In Vivo Whole-Cell Patch-Clamp Methods: Recent Technical Progress and Future Perspectives
Abstract
1. Introduction
2. In Vivo Whole-Cell Recordings from Anesthetized Animals
2.1. Neocortex
2.2. Hippocampus and Other Regions
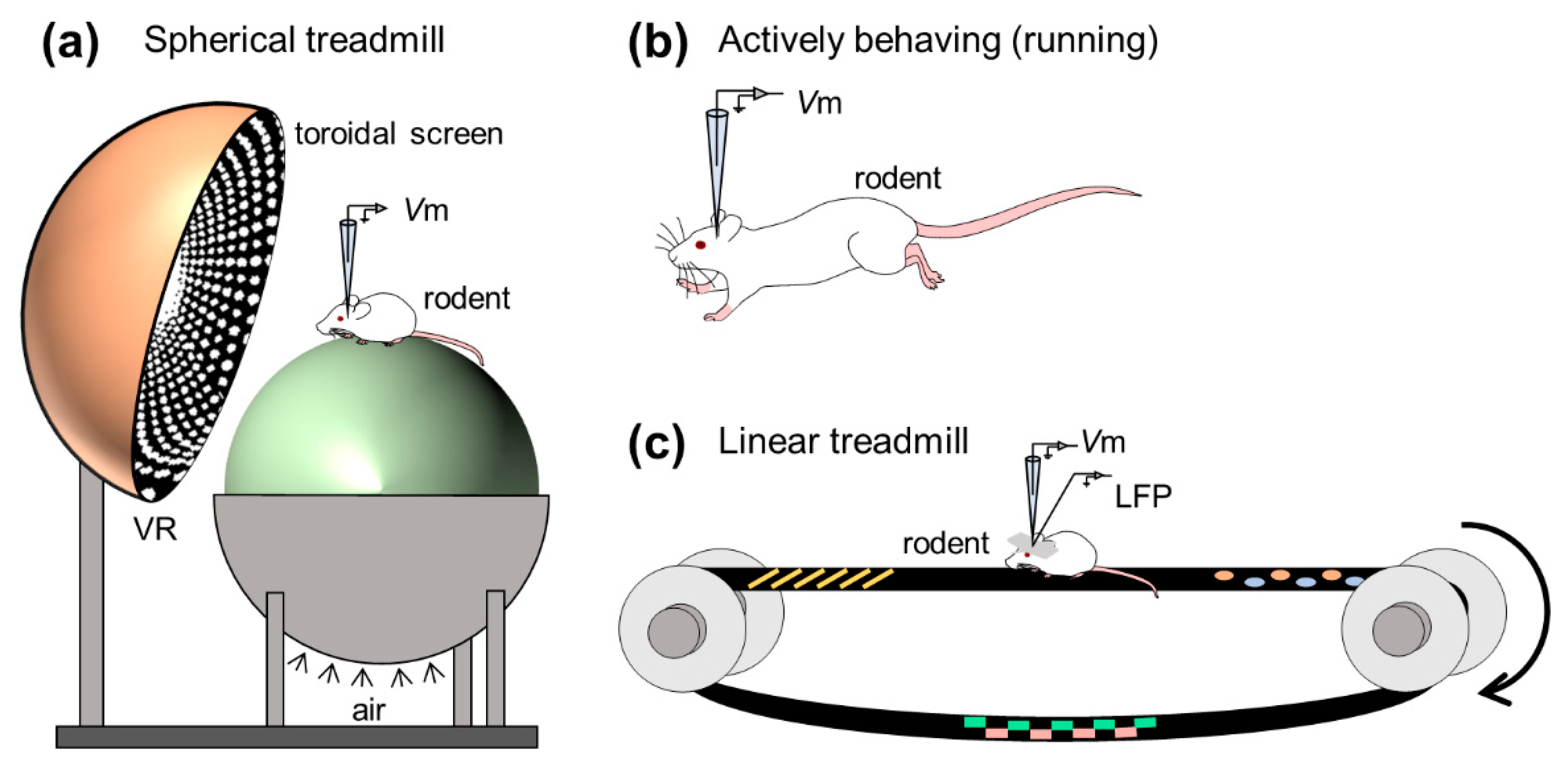
3. In Vivo Whole-Cell Recordings from Awake Animals
3.1. Neocortex
3.2. Hippocampus and Other Regions
4. Hybrid Methodologies with In Vivo Whole-Cell Recording Techniques
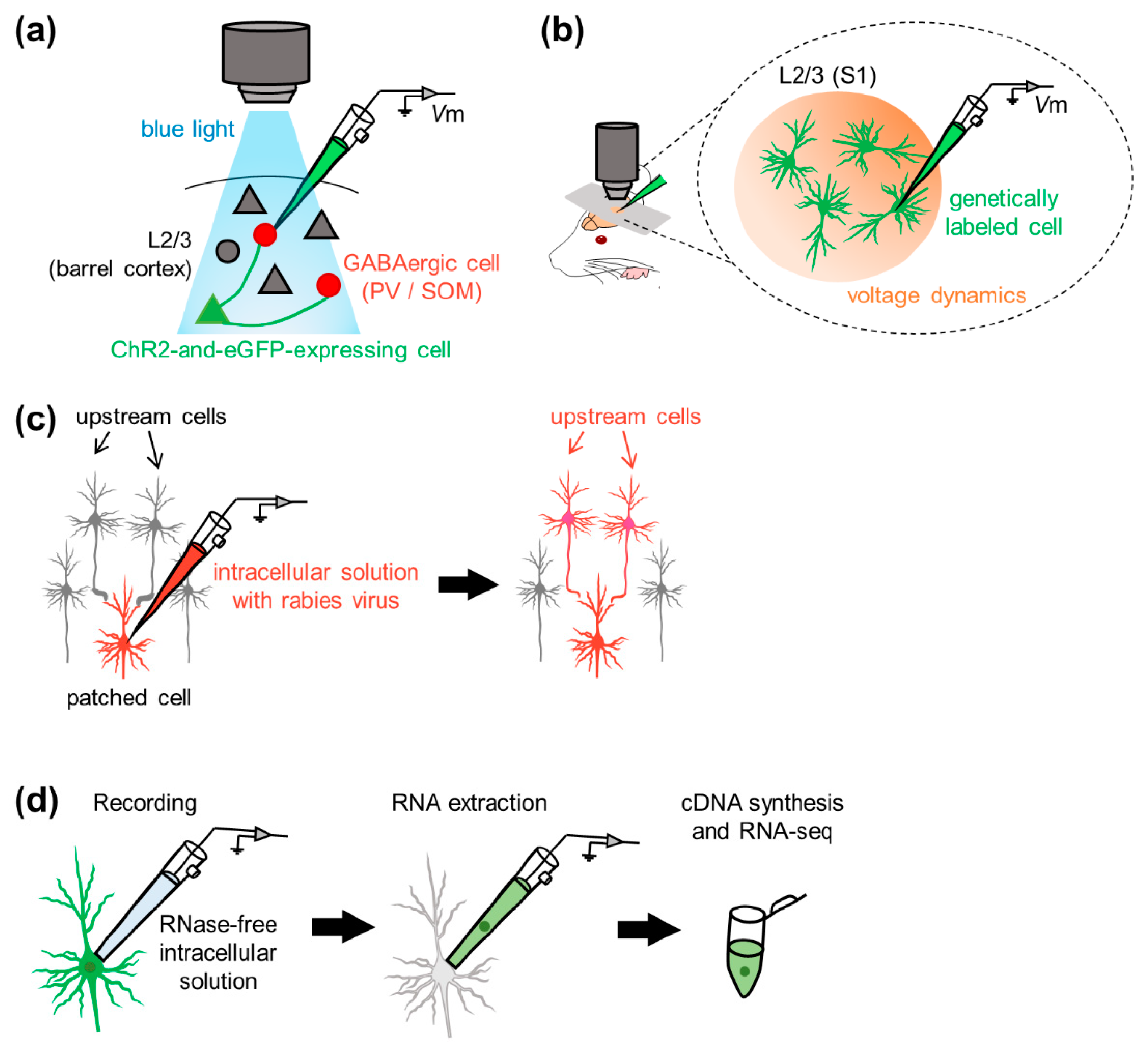
4.1. Optics
4.2. Intracellular Pharmacology
4.3. Gene Manipulation: Transgene Expression and Virus-Aided Connectivity Tracing
4.4. Molecular Characterization: Patch-Seq
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neher, E.; Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 1976, 260, 799–802. [Google Scholar] [CrossRef]
- Van Hook, M.J.; Thoreson, W.B. Whole-Cell Patch-Clamp Recording. In Current Laboratory Methods in Neuroscience Research; Springer: New York, NY, USA, 2014; pp. 353–367. [Google Scholar]
- Neher, E.; Sakmann, B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J. Physiol. 1976, 258, 705–729. [Google Scholar] [CrossRef]
- Horn, R.; Marty, A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988, 92, 145–159. [Google Scholar] [CrossRef]
- Pei, X.; Volgushev, M.; Vidyasagar, T.R.; Creutzfeldt, O.D. Whole cell recording and conductance measurements in cat visual cortex in-vivo. Neuroreport 1991, 2, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Margrie, T.W.; Brecht, M.; Sakmann, B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflügers Arch. 2002, 444, 491–498. [Google Scholar] [CrossRef]
- Nelson, S.; Toth, L.; Sheth, B.; Sur, M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science 1994, 265, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Connors, B.W. Intrinsic Firing Patterns and Whisker-Evoked Synaptic Responses of Neurons in the Rat Barrel Cortex. J. Neurophysiol. 1999, 81, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Brecht, M.; Roth, A.; Sakmann, B. Dynamic Receptive Fields of Reconstructed Pyramidal Cells in Layers 3 and 2 of Rat Somatosensory Barrel Cortex. J. Physiol. 2003, 553, 243–265. [Google Scholar] [CrossRef]
- Manns, I.D.; Sakmann, B.; Brecht, M. Sub- and suprathreshold receptive field properties of pyramidal neurones in layers 5A and 5B of rat somatosensory barrel cortex. J. Physiol. 2004, 556, 601–622. [Google Scholar] [CrossRef]
- Brecht, M.; Sakmann, B. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J. Physiol. 2002, 543, 49–70. [Google Scholar] [CrossRef]
- London, M.; Roth, A.; Beeren, L.; Häusser, M.; Latham, P.E. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature 2010, 466, 123–127. [Google Scholar] [CrossRef]
- Tao, C.; Zhang, G.; Zhou, C.; Wang, L.; Yan, S.; Tao, H.W.; Zhang, L.I.; Zhou, Y.; Xiong, Y. Diversity in Excitation-Inhibition Mismatch Underlies Local Functional Heterogeneity in the Rat Auditory Cortex. Cell Rep. 2017, 19, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Wu, G.K.; Liu, B.; Li, P.; Zhou, M.; Xiao, Z.; Tao, H.W.; Zhang, L.I. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature 2010, 465, 927–931. [Google Scholar] [CrossRef]
- Tao, C.; Zhang, G.; Zhou, C.; Wang, L.; Yan, S.; Zhang, L.I.; Zhou, Y.; Xiong, Y. Synaptic Basis for the Generation of Response Variation in Auditory Cortex. Sci. Rep. 2016, 6, 31024. [Google Scholar] [CrossRef]
- Minamisawa, G.; Takahashi, N.; Matsuki, N.; Ikegaya, Y. Laterality of neocortical slow-wave oscillations in anesthetized mice. Neurosci. Res. 2009, 64, 240–242. [Google Scholar] [CrossRef]
- Furue, H. In Vivo Blind Patch-Clamp Recording Technique. In Patch Clamp Techniques; Okada, Y., Ed.; Springer: Tokyo, Japan, 2012; pp. 171–182. [Google Scholar]
- Kim, U.; Ebner, F.F. Barrels and septa: Separate circuits in rat barrels field cortex. J. Comp. Neurol. 1999, 408, 489–505. [Google Scholar] [CrossRef]
- Dittgen, T.; Nimmerjahn, A.; Komai, S.; Licznerski, P.; Waters, J.; Margrie, T.W.; Helmchen, F.; Denk, W.; Brecht, M.; Osten, P. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 18206–18211. [Google Scholar] [CrossRef]
- Hahn, T.T.G.; McFarland, J.M.; Berberich, S.; Sakmann, B.; Mehta, M.R. Spontaneous persistent activity in entorhinal cortex modulates cortico-hippocampal interaction in vivo. Nat. Neurosci. 2012, 15, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Leitner, F.C.; Melzer, S.; Lütcke, H.; Pinna, R.; Seeburg, P.H.; Helmchen, F.; Monyer, H. Spatially segregated feedforward and feedback neurons support differential odor processing in the lateral entorhinal cortex. Nat. Neurosci. 2016, 19, 935–944. [Google Scholar] [CrossRef]
- Epsztein, J.; Milh, M.; Id Bihi, R.; Jorquera, I.; Ben-Ari, Y.; Represa, A.; Crépel, V. Ongoing Epileptiform Activity in the Post-Ischemic Hippocampus Is Associated with a Permanent Shift of the Excitatory-Inhibitory Synaptic Balance in CA3 Pyramidal Neurons. J. Neurosci. 2006, 26, 7082–7092. [Google Scholar] [CrossRef]
- Hahn, T.T.G.; Sakmann, B.; Mehta, M.R. Differential responses of hippocampal subfields to cortical up-down states. Proc. Natl. Acad. Sci. USA 2007, 104, 5169–5174. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.T.G.; Sakmann, B.; Mehta, M.R. Phase-locking of hippocampal interneurons’ membrane potential to neocortical up-down states. Nat. Neurosci. 2006, 9, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Atallah, B.V.; Scanziani, M. Instantaneous Modulation of Gamma Oscillation Frequency by Balancing Excitation with Inhibition. Neuron 2009, 62, 566–577. [Google Scholar] [CrossRef]
- Ouedraogo, D.W.; Lenck-Santini, P.-P.; Marti, G.; Robbe, D.; Crépel, V.; Epsztein, J. Abnormal UP/DOWN Membrane Potential Dynamics Coupled with the Neocortical Slow Oscillation in Dentate Granule Cells during the Latent Phase of Temporal Lobe Epilepsy. Eneuro 2016, 3. [Google Scholar] [CrossRef]
- Matsumoto, N.; Okamoto, K.; Takagi, Y.; Ikegaya, Y. 3-Hz subthreshold oscillations of CA2 neurons in vivo. Hippocampus 2016, 26, 1570–1578. [Google Scholar] [CrossRef]
