M.A.; Gómez, F.; Solberg, T.; Carabe, A.; Lozano, M. Silicon-based three-dimensional microstructures for radiation dosimetry in hadrontherapy. Appl. Phys. Lett. 2015, 107, 023505. [Google Scholar] [CrossRef]
- Rosenfeld, A.B.; Bradley, P.D.; Cornelius, I.; Kaplan, G.I.; Allen, B.J.; Flanz, J.B.; Goitein, M.; Van Meerbeeck, A.; Schubert, J.; Bailey, J.; et al. A new silicon detector for microdosimetry applications in proton therapy. IEEE Trans. Nucl. Sci. 2000, 47, 1386–1394. [Google Scholar] [CrossRef]
- Cornelius, I.; Siegele, R.; Rosenfeld, A.B.; Cohen, D.D. Ion beam induced charge characterisation of a silicon microdosimeter using a heavy ion microprobe. Nucl. Inst. Methods Phys.s Res. 2002, 190, 335–338. [Google Scholar] [CrossRef]
- Fleta, C.; Guardiola, C.; Esteban, S.; Pellegrini, G.; Quirion, D.; Rodriguez, J.; Gomez, F.; Carabe-Fernández, A.; Lozano, M. First investigations of Ultra-Thin 3D silicon detectors as microdosimeters. Radiother. Oncol. 2014, 110, S36. [Google Scholar] [CrossRef]
- James, B.; Tran, L.T.; Bolst, D.; Peracchi, S.; Davis, J.A.; Prokopovich, D.A.; Guatelli, S.; Petasecca, M.; Lerch, M.; Povoli, M.; et al. SOI Thin Microdosimeters for High LET Single-Event Upset Studies in Fe, O, Xe, and Cocktail Ion Beam Fields. IEEE Trans. Nucl. Sci. 2019, 67, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Conte, V.; Bianchi, A.; Selva, A.; Petringa, G.; Cirrone, G.A.P.; Parisi, A.; Vanhavere, F.; Colautti, P. Microdosimetry at the CATANA 62 MeV proton beam with a sealed miniaturized TEPC. Phys. Med. 2019, 64, 114–122. [Google Scholar] [CrossRef]
- Bianchi, A.; Selva, A.; Colautti, P.; Bortot, D.; Mazzucconi, D.; Pola, A.; Agosteo, S.; Petringa, G.; Cirrone, G.P.; Reniers, B.; et al. Microdosimetry with a sealed mini-TEPC and a silicon telescope at a clinical proton SOBP of CATANA. Radiat. Phys. Chem. 2020, 171, 108730. [Google Scholar] [CrossRef]
- Rollet, S.; Angelone, M.; Magrin, G.; Marinelli, M.; Milani, E.; Pillon, M.; Prestopino, G.; Verona, C.; Verona-Rinati, G. A novel microdosimeter based upon artificial single crystal diamond. IEEE Trans. Nucl. Sci. 2012, 59, 2409–2415. [Google Scholar] [CrossRef] [Green Version]
- Magrin, G. Microdosimetry in ion-beam therapy: Studying and comparing outcomes from different detectors. arXiv 2018, arXiv:1802.06705. [Google Scholar]
- Verona, C.; Magrin, G.; Solevi, P.; Bandorf, M.; Marinelli, M.; Stock, M.; Rinati, G.V. Toward the use of single crystal diamond based detector for ion-beam therapy microdosimetry. Radiat. Meas. 2018, 110, 25–31. [Google Scholar] [CrossRef]
- Manfredotti, C.; Giudice, A.L.; Ricciardi, C.; Paolini, C.; Massa, E.; Fizzotti, F.; Vittone, E. CVD diamond microdosimeters. Nucl. Inst. Methods in Phys. Res. 2001, 458, 360–364. [Google Scholar] [CrossRef]
- Zahradnik, I.A.; Pomorski, M.T.; De Marzi, L.; Tromson, D.; Barberet, P.; Skukan, N.; Bergonzo, P.; Devès, G.; Herault, J.; Kada, W.; et al. scCVD diamond membrane based microdosimeter for hadron therapy. Phys. Status Solidi 2018, 215, 1800383. [Google Scholar] [CrossRef]
- Zahradnik, I.A.; Barberet, P.; Tromson, D.; De Marzi, L.; Pomorski, M.T. A diamond guard ring microdosimeter for ion beam therapy. Rev Sci Inst 2020, 91, 054102. [Google Scholar] [CrossRef]
- Available online: https://www.labzy.com/products/nanomcaii/ (accessed on 9 October 2020).
- Available online: https://www.iba-dosimetry./com/ (accessed on 9 October 2020).