- Sato, M.; Matsumoto, N.; Noguchi, A.; Okonogi, T.; Sasaki, T.; Ikegaya, Y. Simultaneous monitoring of mouse respiratory and cardiac rates through a single precordial electrode. J. Pharmacol. Sci. 2018, 137, 177–186. [Google Scholar] [CrossRef]
- Yagishita, H.; Nishimura, Y.; Noguchi, A.; Shikano, Y.; Ikegaya, Y.; Sasaki, T. Urethane anesthesia suppresses hippocampal subthreshold activity and neuronal synchronization. Brain Res. 2020, 1749, 147137. [Google Scholar] [CrossRef]
- Abe, R.; Sakaguchi, T.; Matsumoto, N.; Matsuki, N.; Ikegaya, Y. Sound-induced hyperpolarization of hippocampal neurons. Neuroreport 2014, 25, 1013–1017. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Wang, Y.; Shen, W.; Wang, Z. Enhancement of synchronized activity between hippocampal CA1 neurons during initial storage of associative fear memory. J. Physiol. 2017, 595, 5327–5340. [Google Scholar] [CrossRef]
- Windels, F.; Yan, S.; Stratton, P.G.; Sullivan, R.; Crane, J.W.; Sah, P. Auditory Tones and Foot-Shock Recapitulate Spontaneous Sub-Threshold Activity in Basolateral Amygdala Principal Neurons and Interneurons. PLoS ONE 2016, 11, e0155192. [Google Scholar] [CrossRef]
- Windels, F.; Crane, J.W.; Sah, P. Inhibition Dominates the Early Phase of Up-States in the Basolateral Amygdala. J. Neurophysiol. 2010, 104, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.W.; Windels, F.; Sah, P. Oscillations in the basolateral amygdala: Aversive stimulation is state dependent and resets the oscillatory phase. J. Neurophysiol. 2009, 102, 1379–1387. [Google Scholar] [CrossRef]
- Davison, I.G.; Ehlers, M.D. Neural Circuit Mechanisms for Pattern Detection and Feature Combination in Olfactory Cortex. Neuron 2011, 70, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Poo, C.; Isaacson, J.S. Odor Representations in Olfactory Cortex: “Sparse” Coding, Global Inhibition, and Oscillations. Neuron 2009, 62, 850–861. [Google Scholar] [CrossRef]
- Poo, C.; Isaacson, J.S. A Major Role for Intracortical Circuits in the Strength and Tuning of Odor-Evoked Excitation in Olfactory Cortex. Neuron 2011, 72, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Brecht, M.; Sakmann, B. Whisker maps of neuronal subclasses of the rat ventral posterior medial thalamus, identified by whole-cell voltage recording and morphological reconstruction. J. Physiol. 2002, 538, 495–515. [Google Scholar] [CrossRef]
- Otomo, K.; Perkins, J.; Kulkarni, A.; Stojanovic, S.; Roeper, J.; Paladini, C.A. In vivo patch-clamp recordings reveal distinct subthreshold signatures and threshold dynamics of midbrain dopamine neurons. Nat. Commun. 2020, 11, 6286. [Google Scholar] [CrossRef]
- Sugiyama, D.; Hur, S.W.; Pickering, A.E.; Kase, D.; Kim, S.J.; Kawamata, M.; Imoto, K.; Furue, H. In vivo patch-clamp recording from locus coeruleus neurones in the rat brainstem. J. Physiol. 2012, 590, 2225–2231. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.-T.; Yuan, W.; Tao, H.W.; Zhang, L.I. Synaptic mechanisms for generating temporal diversity of auditory representation in the dorsal cochlear nucleus. J. Neurophysiol. 2015, 113, 1358–1368. [Google Scholar] [CrossRef]
- Chadderton, P.; Margrie, T.W.; Häusser, M. Integration of quanta in cerebellar granule cells during sensory processing. Nature 2004, 428, 856–860. [Google Scholar] [CrossRef]
- Rancz, E.A.; Ishikawa, T.; Duguid, I.; Chadderton, P.; Mahon, S.; Häusser, M. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature 2007, 450, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Duguid, I.; Branco, T.; London, M.; Chadderton, P.; Häusser, M. Tonic Inhibition Enhances Fidelity of Sensory Information Transmission in the Cerebellar Cortex. J. Neurosci. 2012, 32, 11132–11143. [Google Scholar] [CrossRef]
- Ishikawa, T.; Shimuta, M.; Häusser, M. Multimodal sensory integration in single cerebellar granule cells in vivo. Elife 2015, 4, e12916. [Google Scholar] [CrossRef] [PubMed]
- Duguid, I.; Branco, T.; Chadderton, P.; Arlt, C.; Powell, K.; Häusser, M. Control of cerebellar granule cell output by sensory-evoked Golgi cell inhibition. Proc. Natl. Acad. Sci. USA 2015, 112, 13099–13104. [Google Scholar] [CrossRef]
- Witter, L.; De Zeeuw, C.I. In Vivo Differences in Inputs and Spiking Between Neurons in Lobules VI/VII of Neocerebellum and Lobule X of Archaeocerebellum. Cerebellum 2015, 14, 506–515. [Google Scholar] [CrossRef]
- Arenz, A.; Silver, R.A.; Schaefer, A.T.; Margrie, T.W. The Contribution of Single Synapses to Sensory Representation in Vivo. Science 2008, 321, 977–980. [Google Scholar] [CrossRef]
- Margrie, T.W.; Meyer, A.H.; Caputi, A.; Monyer, H.; Hasan, M.T.; Schaefer, A.T.; Denk, W.; Brecht, M. Targeted Whole-Cell Recordings in the Mammalian Brain In Vivo. Neuron 2003, 39, 911–918. [Google Scholar] [CrossRef]
- Komai, S.; Denk, W.; Osten, P.; Brecht, M.; Margrie, T.W. Two-photon targeted patching (TPTP) in vivo. Nat. Protoc. 2006, 1, 647–652. [Google Scholar] [CrossRef]
- Kitamura, K.; Judkewitz, B.; Kano, M.; Denk, W.; Häusser, M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nat. Methods 2008, 5, 61–67. [Google Scholar] [CrossRef]
- Häusser, M.; Margrie, T.W. Two-Photon Targeted Patching and Electroporation In Vivo. Cold Spring Harb. Protoc. 2014, 2014, pdb–prot080143. [Google Scholar] [CrossRef]
- Ding, R.; Liao, X.; Li, J.; Zhang, J.; Wang, M.; Guang, Y.; Qin, H.; Li, X.; Zhang, K.; Liang, S.; et al. Targeted Patching and Dendritic Ca2+ Imaging in Nonhuman Primate Brain in vivo. Sci. Rep. 2017, 7, 2873. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Leischner, U.; Rochefort, N.L.; Nelken, I.; Konnerth, A. Functional mapping of single spines in cortical neurons in vivo. Nature 2011, 475, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, S.; Bennett, C.; Hestrin, S. Correlation of Synaptic Inputs in the Visual Cortex of Awake, Behaving Mice. Neuron 2018, 99, 1289–1301.e2. [Google Scholar] [CrossRef]
- Jouhanneau, J.-S.; Ferrarese, L.; Estebanez, L.; Audette, N.J.; Brecht, M.; Barth, A.L.; Poulet, J.F.A. Cortical fosGFP Expression Reveals Broad Receptive Field Excitatory Neurons Targeted by POm. Neuron 2014, 84, 1065–1078. [Google Scholar] [CrossRef]
- Jouhanneau, J.-S.; Kremkow, J.; Poulet, J.F.A. Single synaptic inputs drive high-precision action potentials in parvalbumin expressing GABA-ergic cortical neurons in vivo. Nat. Commun. 2018, 9, 1540. [Google Scholar] [CrossRef]
- Jouhanneau, J.-S.; Poulet, J.F.A. Multiple Two-Photon Targeted Whole-Cell Patch-Clamp Recordings From Monosynaptically Connected Neurons in vivo. Front. Synaptic Neurosci. 2019, 11, 15. [Google Scholar] [CrossRef]
- Jouhanneau, J.-S.; Kremkow, J.; Dorrn, A.L.; Poulet, J.F.A. In Vivo Monosynaptic Excitatory Transmission between Layer 2 Cortical Pyramidal Neurons. Cell Rep. 2015, 13, 2098–2106. [Google Scholar] [CrossRef]
- Poulet, J.F.A.; Fernandez, L.M.J.; Crochet, S.; Petersen, C.C.H. Thalamic control of cortical states. Nat. Neurosci. 2012, 15, 370–372. [Google Scholar] [CrossRef]
- Yu, J.; Hu, H.; Agmon, A.; Svoboda, K. Recruitment of GABAergic Interneurons in the Barrel Cortex during Active Tactile Behavior. Neuron 2019, 104, 412–427.e4. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Smith, I.T.; Branco, T.; Häusser, M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature 2013, 503, 115–120. [Google Scholar] [CrossRef]
- Haider, B.; Schulz, D.P.A.; Häusser, M.; Carandini, M. Millisecond Coupling of Local Field Potentials to Synaptic Currents in the Awake Visual Cortex. Neuron 2016, 90, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Funayama, K.; Minamisawa, G.; Matsumoto, N.; Ban, H.; Chan, A.W.; Matsuki, N.; Murphy, T.H.; Ikegaya, Y. Neocortical Rebound Depolarization Enhances Visual Perception. PLoS Biol. 2015, 13, e1002231. [Google Scholar] [CrossRef] [PubMed]
- Funayama, K.; Hagura, N.; Ban, H.; Ikegaya, Y. Functional Organization of Flash-Induced V1 Offline Reactivation. J. Neurosci. 2016, 36, 11727–11738. [Google Scholar] [CrossRef]
- Minamisawa, G.; Funayama, K.; Matsumoto, N.; Matsuki, N.; Ikegaya, Y. Flashing Lights Induce Prolonged Distortions in Visual Cortical Responses and Visual Perception. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Haider, B.; Häusser, M.; Carandini, M. Inhibition dominates sensory responses in the awake cortex. Nature 2013, 493, 97–100. [Google Scholar] [CrossRef]
- Lien, A.D.; Scanziani, M. Cortical direction selectivity emerges at convergence of thalamic synapses. Nature 2018, 558, 80–86. [Google Scholar] [CrossRef]
- Tan, A.Y.Y.; Chen, Y.; Scholl, B.; Seidemann, E.; Priebe, N.J. Sensory stimulation shifts visual cortex from synchronous to asynchronous states. Nature 2014, 509, 226–229. [Google Scholar] [CrossRef]
- Schiemann, J.; Puggioni, P.; Dacre, J.; Pelko, M.; Domanski, A.; van Rossum, M.C.W.; Duguid, I. Cellular Mechanisms Underlying Behavioral State-Dependent Bidirectional Modulation of Motor Cortex Output. Cell Rep. 2015, 11, 1319–1330. [Google Scholar] [CrossRef]
- Guo, Z.V.; Inagaki, H.K.; Daie, K.; Druckmann, S.; Gerfen, C.R.; Svoboda, K. Maintenance of persistent activity in a frontal thalamocortical loop. Nature 2017, 545, 181–186. [Google Scholar] [CrossRef]
- Inagaki, H.K.; Fontolan, L.; Romani, S.; Svoboda, K. Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature 2019, 566, 212–217. [Google Scholar] [CrossRef]
- Bitzenhofer, S.H.; Sieben, K.; Siebert, K.D.; Spehr, M.; Hanganu-Opatz, I.L. Oscillatory Activity in Developing Prefrontal Networks Results from Theta-Gamma-Modulated Synaptic Inputs. Cell Rep. 2015, 11, 486–497. [Google Scholar] [CrossRef]
- DeWeese, M.R.; Zador, A.M. Non-Gaussian Membrane Potential Dynamics Imply Sparse, Synchronous Activity in Auditory Cortex. J. Neurosci. 2006, 26, 12206–12218. [Google Scholar] [CrossRef]
- Lenschow, C.; Brecht, M. Barrel Cortex Membrane Potential Dynamics in Social Touch. Neuron 2015, 85, 718–725. [Google Scholar] [CrossRef]
- Ebbesen, C.L.; Doron, G.; Lenschow, C.; Brecht, M. Vibrissa motor cortex activity suppresses contralateral whisking behavior. Nat. Neurosci. 2017, 20, 82–89. [Google Scholar] [CrossRef]
- Clemens, A.M.; Lenschow, C.; Beed, P.; Li, L.; Sammons, R.; Naumann, R.K.; Wang, H.; Schmitz, D.; Brecht, M. Estrus-Cycle Regulation of Cortical Inhibition. Curr. Biol. 2019, 29, 605–615.e6. [Google Scholar] [CrossRef]
- Poulet, J.F.A.; Petersen, C.C.H. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 2008, 454, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Gentet, L.J.; Avermann, M.; Matyas, F.; Staiger, J.F.; Petersen, C.C.H. Membrane Potential Dynamics of GABAergic Neurons in the Barrel Cortex of Behaving Mice. Neuron 2010, 65, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-J.; Kremkow, J.; Poulet, J.F.A. Translaminar Cortical Membrane Potential Synchrony in Behaving Mice. Cell Rep. 2016, 15, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Pala, A.; Petersen, C.C.H. State-dependent cell-type-specific membrane potential dynamics and unitary synaptic inputs in awake mice. Elife 2018, 7, e35869. [Google Scholar] [CrossRef] [PubMed]
- Kolb, I.; Stoy, W.A.; Rousseau, E.B.; Moody, O.A.; Jenkins, A.; Forest, C.R. Cleaning patch-clamp pipettes for immediate reuse. Sci. Rep. 2016, 6, 35001. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Miyawaki, T.; Morikawa, S.; Susaki, E.A.; Nakashima, A.; Takeuchi, H.; Yamaguchi, S.; Ueda, H.R.; Ikegaya, Y. Visualization and molecular characterization of whole-brain vascular networks with capillary resolution. Nat. Commun. 2020, 11, 1104. [Google Scholar] [CrossRef]
- Matsumoto, N.; Takahara, Y.; Matsuki, N.; Ikegaya, Y. Thoracotomy reduces intrinsic brain movement caused by heartbeat and respiration: A simple method to prevent motion artifact for in vivo experiments. Neurosci. Res. 2011, 71, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.D.; Collman, F.; Dombeck, D.A.; Tank, D.W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 2009, 461, 941–946. [Google Scholar] [CrossRef]