- Agostinelli, S.; Allison, J.; Amako, K.A.; Apostolakis, J.; Araujo, H.; Arce, P. GEANT4—A simulation toolkit. Nucl. Inst. Meth. A 2003, 506, 250–303. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.A. Diamond Microdosimetry for Radioprotection Applications in Space. Doctoral Thesis, University of Wollongong, Wollongong, Australia, 2015. [Google Scholar]
- Bolst, D.; Guatelli, S.; Tran, L.T.; Chartier, L.; Davis, J.; Biasi, G.; Prokopovich, D.A.; Pogossov, A.; Reinhard, M.I.; Petasecca, M. Validation of Geant4 for silicon microdosimetry in heavy ion therapy. Phys. Med. Biol. 2020, 65, 045014. [Google Scholar] [CrossRef]
- Tran, L.T.; Chartier, L.; Bolst, D.; Pogossov, A.; Guatelli, S.; Petasecca, M.; Lerch, M.L.; Prokopovich, D.A.; Reinhard, M.I.; Clasie, B.; et al. Characterization of proton pencil beam scanning and passive beam using a high spatial resolution solid-state microdosimeter. Med. Phys. 2017, 44, 6085–6095. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.E.; Furutani, K.M.; Tran, L.T.; Chartier, L.; Petasecca, M.; Lerch, M.; Prokopovich, D.A.; Reinhard, M.; Perevertaylo, V.L.; Rosenfeld, A.B.; et al. Microdosimetric measurements of a clinical proton beam with micrometer-sized solid-state detector. Med. Phys. 2017, 44, 6029–6037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debrot, E.; Tran, L.; Chartier, L.; Bolst, D.; Guatelli, S.; Vandevoorde, C.; de Kock, E.; Beukes, P.; Symons, J.; Nieto-Camero, J.; et al. SOI microdosimetry and modified MKM for evaluation of relative biological effectiveness for a passive proton therapy radiation field. Phys. Med. Biol. 2018, 63, 235007. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Peeler, C.; Bronk, L.; Geng, C.; Taleei, R.; Randeniya, S.; Ge, S.; Mirkovic, D.; Grosshans, D.; Mohan, R.; et al. Analysis of the track-and dose-averaged LET and LET spectra in proton therapy using the geant4 Monte Carlo code. Med. Phys. 2015, 42, 6234–6247. [Google Scholar] [CrossRef] [PubMed]
- Perl, J.; Shin, J.; Schümann, J.; Faddegon, B.; Paganetti, H. TOPAS: An innovative proton Monte Carlo platform for research and clinical applications. Med. Phys. 2012, 39, 6818–6837. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Giraldo, M.A.; Carabe, A. A critical study of different Monte Carlo scoring methods of dose average linear-energy-transfer maps calculated in voxelized geometries irradiated with clinical proton beams. Phys. Med. Biol. 2015, 60, 2645. [Google Scholar] [CrossRef] [PubMed]
- Granville, D.A.; Sawakuchi, G.O. Comparison of linear energy transfer scoring techniques in Monte Carlo simulations of proton beams. Phys. Med. Biol. 2015, 60, N283. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loto, O.; Zahradnik, I.; Leite, A.M.; De Marzi, L.; Tromson, D.; Pomorski, M. Simultaneous Measurements of Dose and Microdosimetric Spectra in a Clinical Proton Beam Using a scCVD Diamond Membrane Microdosimeter. Sensors 2021, 21, 1314. https://doi.org/10.3390/s21041314
Loto O, Zahradnik I, Leite AM, De Marzi L, Tromson D, Pomorski M. Simultaneous Measurements of Dose and Microdosimetric Spectra in a Clinical Proton Beam Using a scCVD Diamond Membrane Microdosimeter. Sensors. 2021; 21(4):1314. https://doi.org/10.3390/s21041314
Chicago/Turabian StyleLoto, Oluwasayo, Izabella Zahradnik, Amelia Maia Leite, Ludovic De Marzi, Dominique Tromson, and Michal Pomorski. 2021. "Simultaneous Measurements of Dose and Microdosimetric Spectra in a Clinical Proton Beam Using a scCVD Diamond Membrane Microdosimeter" Sensors 21, no. 4: 1314. https://doi.org/10.3390/s21041314
APA StyleLoto, O., Zahradnik, I., Leite, A. M., De Marzi, L., Tromson, D., & Pomorski, M. (2021). Simultaneous Measurements of Dose and Microdosimetric Spectra in a Clinical Proton Beam Using a scCVD Diamond Membrane Microdosimeter. Sensors, 21(4), 1314. https://doi.org/10.3390/s21041314