- Epsztein, J.; Lee, A.K.; Chorev, E.; Brecht, M. Impact of Spikelets on Hippocampal CA1 Pyramidal Cell Activity During Spatial Exploration. Science 2010, 327, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Sakaguchi, T.; Kitajo, K.; Ishikawa, D.; Matsumoto, N.; Matsuki, N.; Ikegaya, Y. Sound-induced modulation of hippocampal θ oscillations. Neuroreport 2014, 25, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, F.; Justus, D.; Sosulina, L.; Kaneko, H.; Beutel, T.; Friedrichs, D.; Schoch, S.; Schwarz, M.; Fuhrmann, M.; Remy, S. Locomotion, Theta Oscillations, and the Speed-Correlated Firing of Hippocampal Neurons Are Controlled by a Medial Septal Glutamatergic Circuit. Neuron 2015, 86, 1253–1264. [Google Scholar] [CrossRef]
- Hulse, B.K.; Lubenov, E.V.; Siapas, A.G. Brain State Dependence of Hippocampal Subthreshold Activity in Awake Mice. Cell Rep. 2017, 18, 136–147. [Google Scholar] [CrossRef]
- Kolb, I.; Talei Franzesi, G.; Wang, M.; Kodandaramaiah, S.B.; Forest, C.R.; Boyden, E.S.; Singer, A.C. Evidence for Long-Timescale Patterns of Synaptic Inputs in CA1 of Awake Behaving Mice. J. Neurosci. 2018, 38, 1821–1834. [Google Scholar] [CrossRef]
- Lee, A.K.; Manns, I.D.; Sakmann, B.; Brecht, M. Whole-Cell Recordings in Freely Moving Rats. Neuron 2006, 51, 399–407. [Google Scholar] [CrossRef]
- Lee, A.K.; Epsztein, J.; Brecht, M. Head-anchored whole-cell recordings in freely moving rats. Nat. Protoc. 2009, 4, 385–392. [Google Scholar] [CrossRef]
- Lee, D.; Shtengel, G.; Osborne, J.E.; Lee, A.K. Anesthetized- and awake-patched whole-cell recordings in freely moving rats using UV-cured collar-based electrode stabilization. Nat. Protoc. 2014, 9, 2784–2795. [Google Scholar] [CrossRef]
- Lee, A.K.; Brecht, M. Elucidating Neuronal Mechanisms Using Intracellular Recordings during Behavior. Trends Neurosci. 2018, 41, 385–403. [Google Scholar] [CrossRef]
- Petersen, C.C.H. Whole-Cell Recording of Neuronal Membrane Potential during Behavior. Neuron 2017, 95, 1266–1281. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, D.; Takahashi, N.; Sasaki, T.; Usami, A.; Matsuki, N.; Ikegaya, Y. Fluorescent pipettes for optically targeted patch-clamp recordings. Neural Netw. 2010, 23, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Piccione, R.; Regalado-Reyes, M.; Fernaud-Espinosa, I.; Kastanauskaite, A.; Tapia-González, S.; León-Espinosa, G.; Rojo, C.; Insausti, R.; Segev, I.; DeFelipe, J. Differential Structure of Hippocampal CA1 Pyramidal Neurons in the Human and Mouse. Cereb. Cortex 2020, 30, 730–752. [Google Scholar] [CrossRef]
- Zhuravleva, Z.N.; Saifullina, V.N.; Zenchenko, C.I. Morphometric Analysis of Hippocampal Pyramidal Neurons in situ and in Grafts Developing in the Anterior Eye Chambers of Young and Aged Wistar Rats. J. Neural Transplant. Plast. 1997, 6, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Epsztein, J.; Brecht, M.; Lee, A.K. Intracellular Determinants of Hippocampal CA1 Place and Silent Cell Activity in a Novel Environment. Neuron 2011, 70, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lin, B.-J.; Lee, A.K. Hippocampal Place Fields Emerge upon Single-Cell Manipulation of Excitability During Behavior. Science 2012, 337, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Rich, P.D.; Liaw, H.-P.; Lee, A.K. Large environments reveal the statistical structure governing hippocampal representations. Science 2014, 345, 814–817. [Google Scholar] [CrossRef]
- Bittner, K.C.; Grienberger, C.; Vaidya, S.P.; Milstein, A.D.; Macklin, J.J.; Suh, J.; Tonegawa, S.; Magee, J.C. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat. Neurosci. 2015, 18, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Bolstad, M.; Lee, A.K. Experience-dependent shaping of hippocampal CA1 intracellular activity in novel and familiar environments. Elife 2017, 6, e23040. [Google Scholar] [CrossRef]
- Grienberger, C.; Milstein, A.D.; Bittner, K.C.; Romani, S.; Magee, J.C. Inhibitory suppression of heterogeneously tuned excitation enhances spatial coding in CA1 place cells. Nat. Neurosci. 2017, 20, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Bittner, K.C.; Milstein, A.D.; Grienberger, C.; Romani, S.; Magee, J.C. Behavioral time scale synaptic plasticity underlies CA1 place fields. Science 2017, 357, 1033–1036. [Google Scholar] [CrossRef]
- Morgan, P.J.; Bourboulou, R.; Filippi, C.; Koenig-Gambini, J.; Epsztein, J. Kv1.1 contributes to a rapid homeostatic plasticity of intrinsic excitability in CA1 pyramidal neurons in vivo. Elife 2019, 8, e49915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Spruston, N.; Magee, J.C. Membrane potential dynamics underlying context-dependent sensory responses in the hippocampus. Nat. Neurosci. 2020, 23, 881–891. [Google Scholar] [CrossRef]
- Zhang, X.; Schlögl, A.; Jonas, P. Selective Routing of Spatial Information Flow from Input to Output in Hippocampal Granule Cells. Neuron 2020, 107, 1212–1225.e7. [Google Scholar] [CrossRef]
- Domnisoru, C.; Kinkhabwala, A.A.; Tank, D.W. Membrane potential dynamics of grid cells. Nature 2013, 495, 199–204. [Google Scholar] [CrossRef]
- Schmidt-Hieber, C.; Häusser, M. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat. Neurosci. 2013, 16, 325–331. [Google Scholar] [CrossRef]
- Buzsáki, G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015, 25, 1073–1188. [Google Scholar] [CrossRef]
- Buzsáki, G. Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience 1989, 31, 551–570. [Google Scholar] [CrossRef]
- Buzsáki, G. Theta oscillations in the hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef]
- Buzsáki, G.; Tingley, D. Space and Time: The Hippocampus as a Sequence Generator. Trends Cogn. Sci. 2018, 22, 853–869. [Google Scholar] [CrossRef]
- Pernía-Andrade, A.J.; Jonas, P. Theta-Gamma-Modulated Synaptic Currents in Hippocampal Granule Cells In Vivo Define a Mechanism for Network Oscillations. Neuron 2014, 81, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, D.; Matsumoto, N.; Sakaguchi, T.; Matsuki, N.; Ikegaya, Y. Operant Conditioning of Synaptic and Spiking Activity Patterns in Single Hippocampal Neurons. J. Neurosci. 2014, 34, 5044–5053. [Google Scholar] [CrossRef] [PubMed]
- Malezieux, M.; Kees, A.L.; Mulle, C. Theta Oscillations Coincide with Sustained Hyperpolarization in CA3 Pyramidal Cells, Underlying Decreased Firing. Cell Rep. 2020, 32, 107868. [Google Scholar] [CrossRef] [PubMed]
- English, D.F.; Peyrache, A.; Stark, E.; Roux, L.; Vallentin, D.; Long, M.A.; Buzsáki, G. Excitation and inhibition compete to control spiking during hippocampal ripples: Intracellular study in behaving mice. J. Neurosci. 2014, 34, 16509–16517. [Google Scholar] [CrossRef]
- Böhm, C.; Peng, Y.; Maier, N.; Winterer, J.; Poulet, J.F.A.; Geiger, J.R.P.; Schmitz, D. Functional Diversity of Subicular Principal Cells during Hippocampal Ripples. J. Neurosci. 2015, 35, 13608–13618. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.; Cid, E.; Averkin, R.G.; Aguilar, J.; Sanchez-Aguilera, A.; Viney, T.J.; Gomez-Dominguez, D.; Bellistri, E.; de la Prida, L.M. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat. Neurosci. 2015, 18, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Hulse, B.K.; Moreaux, L.C.; Lubenov, E.V.; Siapas, A.G. Membrane Potential Dynamics of CA1 Pyramidal Neurons during Hippocampal Ripples in Awake Mice. Neuron 2016, 89, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Weng, S.; Pernía-Andrade, A.J.; Csicsvari, J.; Jonas, P. Phase-Locked Inhibition, but Not Excitation, Underlies Hippocampal Ripple Oscillations in Awake Mice In Vivo. Neuron 2017, 93, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.; de la Prida, L.M. The hippocampus in depth: A sublayer-specific perspective of entorhinal–hippocampal function. Curr. Opin. Neurobiol. 2018, 52, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Šišková, Z.; Justus, D.; Kaneko, H.; Friedrichs, D.; Henneberg, N.; Beutel, T.; Pitsch, J.; Schoch, S.; Becker, A.; von der Kammer, H.; et al. Dendritic Structural Degeneration Is Functionally Linked to Cellular Hyperexcitability in a Mouse Model of Alzheimer’s Disease. Neuron 2014, 84, 1023–1033. [Google Scholar] [CrossRef]
- Jordan, R.; Fukunaga, I.; Kollo, M.; Schaefer, A.T. Active Sampling State Dynamically Enhances Olfactory Bulb Odor Representation. Neuron 2018, 98, 1214–1228.e5. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.; Mathy, A.; Duguid, I.; Häusser, M. Synaptic representation of locomotion in single cerebellar granule cells. Elife 2015, 4, e07290. [Google Scholar] [CrossRef]
- Clemens, A.M.; Wang, H.; Brecht, M. The lateral septum mediates kinship behavior in the rat. Nat. Commun. 2020, 11, 3161. [Google Scholar] [CrossRef]
- Covey, E.; Kauer, J.A.; Casseday, J.H. Whole-Cell Patch-Clamp Recording Reveals Subthreshold Sound-Evoked Postsynaptic Currents in the Inferior Colliculus of Awake Bats. J. Neurosci. 1996, 16, 3009–3018. [Google Scholar] [CrossRef]
- Xie, R.; Gittelman, J.X.; Pollak, G.D. Rethinking Tuning: In Vivo Whole-Cell Recordings of the Inferior Colliculus in Awake Bats. J. Neurosci. 2007, 27, 9469–9481. [Google Scholar] [CrossRef]
- Xie, R.; Gittelman, J.X.; Li, N.; Pollak, G.D. Whole cell recordings of intrinsic properties and sound-evoked responses from the inferior colliculus. Neuroscience 2008, 154, 245–256. [Google Scholar] [CrossRef]
- Li, N.; Gittelman, J.X.; Pollak, G.D. Intracellular Recordings Reveal Novel Features of Neurons That Code Interaural Intensity Disparities in the Inferior Colliculus. J. Neurosci. 2010, 30, 14573–14584. [Google Scholar] [CrossRef] [PubMed]
- Gittelman, J.X.; Pollak, G.D. It’s About Time: How Input Timing Is Used and Not Used To Create Emergent Properties in the Auditory System. J. Neurosci. 2011, 31, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Avermann, M.; Gentet, L.J.; Zhang, F.; Deisseroth, K.; Petersen, C.C.H. In Vivo Optogenetic Stimulation of Neocortical Excitatory Neurons Drives Brain-State-Dependent Inhibition. Curr. Biol. 2011, 21, 1593–1602. [Google Scholar] [CrossRef]
- Lien, A.D.; Scanziani, M. Tuned thalamic excitation is amplified by visual cortical circuits. Nat. Neurosci. 2013, 16, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Pala, A.; Petersen, C.C.H. In Vivo Measurement of Cell-Type-Specific Synaptic Connectivity and Synaptic Transmission in Layer 2/3 Mouse Barrel Cortex. Neuron 2015, 85, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, K.; Lien, A.D.; Scanziani, M. Distinct recurrent versus afferent dynamics in cortical visual processing. Nat. Neurosci. 2015, 18, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Valeeva, G.; Tressard, T.; Mukhtarov, M.; Baude, A.; Khazipov, R. An Optogenetic Approach for Investigation of Excitatory and Inhibitory Network GABA Actions in Mice Expressing Channelrhodopsin-2 in GABAergic Neurons. J. Neurosci. 2016, 36, 5961–5973. [Google Scholar] [CrossRef]
- Zucca, S.; Griguoli, M.; Malézieux, M.; Grosjean, N.; Carta, M.; Mulle, C. Control of Spike Transfer at Hippocampal Mossy Fiber Synapses In Vivo by GABA A and GABA B Receptor-Mediated Inhibition. J. Neurosci. 2017, 37, 587–598. [Google Scholar] [CrossRef]
- Kato, H.K.; Asinof, S.K.; Isaacson, J.S. Network-Level Control of Frequency Tuning in Auditory Cortex. Neuron 2017, 95, 412–423.e4. [Google Scholar] [CrossRef]
- González-Rueda, A.; Pedrosa, V.; Feord, R.C.; Clopath, C.; Paulsen, O. Activity-Dependent Downscaling of Subthreshold Synaptic Inputs during Slow-Wave-Sleep-like Activity In Vivo. Neuron 2018, 97, 1244–1252.e5. [Google Scholar] [CrossRef]
- Petersen, C.C.H.; Hahn, T.T.G.; Mehta, M.; Grinvald, A.; Sakmann, B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc. Natl. Acad. Sci. USA 2003, 100, 13638–13643. [Google Scholar] [CrossRef]
- Kitamura, K.; Häusser, M. Dendritic Calcium Signaling Triggered by Spontaneous and Sensory-Evoked Climbing Fiber Input to Cerebellar Purkinje Cells In Vivo. J. Neurosci. 2011, 31, 10847–10858. [Google Scholar] [CrossRef]
- Deubner, J.; Coulon, P.; Diester, I. Optogenetic approaches to study the mammalian brain. Curr. Opin. Struct. Biol. 2019, 57, 157–163. [Google Scholar] [CrossRef]
- Adamantidis, A.R.; Zhang, F.; de Lecea, L.; Deisseroth, K. Optogenetics: Opsins and Optical Interfaces in Neuroscience. Cold Spring Harb. Protoc. 2014, 2014, pdb–top083329. [Google Scholar] [CrossRef][Green Version]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef]
- Boyden, E.S. Optogenetics and the future of neuroscience. Nat. Neurosci. 2015, 18, 1200–1201. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.; Gauron, C.; Volovitch, M.; Bensimon, D.; Jullien, L.; Vriz, S. How to control proteins with light in living systems. Nat. Chem. Biol. 2014, 10, 533–541. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, R.S.; Bedbrook, C.N.; Arnold, F.H. Recent advances in engineering microbial rhodopsins for optogenetics. Curr. Opin. Struct. Biol. 2015, 33, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Arlt, C.; Häusser, M. Microcircuit Rules Governing Impact of Single Interneurons on Purkinje Cell Output In Vivo. Cell Rep. 2020, 30, 3020–3035.e3. [Google Scholar] [CrossRef]
- Bureau, I.; Shepherd, G.M.G.; Svoboda, K. Precise Development of Functional and Anatomical Columns in the Neocortex. Neuron 2004, 42, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, W.; Tremblay, R.; Rudy, B. Channelrhodopsin-Assisted Patching: In Vivo Recording of Genetically and Morphologically Identified Neurons throughout the Brain. Cell Rep. 2014, 9, 2304–2316. [Google Scholar] [CrossRef]
- van Welie, I.; Roth, A.; Ho, S.S.N.; Komai, S.; Häusser, M. Conditional Spike Transmission Mediated by Electrical Coupling Ensures Millisecond Precision-Correlated Activity among Interneurons In Vivo. Neuron 2016, 90, 810–823. [Google Scholar] [CrossRef]
- Chen, X.; Kovalchuk, Y.; Adelsberger, H.; Henning, H.A.; Sausbier, M.; Wietzorrek, G.; Ruth, P.; Yarom, Y.; Konnerth, A. Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels. Proc. Natl. Acad. Sci. USA 2010, 107, 12323–12328. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.C.H.; Grinvald, A.; Sakmann, B. Spatiotemporal Dynamics of Sensory Responses in Layer 2/3 of Rat Barrel Cortex Measured In Vivo by Voltage-Sensitive Dye Imaging Combined with Whole-Cell Voltage Recordings and Neuron Reconstructions. J. Neurosci. 2003, 23, 1298–1309. [Google Scholar] [CrossRef]
- Grinvald, A.; Lieke, E.; Frostig, R.D.; Gilbert, C.D.; Wiesel, T.N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 1986, 324, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Kleinfeld, D.; Delaney, K.R. Distributed representation of vibrissa movement in the upper layers of somatosensory cortex revealed with voltage-sensitive dyes. J. Comp. Neurol. 1996, 375, 89–108. [Google Scholar] [CrossRef]
- Cohen, L.B.; Keynes, R.D.; Hille, B. Light Scattering and Birefringence Changes during Nerve Activity. Nature 1968, 218, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, B.M.; Davila, H.V.; Cohen, L.B. Optical Recording of Impulses in Individual Neurones of an Invertebrate Central Nervous System. Nature 1973, 246, 508–509. [Google Scholar] [CrossRef]
- Grinvald, A.; Anglister, L.; Freeman, J.A.; Hildesheim, R.; Manker, A. Real-time optical imaging of naturally evoked electrical activity in intact frog brain. Nature 1984, 308, 848–850. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, S.; Wang, Z. In vivo whole-cell recording with high success rate in anaesthetized and awake mammalian brains. Mol. Brain 2016, 9, 86. [Google Scholar] [CrossRef]
- Atherton, L.A.; Burnell, E.S.; Mellor, J.R. Assessment of Methods for the Intracellular Blockade of GABAA Receptors. PLoS ONE 2016, 11, e0160900. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, C.; Okamoto, K.; Mochizuki, Y.; Urakubo, H.; Funayama, K.; Ishikawa, T.; Kashima, T.; Ouchi, A.; Szymanska, A.F.; Ishii, S.; et al. GABAergic inhibition reduces the impact of synaptic excitation on somatic excitation. Neurosci. Res. 2019, 146, 22–35. [Google Scholar] [CrossRef]
- Palmer, L.M.; Shai, A.S.; Reeve, J.E.; Anderson, H.L.; Paulsen, O.; Larkum, M.E. NMDA spikes enhance action potential generation during sensory input. Nat. Neurosci. 2014, 17, 383–390. [Google Scholar] [CrossRef]
- Liu, H.; Lan, Y.; Bing, Y.-H.; Chu, C.-P.; Qiu, D.-L. N-methyl-D-Aspartate Receptors Contribute to Complex Spike Signaling in Cerebellar Purkinje Cells: An In vivo Study in Mice. Front. Cell. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Wu, M.-C.; Bing, Y.-H.; Chu, C.-P.; Qiu, D.-L. Ethanol modulates facial stimulation-evoked outward currents in cerebellar Purkinje cells in vivo in mice. Sci. Rep. 2016, 6, 30857. [Google Scholar] [CrossRef]
- Jin, X.-H.; Wang, H.-W.; Zhang, X.-Y.; Chu, C.-P.; Jin, Y.-Z.; Cui, S.-B.; Qiu, D.-L. Mechanisms of Spontaneous Climbing Fiber Discharge-Evoked Pauses and Output Modulation of Cerebellar Purkinje Cell in Mice. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Wu, M.-C.; Shi, J.-D.; Xu, Y.-H.; Chu, C.-P.; Cui, S.-B.; Qiu, D.-L. Ethanol Modulates the Spontaneous Complex Spike Waveform of Cerebellar Purkinje Cells Recorded in vivo in Mice. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Sáez, M.; Ketzef, M.; Alegre-Cortés, J.; Reig, R.; Silberberg, G. A New Micro-holder Device for Local Drug Delivery during In Vivo Whole-cell Recordings. Neuroscience 2018, 381, 115–123. [Google Scholar] [CrossRef]
- Marshel, J.H.; Mori, T.; Nielsen, K.J.; Callaway, E.M. Targeting Single Neuronal Networks for Gene Expression and Cell Labeling In Vivo. Neuron 2010, 67, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Rancz, E.A.; Franks, K.M.; Schwarz, M.K.; Pichler, B.; Schaefer, A.T.; Margrie, T.W. Transfection via whole-cell recording in vivo: Bridging single-cell physiology, genetics and connectomics. Nat. Neurosci. 2011, 14, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Fort, M.; Rousseau, C.V.; Niedworok, C.J.; Wickersham, I.R.; Rancz, E.A.; Brown, A.P.Y.; Strom, M.; Margrie, T.W. The Stimulus Selectivity and Connectivity of Layer Six Principal Cells Reveals Cortical Microcircuits Underlying Visual Processing. Neuron 2014, 83, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E.M.; Luo, L. Monosynaptic Circuit Tracing with Glycoprotein-Deleted Rabies Viruses. J. Neurosci. 2015, 35, 8979–8985. [Google Scholar] [CrossRef]
- Albisetti, G.W.; Ghanem, A.; Foster, E.; Conzelmann, K.-K.; Zeilhofer, H.U.; Wildner, H. Identification of Two Classes of Somatosensory Neurons That Display Resistance to Retrograde Infection by Rabies Virus. J. Neurosci. 2017, 37, 10358–10371. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Morimoto, N.; Akaike, A.; Osakada, F. Multiplex Neural Circuit Tracing With G-Deleted Rabies Viral Vectors. Front. Neural Circuits 2020, 13. [Google Scholar] [CrossRef]
- Schwarz, M.K.; Remy, S. Rabies virus-mediated connectivity tracing from single neurons. J. Neurosci. Methods 2019, 325, 108365. [Google Scholar] [CrossRef] [PubMed]
- Lavin, T.K.; Jin, L.; Wickersham, I.R. Monosynaptic tracing: A step-by-step protocol. J. Chem. Neuroanat. 2019, 102, 101661. [Google Scholar] [CrossRef]
- Ginger, M.; Haberl, M.; Conzelmann, K.-K.; Schwarz, M.K.; Frick, A. Revealing the secrets of neuronal circuits with recombinant rabies virus technology. Front. Neural Circuits 2013, 7. [Google Scholar] [CrossRef]
- Osakada, F.; Mori, T.; Cetin, A.H.; Marshel, J.H.; Virgen, B.; Callaway, E.M. New Rabies Virus Variants for Monitoring and Manipulating Activity and Gene Expression in Defined Neural Circuits. Neuron 2011, 71, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Fuzik, J.; Zeisel, A.; Máté, Z.; Calvigioni, D.; Yanagawa, Y.; Szabó, G.; Linnarsson, S.; Harkany, T. Integration of electrophysiological recordings with single-cell RNA-seq data identifies neuronal subtypes. Nat. Biotechnol. 2016, 34, 175–183. [Google Scholar] [CrossRef]
- Cadwell, C.R.; Sandberg, R.; Jiang, X.; Tolias, A.S. Q&A: Using Patch-seq to profile single cells. BMC Biol. 2017, 15, 58. [Google Scholar] [CrossRef]
- Cadwell, C.R.; Scala, F.; Li, S.; Livrizzi, G.; Shen, S.; Sandberg, R.; Jiang, X.; Tolias, A.S. Multimodal profiling of single-cell morphology, electrophysiology, and gene expression using Patch-seq. Nat. Protoc. 2017, 12, 2531–2553. [Google Scholar] [CrossRef]
- Toledo-Rodriguez, M.; Markram, H. Single-Cell RT-PCR, a Technique to Decipher the Electrical, Anatomical, and Genetic Determinants of Neuronal Diversity. In Patch-Clamp Methods and Protocols; Martina, M., Taverna, S., Eds.; Humana Press: New York, NY, USA, 2014; pp. 143–158. [Google Scholar]
- Li, Y.; Xu, J.; Liu, Y.; Zhu, J.; Liu, N.; Zeng, W.; Huang, N.; Rasch, M.J.; Jiang, H.; Gu, X.; et al. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat. Neurosci. 2017, 20, 559–570. [Google Scholar] [CrossRef]
- Hashikawa, Y.; Hashikawa, K.; Rossi, M.A.; Basiri, M.L.; Liu, Y.; Johnston, N.L.; Ahmad, O.R.; Stuber, G.D. Transcriptional and Spatial Resolution of Cell Types in the Mammalian Habenula. Neuron 2020, 106, 743–758.e5. [Google Scholar] [CrossRef]
- Lipovsek, M.; Bardy, C.; Cadwell, C.R.; Hadley, K.; Kobak, D.; Tripathy, S.J. Patch-seq: Past, Present, and Future. J. Neurosci. 2021, 41, 937–946. [Google Scholar] [CrossRef]
- Lee, B.R.; Budzillo, A.; Hadley, K.; Miller, J.A.; Jarsky, T.; Baker, K.; Hill, D.; Kim, L.; Mann, R.; Ng, L.; et al. Scaled, high fidelity electrophysiological, morphological, and transcriptomic cell characterization. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cadwell, C.R.; Palasantza, A.; Jiang, X.; Berens, P.; Deng, Q.; Yilmaz, M.; Reimer, J.; Shen, S.; Bethge, M.; Tolias, K.F.; et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat. Biotechnol. 2016, 34, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.J.; Toker, L.; Bomkamp, C.; Mancarci, B.O.; Belmadani, M.; Pavlidis, P. Assessing Transcriptome Quality in Patch-Seq Datasets. Front. Mol. Neurosci. 2018, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Zhang, G.; Xiong, Y.; Zhou, Y. Functional dissection of synaptic circuits: In vivo patch-clamp recording in neuroscience. Front. Neural Circuits 2015, 9, 23. [Google Scholar] [CrossRef]
- Tang, Q.; Tsytsarev, V.; Liang, C.-P.; Akkentli, F.; Erzurumlu, R.S.; Chen, Y. In Vivo Voltage-Sensitive Dye Imaging of Subcortical Brain Function. Sci. Rep. 2015, 5, 17325. [Google Scholar] [CrossRef]
- Siegel, M.S.; Isacoff, E.Y. A Genetically Encoded Optical Probe of Membrane Voltage. Neuron 1997, 19, 735–741. [Google Scholar] [CrossRef]
- Abdelfattah, A.S.; Kawashima, T.; Singh, A.; Novak, O.; Liu, H.; Shuai, Y.; Huang, Y.C.; Campagnola, L.; Seeman, S.C.; Yu, J.; et al. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 2019, 365, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Piatkevich, K.D.; Bensussen, S.; Tseng, H.; Shroff, S.N.; Lopez-Huerta, V.G.; Park, D.; Jung, E.E.; Shemesh, O.A.; Straub, C.; Gritton, H.J.; et al. Population imaging of neural activity in awake behaving mice. Nature 2019, 574, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Adam, Y.; Kim, J.J.; Lou, S.; Zhao, Y.; Xie, M.E.; Brinks, D.; Wu, H.; Mostajo-Radji, M.A.; Kheifets, S.; Parot, V.; et al. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 2019, 569, 413–417. [Google Scholar] [CrossRef]
- Jin, L.; Han, Z.; Platisa, J.; Wooltorton, J.R.A.; Cohen, L.B.; Pieribone, V.A. Single Action Potentials and Subthreshold Electrical Events Imaged in Neurons with a Fluorescent Protein Voltage Probe. Neuron 2012, 75, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; St-Pierre, F. Genetically Encoded Voltage Indicators: Opportunities and Challenges. J. Neurosci. 2016, 36, 9977–9989. [Google Scholar] [CrossRef]
- Hochbaum, D.R.; Zhao, Y.; Farhi, S.L.; Klapoetke, N.; Werley, C.A.; Kapoor, V.; Zou, P.; Kralj, J.M.; Maclaurin, D.; Smedemark-Margulies, N.; et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 2014, 11, 825–833. [Google Scholar] [CrossRef]
- Lou, S.; Adam, Y.; Weinstein, E.N.; Williams, E.; Williams, K.; Parot, V.; Kavokine, N.; Liberles, S.; Madisen, L.; Zeng, H.; et al. Genetically Targeted All-Optical Electrophysiology with a Transgenic Cre-Dependent Optopatch Mouse. J. Neurosci. 2016, 36, 11059–11073. [Google Scholar] [CrossRef]
- Fan, L.Z.; Kheifets, S.; Böhm, U.L.; Wu, H.; Piatkevich, K.D.; Xie, M.E.; Parot, V.; Ha, Y.; Evans, K.E.; Boyden, E.S.; et al. All-Optical Electrophysiology Reveals the Role of Lateral Inhibition in Sensory Processing in Cortical Layer 1. Cell 2020, 180, 521–535.e18. [Google Scholar] [CrossRef]
- Kannan, M.; Vasan, G.; Pieribone, V.A. Optimizing Strategies for Developing Genetically Encoded Voltage Indicators. Front. Cell. Neurosci. 2019, 13, 53. [Google Scholar] [CrossRef]
- Harris, N.C.; Constanti, A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J. Neurophysiol. 1995, 74, 2366–2378. [Google Scholar] [CrossRef]
- Annecchino, L.A.; Schultz, S.R. Progress in automating patch clamp cellular physiology. Brain Neurosci. Adv. 2018, 2, 239821281877656. [Google Scholar] [CrossRef]
- Annecchino, L.A.; Morris, A.R.; Copeland, C.S.; Agabi, O.E.; Chadderton, P.; Schultz, S.R. Robotic Automation of In Vivo Two-Photon Targeted Whole-Cell Patch-Clamp Electrophysiology. Neuron 2017, 95, 1048–1055.e3. [Google Scholar] [CrossRef]
- Kodandaramaiah, S.B.; Flores, F.J.; Holst, G.L.; Singer, A.C.; Han, X.; Brown, E.N.; Boyden, E.S.; Forest, C.R. Multi-neuron intracellular recording in vivo via interacting autopatching robots. Elife 2018, 7, e24656. [Google Scholar] [CrossRef]
- Holst, G.L.; Stoy, W.; Yang, B.; Kolb, I.; Kodandaramaiah, S.B.; Li, L.; Knoblich, U.; Zeng, H.; Haider, B.; Boyden, E.S.; et al. Autonomous patch-clamp robot for functional characterization of neurons in vivo: Development and application to mouse visual cortex. J. Neurophysiol. 2019, 121, 2341–2357. [Google Scholar] [CrossRef]
- Suk, H.-J.; Boyden, E.S.; van Welie, I. Advances in the automation of whole-cell patch clamp technology. J. Neurosci. Methods 2019, 326, 108357. [Google Scholar] [CrossRef]
- Suk, H.-J.; van Welie, I.; Kodandaramaiah, S.B.; Allen, B.; Forest, C.R.; Boyden, E.S. Closed-Loop Real-Time Imaging Enables Fully Automated Cell-Targeted Patch-Clamp Neural Recording In Vivo. Neuron 2017, 95, 1037–1047.e11. [Google Scholar] [CrossRef]
- Kodandaramaiah, S.B.; Franzesi, G.T.; Chow, B.Y.; Boyden, E.S.; Forest, C.R. Automated whole-cell patch-clamp electrophysiology of neurons in vivo. Nat. Methods 2012, 9, 585–587. [Google Scholar] [CrossRef]
- Kodandaramaiah, S.B.; Holst, G.L.; Wickersham, I.R.; Singer, A.C.; Franzesi, G.T.; McKinnon, M.L.; Forest, C.R.; Boyden, E.S. Assembly and operation of the autopatcher for automated intracellular neural recording in vivo. Nat. Protoc. 2016, 11, 634–654. [Google Scholar] [CrossRef]
- Singer, A.C.; Talei Franzesi, G.; Kodandaramaiah, S.B.; Flores, F.J.; Cohen, J.D.; Lee, A.K.; Borgers, C.; Forest, C.R.; Kopell, N.J.; Boyden, E.S. Mesoscale-duration activated states gate spiking in response to fast rises in membrane voltage in the awake brain. J. Neurophysiol. 2017, 118, 1270–1291. [Google Scholar] [CrossRef]
- Stoy, W.A.; Kolb, I.; Holst, G.L.; Liew, Y.; Pala, A.; Yang, B.; Boyden, E.S.; Stanley, G.B.; Forest, C.R. Robotic navigation to subcortical neural tissue for intracellular electrophysiology in vivo. J. Neurophysiol. 2017, 118, 1141–1150. [Google Scholar] [CrossRef]
- Zhang, R.; Du, J. In Vivo Whole-Cell Patch-Clamp Recording in the Zebrafish Brain. In Zebrafish; Kawakami, K., Patton, E.E., Orger, M., Eds.; Humana Press: New York, NY, USA, 2016; pp. 281–291. [Google Scholar]
- Chang, W.; Pedroni, A.; Hohendorf, V.; Giacomello, S.; Hibi, M.; Köster, R.W.; Ampatzis, K. Functionally distinct Purkinje cell types show temporal precision in encoding locomotion. Proc. Natl. Acad. Sci. USA 2020, 117, 17330–17337. [Google Scholar] [CrossRef]
- Roy, A.; Osik, J.J.; Meschede-Krasa, B.; Alford, W.T.; Leman, D.P.; Van Hooser, S.D. Synaptic and intrinsic mechanisms underlying development of cortical direction selectivity. Elife 2020, 9, e58509. [Google Scholar] [CrossRef]
- Ferster, D.; Jagadeesh, B. EPSP-IPSP interactions in cat visual cortex studied with in vivo whole- cell patch recording. J. Neurosci. 1992, 12, 1262–1274. [Google Scholar] [CrossRef]
- Volgushev, M.; Pei, X.; Vidyasagar, T.R.; Creutzfeldt, O.D. Excitation and inhibition in orientation selectivity of cat visual cortex neurons revealed by whole-cell recordings in vivo. Vis. Neurosci. 1993, 10, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Schramm, A.E.; Marinazzo, D.; Gener, T.; Graham, L.J. The Touch and Zap Method for In Vivo Whole-Cell Patch Recording of Intrinsic and Visual Responses of Cortical Neurons and Glial Cells. PLoS ONE 2014, 9, e97310. [Google Scholar] [CrossRef]
- Lindsay, T.H.; Thiele, T.R.; Lockery, S.R. Optogenetic analysis of synaptic transmission in the central nervous system of the nematode Caenorhabditis elegans. Nat. Commun. 2011, 2, 306. [Google Scholar] [CrossRef]
- Davie, J.T.; Kole, M.H.P.; Letzkus, J.J.; Rancz, E.A.; Spruston, N.; Stuart, G.J.; Häusser, M. Dendritic patch-clamp recording. Nat. Protoc. 2006, 1, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Matsuki, N.; Ikegaya, Y. Targeted axon-attached recording with fluorescent patch-clamp pipettes in brain slices. Nat. Protoc. 2012, 7, 1228–1234. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, A.; Ikegaya, Y.; Matsumoto, N. In Vivo Whole-Cell Patch-Clamp Methods: Recent Technical Progress and Future Perspectives. Sensors 2021, 21, 1448. https://doi.org/10.3390/s21041448
Noguchi A, Ikegaya Y, Matsumoto N. In Vivo Whole-Cell Patch-Clamp Methods: Recent Technical Progress and Future Perspectives. Sensors. 2021; 21(4):1448. https://doi.org/10.3390/s21041448
Chicago/Turabian StyleNoguchi, Asako, Yuji Ikegaya, and Nobuyoshi Matsumoto. 2021. "In Vivo Whole-Cell Patch-Clamp Methods: Recent Technical Progress and Future Perspectives" Sensors 21, no. 4: 1448. https://doi.org/10.3390/s21041448
APA StyleNoguchi, A., Ikegaya, Y., & Matsumoto, N. (2021). In Vivo Whole-Cell Patch-Clamp Methods: Recent Technical Progress and Future Perspectives. Sensors, 21(4), 1448. https://doi.org/10.3390/s21041448





